Abstract
AIM: To compare the predictive power of different endothelial progenitor cell (EPC) phenotypic markers for future cardiovascular events.
METHODS: Peripheral blood was collected from 76 consecutive patients with acute coronary syndromes (ACS) who underwent percutaneous coronary intervention in our institute. The various EPC phenotypes of peripheral blood mononuclear cells were CD34+CD133+, CD34+KDR+, and CD 133+KDR+. The outcome endpoint included cardiovascular mortality, recurrent ACS, and hospitalization for decompensated heart failure during a 24-mo follow-up period.
RESULTS: CD34+CD133+ cells (P = 0.034), but not CD34+KDR+ (P = 0.35) or CD 133+KDR+ cells (P = 0.19), were found to predict recurrent ACS. We found no correlation between EPCs measured by any of the three phenotypic combinations of accepted CD markers and the total combination of these separate outcomes.
CONCLUSION: The EPC CD34+CD133+ phenotype, but not the CD34+KDR+ or the CD 133+KDR+ phenotypes, is predictive of future adverse cardiovascular outcomes.
Keywords: Stem cells, Endothelial progenitor cells, Acute coronary syndrome, Biomarkers
INTRODUCTION
Endothelial progenitor cells (EPCs) are a scarce population of bone-derived cells that can play an important role in neoangiogenesis after tissue ischemia has occurred[1,2]. EPCs are positive for CD34 or the more immature marker protein CD133. Recent studies have shown that expression of the CD34 surface antigen is shared by EPCs, hematopoietic progenitor cells, as well as mature endothelial cells[3].
As they mature, EPCs lose the CD133 marker and acquire vascular endothelial growth factor (VEGF) receptor-2, also known as KDR[4-6]. Circulating numbers of EPCs correlate negatively with risk factors for atherosclerosis and with disorders associated with vascular dysfunction[7-9]. In acute coronary syndrome (ACS) patients, there appears to be a trend toward an elevated number of EPCs, suggesting that these cells are possibly mobilized in an attempt to participate in vessel repair after severe ischemia[10-12].
While there is strong evidence to link a reduced number of EPCs to cardiovascular risk factors or disorders, the relationship between levels of EPCs and cardiovascular outcomes is not clear. A recently published large prospective observational study in patients with stable coronary artery disease (CAD) confirmed by angiography showed that a low number of circulating CD34+KDR+ EPCs is associated with a significantly higher risk of death from cardiovascular disease, a first major cardiovascular event, revascularization and hospitalization in comparison to patients with high EPC numbers. However, no significant association was detected between EPC levels and acute myocardial infarction (MI) and death from any cause[13].
Similarly, Schmidt-Lucke et al[14] found, in a mixed population of patients with CAD and healthy individuals, that reduced numbers of EPCs, also characterized by fluorescence-activated cell sorting (FACS) analysis as CD34+KDR+ EPCs, were a significant independent predictor of adverse cardiovascular events over a median follow-up period of 10 mo.
Although attractive, a major obstacle in incorporating FACS analysis of EPCs as a practical biomarker in cardiovascular risk assessment is the lack of fully corroborated and mutually comparative methods for characterizing the putative EPCs[6,15]. Thus, different investigators employ different FACS marker combinations for assessment of EPCs: CD34+KDR+[16,17], CD34+133+[18] or CD34+CD133+KDR+[5]. Both KDR- and CD133-positive cells were shown to differentiate into endothelial cells and were thus suggested as identifying membrane antigens[6].
The purpose of this study was to compare the predictive power of different EPC populations with regard to future adverse cardiovascular events in patients with ACS undergoing coronary angiography.
MATERIALS AND METHODS
Study subjects
We studied a total of 76 consecutive patients with ACS (33 patients had ST-elevation MI, 43 had non-ST-elevation MI), who underwent coronary angiography in our institution. There were 53 males and 23 females, aged 42-86 years (median, 68 years). Table 1 summarizes the demographic and clinical characteristics of the patient population. The institutional ethics committee approved the study and informed consent was obtained from all patients.
Table 1.
Baseline characteristics of the patient population and drug treatment n (%)
| Characteristics | n = 76 |
| Demographic data | |
| Male/female | 53 (70)/23 (30) |
| Median age (range, yr) | 69 (42-86) |
| Current smoker | 35 (46) |
| Comorbidities | |
| Hypertension | 48 (63) |
| Diabetes mellitus | 20 (26) |
| Hyperlipidemia | 39 (51) |
| Peripheral vascular disease | 4 (5.3) |
| CVA/TIA | 5 (6.3) |
| Drug treatment | |
| Statin | 71 (93.4) |
| Beta blocker | 71 (93.4) |
| ACEI/ARB | 75 (99) |
| Spironolactone | 40 (69) |
| Diuretics | 9 (12) |
| CCBs | 5 (6.6) |
| Nitrates | 12 (16) |
CVA: Cardiovascular accident; TIA: Transient ischemic attack; ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; CCB: Calcium channel blocker.
Preparation of blood samples
Blood samples were drawn immediately after insertion of a femoral sheath. Peripheral blood mononuclear cells (PBMNCs) were isolated from 30 mL of freshly drawn heparinized blood using Isopaque-Ficoll (Amersham Biosciences, Buckinghamshire, United Kingdom) gradient centrifugation.
Flow cytometry evaluation
The number of circulating EPC was assessed by FACS analysis by staining 5 million cells for three-color FACS analysis employing the following monoclonal antibodies: fluorescein isothiocyanate-anti-CD34 (IQ products), allophycocyanin-anti VEGF-receptor 2 (KDR, R&D systems) and phycoerythrin-anti-CD133 (R&D systems). The various EPC phenotypes assessed were CD34+CD133+, CD34+KDR+, and CD 133+KDR+.
Follow-up
Information on vital status, reinfarction, recurrent percutaneous coronary intervention, and cardiovascular events was collected using hospital records and telephone interviews. Telephone follow-up each 6 mo was performed for a maximum period of 40 mo. The outcomes censored were either a recurrent ACS event (MI or unstable angina) or mortality, and hospitalization due to acute decompensated heart failure.
Statistical analysis
All data were summarized and displayed as mean and standard deviation for the continuous variables and as the number of patients and percentage in each group for categorical variables.
Because of the relatively small number of patients and outcome events, and the relatively long follow-up period, the comparison of the rate of events for each outcome between the groups according to EPC phenotype combination categories was performed by log-rank statistics with the Kaplan-Meier estimate.
For a sample of 80 patients, in order to detect a survival difference from 70% to 90% with α = 0.05 at the end of the study, we calculated a power (1 - β) of 60%.
In order to evaluate the performance of classification schemes of the different variables and to compare the classification of the different outcome measures, we used a receiver operated characteristic curve analysis. We calculated the area under the curve to compare the classifiers and the asymptotic statistical significance to reject the hypothesis that the curve is similar to the reference line, which is a random classifier.
All above analyses were considered significant at P < 0.05 (two-tailed). The SPSS statistical package was used to perform all statistical evaluations (SSPS Inc., Chicago, IL, USA).
RESULTS
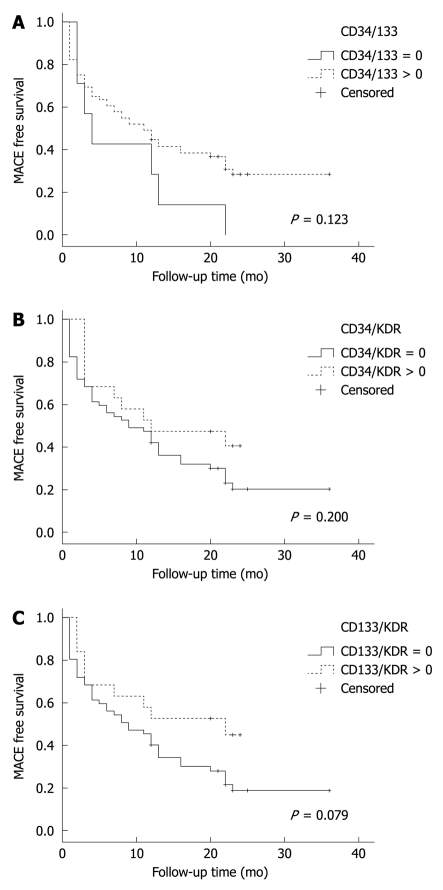
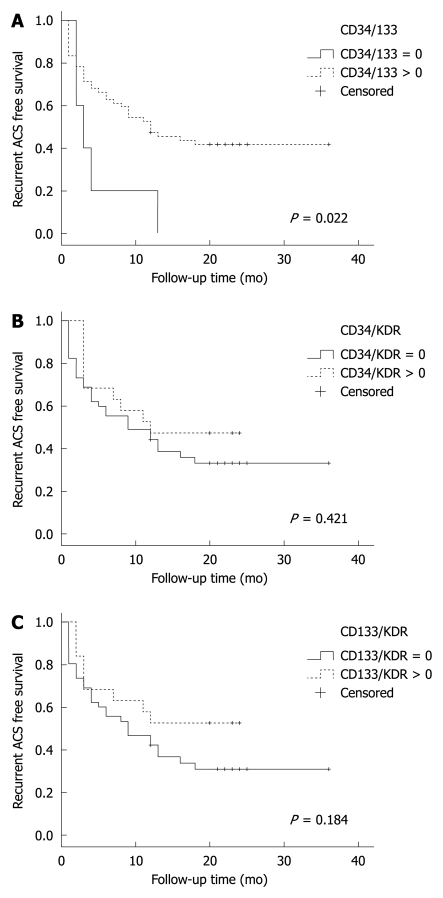
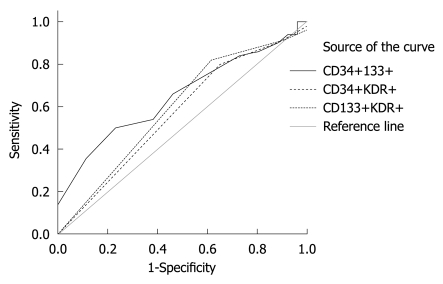
Table 2 shows various EPC combinations stratified by the events censored [total major adverse cardiac event (MACE) or secondary outcomes]. Since the relative EPC numbers were small (0%-0.1% of PBMNCs) and in most cases equal to zero, we chose a binary method of value report, whereby EPCs were categorized as either 0 if non-measurable or 1 if greater than zero. The correlations between the various EPC phenotypes and MACE-free or ACS-free survival are shown in Figures 1, 2 and 3. We found that recurrent ACS was predicted significantly by the CD34+CD133+ combination (P = 0.034; Figures 2 and 3), but not by the CD34+KDR+ (P = 0.35) or by the CD133+KDR+ (P = 0.19) combinations (Figure 1). However, this positive correlation was found to be relatively weak (area under curve = 0.65).
Table 2.
Different endothelial progenitor cell combinations stratified by outcomes n (%)
| Total mortality | Recurrent UA/MI | ADHF | Any MACE | |
| CD34+CD133+ = 0 (n = 7) | 1 (14.3) | 7 (100) | 1 (14.3) | 7 (100) |
| CD34+CD133+ > 0 (n = 69) | 6 (8.7) | 43 (62.3) | 12 (17.4) | 47 (68.1) |
| CD34+KDR+ = 0 (n = 57) | 6 (10.5) | 40 (70.2) | 11 (19.3) | 43 (75.4) |
| CD34+KDR+ > 0 (n = 17) | 1 (5.3) | 10 (52.6) | 2 (10.5) | 11 (57.9) |
| CD133+KDR+ = 0 (n = 57) | 6 (10.5) | 41 (71.9) | 12 (21.1) | 44 (77.2) |
| CD133+KDR+ > 0 (n = 19) | 1 (5.3) | 9 (47.4) | 1 (5.3) | 10 (52.6) |
UA: Unstable angina; MI: Myocardial infarction; ADHF: Acute decompensated heart failure; MACE: Major adverse cardiac event.
Figure 1.
Kaplan-Meier survival chart for the outcome of total major adverse cardiac event stratified by positive (dashed line) and negative (solid line) fluorescence-activated cell sorting analysis. A: CD34+CD133+ cells; B: CD34+KDR+; C: CD133+KDR+. MACE: Major adverse cardiac event.
Figure 2.
Kaplan-Meier event free survival for the secondary endpoint of acute coronary syndrome (recurrent myocardial infarction or unstable angina) stratified by patients with positive fluorescence-activated cell sorting analysis (dashed line) vs patients with negative fluorescence-activated cell sorting analysis (solid line). A: CD34+CD133+ cells; B: CD34+KDR+; C: CD133+KDR+. ACS: Acute coronary syndrome.
Figure 3.
Receiver operating characteristics curve analysis for the endpoint of acute coronary syndrome (recurrent myocardial infarction or unstable angina) as a function of the various endothelial progenitor cell populations. Only CD34+133+ correlated significantly with this outcome (P = 0.034); the P-value for CD34+KDR+ was 0.35 and for CD133+KDR+ was 0.19).
We did not find any significant correlation between the various EPC combinations and total MACE (Figure 3).
DISCUSSION
The factors regulating EPC numbers in acute MI include VEGF[19], interleukin-8[20] and stromal cell-derived factor-1[21]. However, one of the major limitations in studying EPCs is the lack of unifying phenotypic markers that are employed by different investigators. Indeed, the surface marker profile changes during the process of mobilization and maturation. For example, CD34-133+ progenitors differentiate into CD34+133+ EPCs that possess more pronounced angiogenic properties[22]. This leads to confounding results and an inability to perform cross-sectional comparative analyses between different studies.
There are two reports in which circulating EPC were shown to predict outcome in patients with ACS[13,14]. However, both studies were performed in different populations of patients and both used a single phenotype in FACS analysis (CD34+KDR+ cells). We have previously shown that different methods used to assess EPC in humans are not correlated[15]. Thus, it is of interest to assess the relative outcome predictive power of different phenotypic combinations in patients with ACS.
Herein, we failed to detect a significant association between EPCs measured by any of three phenotypic combinations of accepted CD markers and total MACE according to the log-rank statistics with the Kaplan-Meier method. In line with previous studies[13,14], however, we found that a lower number of EPCs (defined as CD34+CD133+) was predictive of recurrent ACS in the population we studied. However, CD34+KDR+ EPCs tested in the aforementioned studies were not found to associate with recurrent ACS or MACE in our study. The apparent discrepancy between our study, showing that CD34+CD133+ but not CD34+KDR+, exhibited a predictive value on outcome in ACS patients could be partially attributed to the negligible number of CD34+KDR+ EPCs in blood samples and the relatively small number of patients. Furthermore, EPC numbers assessed by FACS analysis are extremely low and therefore interobserver variability in assessing their quantity is considerable. We thus chose to differentiate ACS subjects as those having detectable and non-detectable numbers of EPCs. We believe that this approach partially overcomes the inherent need for subjective gating in the FACS analysis that may influence the results and limits potential error stemming from the fact that the numbers of CD34+CD133+ EPCs were considerably higher than those of CD34+KDR+ and CD 133+KDR+.
Why would CD34+CD133+ EPCs be more reflective of a recurrent ACS event than other markers? A low number of EPCs may be associated with a compromised ability to form new blood vessels and restore endothelial integrity by vasculogenesis. An intact vasculogenic process may be required to preserve endothelial function and thus prevent plaque rupture with subsequent progression towards ACS. In recent years, it has become apparent that the most important mechanism by which EPCs promote angiogenesis and vasculogenesis is by paracrine secretion of proangiogenic cytokines. As EPCs become committed to the endothelial lineage, they lose CD133 and acquire KDR and this transition is associated with phenotypic properties more closely related to a mature endothelium but a reduced paracrine capacity. The early EPCs (CD34+CD133+) therefore are probably more potent in elaborating a panel of proangiogenic and vasculogenic cytokines as compared to the more mature EPCs. According to this hypothesis, early EPCs are more powerful in their ability to preserve endothelial integrity and thus prevent stent thrombosis and plaque rupture both of which result in recurrent ACS. Indeed, our findings support the notion that a reduced number of early rather than mature EPCs is predictive of recurrent ACS.
In summary, we have found that in the setting of ACS, circulating CD34+CD133+ EPCs are potentially prognostic of cardiovascular outcome. Further studies in larger numbers of patients are needed in order to establish the feasibility of using certain EPC populations as potential biomarkers of cardiovascular events. Confirmation of the CD34+CD133+ phenotype combination as a significant adverse biomarker in ACS would then engender further research into the putative mechanism, and is likely to enhance our understanding of the role of this ambiguous population of hematopoietic progenitor cells in post-ischemic vasculogenesis.
COMMENTS
Background
Precise phenotypic definition of endothelial progenitor cells (EPCs) is currently controversial and relates to different maturation stages of these cells. Scattered reports in the literature found an association between low levels of EPCs as defined by CD34 and KDR membrane antigen markers and adverse cardiovascular events, but the applicability of these findings to other phenotypic definitions of these cells is unknown.
Research frontiers
To compare the predictive power of different EPC populations with regard to future adverse cardiovascular events in patients with acute coronary syndrome (ACS) undergoing coronary angiography.
Innovations and breakthroughs
Given the controversy pertaining to the precise EPC phenotype definition, we sought to evaluate the cardiovascular outcome predictive value of several accepted antigen marker combinations of EPCs in light of the only CD34+KDR+ phenotypic combination that had been examined previously.
Applications
By demonstrating differences among the various EPC phenotypic combinations in cardiovascular outcome predictive ability, the study could improve current understanding of the biology of these cells.
Terminology
EPCs are a scarce population of progenitor cells derived from the bone marrow that play an important role in vascular regeneration after ischemia. They have been characterized by the presence of different combinations of certain membrane antigen markers, including CD34, CD133 and KDR.
Peer review
The topic is new, and could be of interest to readers. The study is well described, methods are accurately reported, and results clearly showed. However, clinical implications of these findings and the opportunities for developing new possible diagnostic and therapeutic options might be better deepened in the discussion section.
Footnotes
Peer reviewer: Cristina Vassalle, PhD, G. Monasterio Foundation and Institute of Clinical Physiology, Via Moruzzi 1, I-56124, Pisa, Italy
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 3.Zammaretti P, Zisch AH. Adult 'endothelial progenitor cells'. Renewing vasculature. Int J Biochem Cell Biol. 2005;37:493–503. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 5.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 6.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 8.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 11.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 13.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 15.George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12:331–335. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 16.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 17.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 18.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 20.Schömig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schömig A, Ott I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–1037. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 21.Wojakowski W, Tendera M, Michałowska A, Majka M, Kucia M, Maślankiewicz K, Wyderka R, Ochała A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]