Abstract
Natural killer (NK) cells play a central role in host defense against various pathogens. Functional defects of NK cells in HIV-1 infection as a direct effect of abnormal expression or function of inhibitory NK receptors (iNKRs), activating natural cytotoxicity receptors (NCRs), and NKG2D have not yet been described. This study demonstrates an expansion of the functionally defective CD56-/CD16+ population of NK cells in viremic versus aviremic patients. We also demonstrate that in HIV-infected viremic patients, expression of iNKRs was well conserved and that in most cases, there was a trend toward increased expression on NK cells as compared with healthy donors. It was also demonstrated that the major activating NK receptors, with the exception of NKG2D, were significantly down-regulated. In contrast, the expression of iNKRs and activating receptors in HIV-infected individuals whose viremia was suppressed to below detectable levels by highly active antiretroviral therapy for 2 years or longer was comparable to that of healthy donors. Functional tests confirmed that the abnormal expression of the activating receptors and of iNKRs was associated with a markedly impaired NK cytolytic function. This phenomenon is not attributed to a direct HIV-1 infection of NK cells; thus, this study may provide insight into the mechanisms of impaired host defenses in HIV-1 viremic patients.
Natural killer (NK) cells are an important component of the innate immune system. They are characterized by the lack of expression of conventional receptors for antigen, such as surface Ig or T cell receptor (TCR); however, they are able to distinguish between normal MHC class I (MHC-I)-positive cells and cells that have lost their expression as a consequence of tumor transformation or viral infection (1). A delicate balance between opposite signals delivered by the MHC-I-specific inhibitory NK receptors (iNKRs) and by the activating receptors, referred to as natural cytotoxicity receptors (NCRs) and NKG2D, regulates NK cell cytotoxicity. Under normal conditions, the iNKRs are dominant over the NCRs and NKG2D (2, 3). In humans, the iNKRs expressed on NK cells are divided into different groups: (i) killer Ig-like receptors (KIRs) that recognize different allelic groups of classical human leukocyte antigens of class I (HLA-A, -B, and -C); (ii) LIR1/ILT2 that binds HLA-A, -B, -C, and -G; and (iii) NKG2A/CD94 that binds nonclassical HLA-E and belongs to the family of the C-type lectin proteins (3, 4). Certain surface receptors that trigger NK cell killing have recently been identified and labeled as NCRs. They include NKp46, NKp30, and NKp44; the first two are constitutively expressed only on resting and activated NK cells, whereas NKp44 is only present on activated cells (3). Very little is known about the ligands of these NCRs; it has been suggested that they can be expressed on cells “stressed” by viral infection or tumor transformation (5). Another important activating receptor is NKG2D, also expressed on a subset of cytolytic T lymphocytes, for which the ligands have been designated (6). There are also coreceptors, including 2B4, NKp80, and NTBA, that work synergistically with NCRs in driving NK cytotoxicity (3, 5). Understanding the balance between inhibitory and activating molecules expressed on NK cells has allowed us to understand the mechanism by which NK cells are able to eliminate tumor or virus-infected cells that either lack or down-regulate their self-MHC-I molecules.
Various aspects of NK cell function have been analyzed during HIV infection. In this regard, NK cells of HIV-infected individuals have been demonstrated to be an important source of CC-chemokines, such as RANTES and macrophage inflammatory proteins 1α and 1β, that potently suppress replication of R5 strains of HIV (7, 8). Furthermore, there is an inverse correlation between the level of plasma viremia and the ability of NK cells and NK cell-derived supernatants to suppress endogenous HIV replication in CD4+ T cells ex vivo (9). It has also been reported that the CD56dim/CD16+ NK subset is decreased in HIV-infected individuals, whereas the CD56bright population is conserved (10). Moreover, the CD56-/CD16+ subset of NK cells, which is present in an extremely low percentage in uninfected individuals, is common in HIV-1-seropositive subjects (11).
In this study, we performed a phenotypic and functional evaluation of NK cells in viremic patients and in patients whose viremia had been suppressed to below detectable levels with highly active antiretroviral therapy (HAART). We find a striking perturbation of the differential expression of iNKRs and activating receptors on NK cells of viremic compared with aviremic patients that correlates with clear-cut abnormalities in the function of these cells.
Methods
Study Subjects. Two cohorts of 17 viremic patients and 16 aviremic HIV-infected patients were studied (Table 1). The viremic group was composed of 6 patients who were naïve for treatment and 11 who had formerly been receiving HAART but whose treatment regimen had been discontinued. Within the group of the six treatment-naïve viremic patients, none were experiencing primary HIV-1 infection. No difference in terms of the expression and function of NK receptors was found between treated and untreated viremic patients. The aviremic group had been receiving HAART continually for at least 24 months, and all 16 aviremic patients included in this cohort had initiated HAART early in the course of infection before the establishment of chronic HIV-1 infection. Peripheral blood mononuclear cells (PBMCs) were obtained by apheresis, and all patients were enrolled in specific protocols approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board. As negative controls, we used PBMCs from 13 healthy donors seronegative for HIV-1 (Table 1) that were obtained by apheresis generously provided by the Transfusion Medicine Department of the National Institutes of Health Clinical Center. There were no statistical differences among the three groups of study subjects in the median number and percent of NK cells in peripheral blood, and the number of CD4+ T cells was never below 200/mm3 in any HIV-infected subject.
Table 1. Profile of viremic and aviremic HIV-infected patients and healthy donors.
| Group* | n | Viral load, copies RNA per ml† | NK cells, %‡ | CD4+ cells/μl |
|---|---|---|---|---|
| Viremic patients | 17 | 32,831 ± 44,447 | 9 | 556 ± 247 |
| Aviremic patients HAART | 16 | <50 | 8.5 | 520 ± 287 |
| Healthy donors | 13 | — | 11 | 1,455 ± 293 |
Results are presented as mean ± SD or median for NK cells.
Highly active antiretroviral therapy (HAART) included at least one protease inhibitor and/or two nucleoside reverse-transcriptase inhibitors and one nonnucleoside reverse-transcriptase inhibitor
Plasma viremia was analyzed by an ultrasensitive bDNA assay with a lower limit detection of 50 copies per ml of plasma
NK cells are expressed as median percent of total lymphocytes derived from PBMCs
Isolation and Culture of NK Cells. To obtain peripheral blood lymphocytes (PBLs), PBMCs were isolated over Ficoll–Hypaque gradients (LSM, ICN). Enrichment of NK cells from PBLs was performed by using column-based cell separation techniques (StemCell Technologies, Vancouver) as described (9–12). The purity of NK cells was ≥97%. To activate freshly isolated NK cells, we cultured them for 6 days in 96-well round-bottom plates at a concentration of 106 cells per ml with RPMI medium 1641 supplemented with penicillin/streptomycin, l-glutamine (GIBCO), 10% FCS (HyClone), and recombinant IL-2 (rIL-2; Roche Molecular Biochemicals) at 200 units/ml.
Monoclonal Antibodies. The following mAbs were provided by A.M. and D. Pende (University of Genoa): 289 (IgG2a anti-CD3), C218 and FS280 (IgG1 and IgG2a anti-CD56, respectively), KD1 (IgG2a anti-CD16), Gl183 (IgG1 anti-p58.2), 11pb6 (IgG1 anti-p58.1), Z27 (IgG1 anti-p70), F278 (IgG1 anti-LIR1/ILT2), Z270 (IgG1 anti-NKG2A), Xa185 (IgG1 anti-CD94), Bab 281 and KL247 (IgG1 and IgM anti-NKp46, respectively), Az20 and F252 (IgG1 and IgM anti-NKp44, respectively), Z231 (IgG1 anti-NKp44), On72 and Bat221 (IgG1 anti-NKG2D), pp35 (IgG1 anti-2B4), Ma152 (IgG1 anti-NKp80), and Ma127 (IgG1 anti-NTB-A). Anti-CD4 (IgG1), anti-TCRα/β (IgG1), anti-TCRγ/δ (IgG1), anti-CD19 (IgG1), and anti-CD14 (IgG1) were purchased from Becton Dickinson.
Flow Cytometric Analysis and Cytolytic Activity. For one- or two-color cytofluorometric analysis (FACSCalibur, Becton Dickinson), freshly isolated and rIL2-activated NK cells were stained with the appropriate mAbs followed by phycoerythrin (PE)- or FITC-conjugated isotype-specific goat anti-mouse second reagent (Southern Biotechnology Associates and IDLabs, London, ON, Canada). Second appropriate anti-isotypic mAbs conjugated with PE and/or FITC were used as negative controls. After 6 days of activation with rIL2, NK cells were tested for cytolytic activity in a 4-h 51Cr release assay as described (13). The different effector/target (E/T) ratios are indicated in the figures. The saturating concentrations of the various mAbs added were 0.5 μg/ml for redirected killing assay performed with FcγR+ P815 target cell lines.
Detection of HIV-1 DNA by Real-Time PCR. HIV DNA was quantitated as described (14) and adapted for real-time PCR technology. Briefly, genomic DNA was extracted from fresh or frozen PBMCs or NK cells by using the Puregene DNA extraction kit (Gentra Systems). One microgram of genomic DNA was amplified in triplicate by using primers and a probe specific for the RU5 region of the HIV-1 5′ LTR. Because these primers target a highly conserved region of HIV-1, viral DNA can be detected during the early stages of virus replication and as integrated proviral DNA.
The primer sequences are as follows: RU5–5′,5′-GGTCTCTCT-GGTTAGACCAGAT-3′; RU5–3′, 5′-CTGCTAGAGATTTTCCACACTG-3′; and RU5 probe, 5′-FAM-AGTAGTGTGTGCCCGTCTAGG-TAMRA-3′. PCR cycling conditions with the 7700 SDS (Applied Biosystems) include a 94°C denaturation for 2 min followed by 45 cycles of 94°C for 15 sec and 58°C for 1 min. A standard curve derived from limiting dilutions of ACH2 genomic DNA was used to quantitate HIV copy numbers. Values are given as the average HIV-1 copy per microgram of DNA. The lowest limit of detection was 10 copies per microgram of DNA.
IFN-γ and Transforming Growth Factor β (TGF-β) Detection. Culture supernatants from NK cells were tested after 6 h (freshly isolated cells) and 6 days (IL-2-activated cells) for levels of IFN-γ and TGF-β secretion by ELISA (R & D Systems).
Statistical Analysis. The medians and distributions for each variable of the three cohorts were compared by using the Kruskal–Wallis test with the Wilcoxon two-sample test. The Bonferroni method was used to adjust P values for multiple testing.
Results
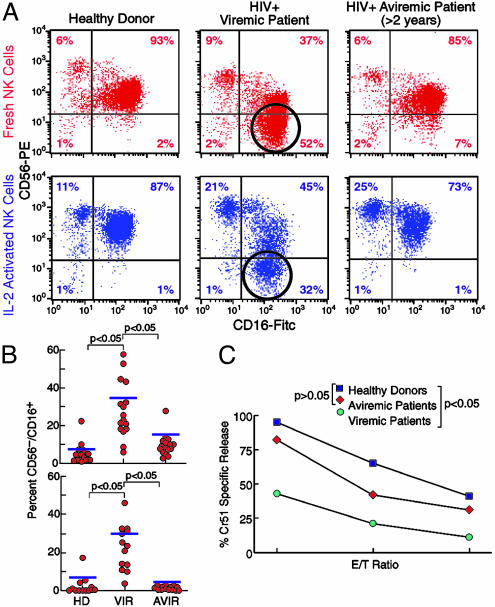
Phenotypic and Functional Analyses of NK Cells Derived from HIV-1-Positive Viremic or HAART-Treated Aviremic Patients. Freshly drawn NK cell populations, purified from purified blood lymphocytes of both cohorts of HIV-infected individuals and healthy donors were negative for CD3, CD4, TCRα/β, TCRγ/δ, CD19, and CD14, and were analyzed by double cytofluorometric analysis for the expression of the CD56 and CD16 cell surface markers (Fig. 1 A and B). In the cohort of viremic patients, the CD56dim/CD16+ subset was sharply decreased as compared with NK cells derived from healthy donors. Of note is that in these viremic individuals, a large subset of cells (20–55% of the total NK cells) manifested the CD56-/CD16+ phenotype, which is rare in normal individuals (P < 0.05). In contrast, aviremic HIV-infected individuals treated with HAART for >2 years displayed a NK cell surface phenotype similar to that of normal individuals (P > 0.05). Indeed, the majority of NK cells in aviremic patients were CD56+/CD16+, whereas only a minor subset was characterized by the CD56-/CD16+ phenotype. In all groups of individuals, the population characterized by the CD56bright/CD16- phenotype was well represented (6–9% of the total NK cells) in freshly isolated NK cells, and no significant differences were noted within the three cohorts in either fresh-by-isolation or IL-2-activated NK cells. Cell surface distribution of CD56 versus CD16 was also analyzed in NK cells cultured in the presence of rIL-2. After 6 days of culture, NK cells derived from normal donors and from both cohorts of HIV-infected individuals expressed the early activation marker CD69 (data not shown). Interestingly, although the CD56 surface density increased once cells were activated, the CD56-/CD16+ subset still represented a high proportion of the NK cells in HIV-infected viremic patients (15–45%) (P < 0.05) (Fig. 1 A and B).
Fig. 1.
Phenotypic analysis and functional capability of NK cells. (A) Double fluorescence cytofluorometric analysis with NK cells stained with CD56 PE/CD16 FITC and characterization of the abnormal CD56-/CD16+ subset in fresh (red dot plots) and activated (blue dot plots) NK cells with statistical analysis. This population is markedly represented in HIV-1-positive viremic patients (open circles) and is very minimally represented in healthy donors and in HIV-1-positive aviremic individuals. (B) Graphs of statistical dot plots showing the percentage of the CD56-/CD16+ NK cells subset (freshly isolated, Upper; activated, Lower) in all of the subjects within the three groups studied with median (blue bars) and P value between the cohorts of healthy donors versus viremic patients and viremic versus aviremic patients. HD, healthy donors; VIR, viremic patients; AVIR, aviremic patients. (C) Spontaneous cytolytic activity of NK cells activated with rIL-2 in vitro for 6 days against K562 cell lines at different E/T ratios (2:1, 1:1, and 0.5:1) (Lower). Averages were calculated on 13 healthy donors (black squares) versus 17 viremic patients (green circles) and 16 aviremic patients (red diamonds) with statistical analysis (Upper).
rIL-2-activated NK cells from both cohorts of HIV-infected individuals were analyzed for cytolytic activity against the NK-susceptible K562 erythroleukemia cell line. As shown in Fig. 1C, the mean cytolytic activity of NK cells from HAART-treated aviremic patients was only slightly reduced as compared with that of normal controls. In contrast, the ability to kill K562 target cells was clearly reduced in NK cells from viremic patients at all E/T ratios used (P < 0.05).
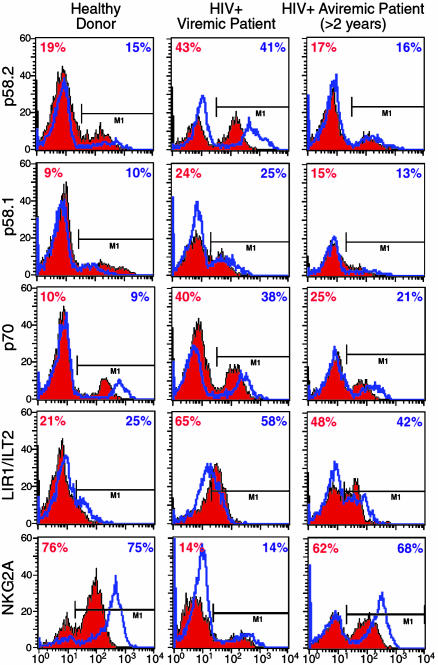
Surface Expression and Function of HLA Class I-Specific NK Cell Receptors in NK Cells from HIV-Infected Individuals. In our study, we evaluated the surface expression of different iNKRs specific for classical or nonclassical HLA class I molecules. These included KIRs such as KIR2DL2/p58.2, KIR2DL1/p58.1, KIR3DL1/p70 (specific for HLA-C 1, 3, 7, and 8, HLA-C 2, 4, 5, and 6 and HLA-Bw4, respectively), LIR1/ILT2 (recognizing HLA-A, -B, -C, and -G) and the CD94/NKG2A heterodimer specific for HLA-E (6–8). NKG2A is always coupled with CD94, a molecule that itself does not bind any MHC and does not trigger any function; however, it is necessary for surface expression of the NKG2 family, either inhibitory (NKG2A) or activating (NKG2C) receptors (15). The expression of the three KIRs examined and LIR1/ILT2 on freshly isolated and IL-2-activated NK cells (Fig. 2) was not only well conserved in the viremic patients compared with the healthy donors, but, as a trend, they all showed an increment; within the viremic cohort the increase for p58.2/KIR2DL2 was statistically significant under both conditions (P < 0.05). Of note, in those patients in whom viremia was suppressed to below detectable levels by HAART for more than 2 years, the surface patterns of the same receptors were comparable to those in the cohort of the uninfected individuals. This phenomenon was not observed with NKG2A, which showed a statistically significant trend toward decrease in viremic patients compared with healthy donors (P < 0.05). The levels of CD94 did not parallel the decrease in NKG2A in viremic patients, and we did not find any difference in the three study groups (data not shown).
Fig. 2.
Expression of iNKRs. Graphs show cytofluorometric analyses of percent of surface expression on freshly isolated (red histograms) and IL-2-activated (blue lines) NK cells in single representative individuals for 13 healthy donors (Left), 17 viremic patients (Center), and 16 aviremic patients suppressed by HAART for >2 years (Right) of the following iNKRs: p58.2, p58.1, p70, LIR1/ILT2, and NKG2A.
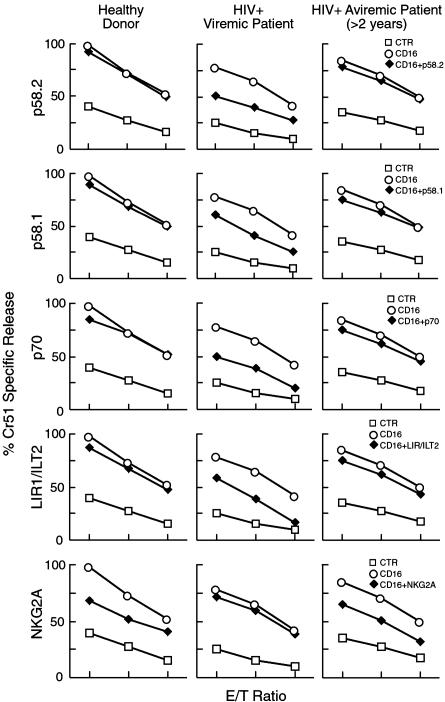
We then analyzed the potential relationship between the expression of various HLA-specific NK receptors and NK-mediated cytotoxic function. To this end, NK cells were analyzed in redirected killing assays against the FcγR+ P815 target cell line in the presence of anti-CD16 mAb, used alone or in combination with mAb specific for one or another HLA-specific NK receptors. In this assay, one would expect that a mAb that would bind to an inhibitory NK receptor and thus serve as an agonist for that receptor would result in an inhibition of cytotoxicity that reflected the relative expression of this inhibitory receptor compared with that of healthy donors. As shown in Fig. 3, in patients with high HIV-1 viral load, which was associated with an increased expression of KIR+ and LIR1/ILT2+ NK cells, mAb-mediated engagement of one or another KIR or of LIR1/ILT2 resulted in greater inhibition of CD16-induced cytotoxicity as compared with normal or aviremic HIV-infected individuals (P < 0.05 for p58.2 and p70). Indeed, the little inhibition observed in normal individuals is likely attributable to the small fraction of NK cells expressing one or another KIR, whereas in HIV-infected patients the incremented inhibitory effects are in line with the higher percent of NK cells expressing a KIR+ phenotype. These data also suggest that the increment in KIR+ NK cells is essentially attributable to the expression of inhibitory rather than activating KIRs. In line with the decreased expression on NK cells of viremic patients, NKG2A displayed a reduced capability to down-regulate the CD16-mediated lysis of target cells.
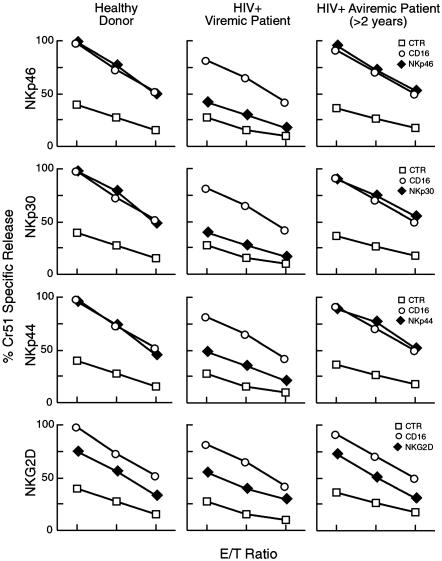
Fig. 3.
Functional evaluation of iNKRs. Graphs show the functional evaluation of surface expression of the following iNKRs by a redirected killing assay using an FcγR+ P815 target cell line. The following receptors were evaluated: p58.2, p58.1, p70, LIR1/ILT2, and NKG2A. Data are presented as an average of all of the data collected from the uninfected individuals (Left), viremic HIV-infected subjects (Center), and aviremic HIV-infected subjects (Right). E/T ratios are 5:1, 2:1, and 0.5:1. Every graph shows the baseline lysis (open squares), the maximal lysis triggered by anti-CD16 IgG mAb (open circles), and the inhibition of killing driven by the relevant inhibitory NK receptors cotriggered with anti-CD16 IgG mAb (filled diamonds).
Both surface expression and function of the KIR3DL2/p140 inhibitory receptor (specific for HLA-A3 and -A11), was comparable in both cohorts of HIV-infected individuals and in healthy donors (data not shown).
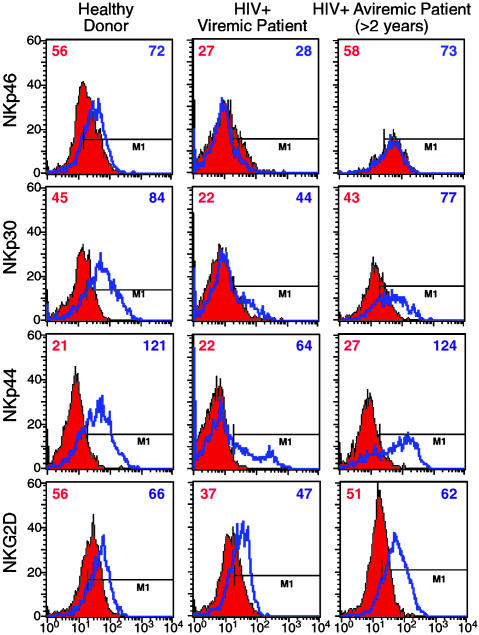
Surface Expression and Function of Different Non-HLA-Specific Activating NK Cell Receptors on NK Cells from HIV-Infected Individuals. We next analyzed the surface expression of NKG2D and NCRs NKp46, NKp30, and NKp44. As shown in Fig. 4, cell surface expression of these markers in both freshly isolated and IL-2-activated NK cells was comparable in HIV-infected aviremic patients and in healthy controls. In contrast, in viremic patients the majority of freshly isolated peripheral blood NK cells displayed the NKp46dim, NKp30dim phenotype and were characterized by statistically significant decreases in surface densities of these receptors (P < 0.05). Also, the surface expression of the NKp44 molecule (that is selectively expressed by activated and not resting NK cells) was decreased in this cohort of patients (P < 0.05). Indeed, most activated NK cells expressed low NKp44 surface densities (NKp44dim phenotype), whereas only a minor subset expressed levels of this molecule comparable to that of normal donors. Only minor differences were detected in NKG2D surface densities between viremic and aviremic patients (P > 0.05).
Fig. 4.
Expression of NCRs and NKG2D. Graphs show cytofluorometric analyses of pattern of surface expression (geometric mean fluorescence) of NCRs on freshly isolated (red histograms) and IL-2-activated (blue lines) NK cells in single representative individuals for 13 healthy donors (Left), 17 viremic patients (Center), and 16 aviremic patients suppressed by HAART for >2 years (Right) of the following activating NK receptors: NKp46, NKp30, NKp44, and NKG2D.
To evaluate the functional capability of these receptors to induce NK-mediated cytotoxicity, rIL-2-activated NK cells were analyzed in redirected killing assays against FcγR+ P815 target cells in the presence of mAbs specific for one or another activating NK receptors. In this redirected killing assay, one would expect that a mAb that would bind to an activating receptor and thus serve as an agonist for that receptor would result in an increase in cytotoxicity that reflected the relative expression of this activating receptor compared with that of healthy donors. No significant differences could be detected in the triggering capability of all these molecules in aviremic patients versus normal subjects, thus reflecting the similarities in expression of these receptors in aviremic patients and healthy donors. In contrast, in NK cells derived from the viremic cohort the three NCRs (and to a lesser extent NKG2D) displayed a clearly reduced ability to enhance NK-mediated cytotoxicity compared with that of healthy donors, reflecting the decreased expression of these activating receptors (Fig. 5) (P < 0.05 for NKp46, NKp30, and NKp44; P > 0.05 for NKG2D).
Fig. 5.
Functional evaluation of NCRs and NKG2D. Graphs show the functional evaluation of surface expression of the following activating NK receptors by a redirected killing assay using an FcγR+ P815 target cell line: NKp46, NKp30, NKp44, and NKG2D. Data are presented as an average of all of the data collected from the uninfected individuals (Left), viremic HIV-infected subjects (Center), and aviremic HIV-Infected subjects (Right). E/T ratios are 5:1, 2:1, and 0.5:1. Every graph shows the baseline lysis (open squares), the maximal lysis triggered by anti-CD16 IgG-mAb (open circles), and the killing driven by the relevant activating NK receptors (filled diamonds).
Both the expression and function of NKp80, 2B4, and NTB-A, three coreceptor molecules expressed by NK cells, were comparable in normal donors and in the two cohorts of HIV-infected patients (data not shown).
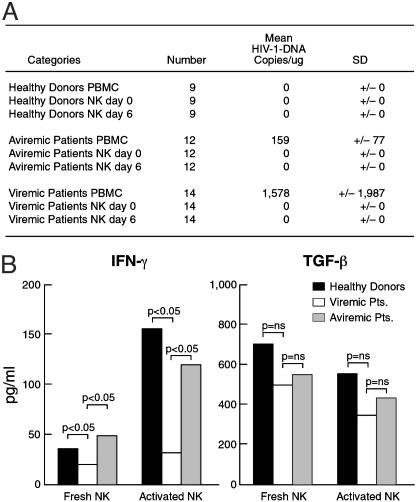
PCR Analysis for the Presence of HIV-1 DNA and Secretion of IFN-γ and TGF-β in NK Cells. It is well known that entry of HIV-1 into its target cell requires expression of both CD4 and a particular chemokine receptor on the cell surface of potential target cells (16). Because freshly isolated NK cells do not express CD4 (data not shown), one would not expect NK cells to become infected with HIV. However, a recent study reported HIV-1 infection of NK cells (17), and this prompted us to clarify whether the altered expression of several NK cell surface receptors could be the result of direct HIV-1 infection of NK cells. NK cells derived from HIV-infected patients and healthy donors express the CCR5 and/or CXCR4 chemokine receptors (data not shown), as reported by other groups (18, 19). To determine whether the NK cell abnormalities observed in our study were not caused by a direct infection of NK cells by HIV, we performed PCR analysis on freshly isolated and activated NK cells derived from HIV-infected patients. To this end, genomic DNA extracted from PBMCs (as positive control) and from freshly isolated and activated purified NK cells of the same 14 viremic and 12 aviremic patients was amplified by using primers specific for a highly conserved region of HIV-1. PBMCs and purified NK cells from healthy individuals were used as negative controls. Purification of NK cells was ≥97%. In no instance could we detect viral DNA amplification in NK cells from the viremic and aviremic patients analyzed (Fig. 6A).
Fig. 6.
PCR for HIV-1 DNA and secretion of IFN-γ and TGF-β. (A) Copies per microgram (mean) with standard deviation of HIV-1 on PBMCs and fresh and activated purified NK cells in the three cohorts of 9 healthy donors (first group), 12 aviremic patients (second group), and 14 viremic patients (third group). (B) Secretion of IFN-γ (Left) and TGF-β (Right) measured in NK cell culture supernatant after 6 h (freshly isolated NK cells) and 6 days of culture in rIL-2 (activated NK cells) in healthy donors (open bars), viremic patients (filled bars), and aviremic patients (stippled bars).
Next we analyzed the production of IFN-γ and TGF-β by NK cells from both cohorts of HIV-infected patients (Fig. 6B). Freshly isolated and activated NK cells from healthy donors and from both viremic and aviremic patients secreted similar amounts of TGF-β. In contrast, a significant reduction in terms of IFN-γ production was detected in freshly isolated and activated NK cells from viremic patients (P < 0.05).
Discussion
This study demonstrates that HIV-infected viremic patients manifest perturbations in the differential expression of iNKRs and activating NK receptors and that these perturbations are associated with abnormalities in the cytolytic function of these cells. In contrast, the phenotypic and functional profile of NK cells of HIV-infected patients who have been rendered aviremic by HAART for >2 years are similar to those of healthy donors.
There have been a number of reports of abnormalities in NK cell function in HIV-infected individuals (20, 21). The mechanisms of these dysfunctions are not entirely clear and are most likely multifactorial. The ability of an NK cell to kill relevant targets such as virally infected or tumor cells depends on a delicate balance of the patterns of expression of iNKRs and activating receptors (4, 5). In recent years several mechanisms potentially responsible for the regulation of NK cell receptor expression have been proposed (1, 3, 6); however, a number of questions remain unanswered related to the ligands of NCRs and the array of soluble factors that may be involved in the expression and modulation of the receptors that drive NK cell function. In this study, we have demonstrated that NK cell cytotoxicity against K562 target cells was decreased in viremic HIV-infected individuals compared with that in healthy donors and aviremic patients. This impaired function has also been observed in the early stages of HIV infection and in patients with advanced HIV disease, and the defect progressed over time (11, 22). Two studies (23, 24) confirmed that freshly isolated NK cells from HIV-infected individuals had a decreased capacity to kill K562 cells compared with uninfected subjects; however, NK cells from patients with low or undetectable levels of plasma viremia surprisingly manifested the least NK cytolytic activity against K562 (23). A possible explanation for this observation was that HIV directly stimulated NK cell activity, as has been seen with other viruses (23), and this phenomenon has also been reported in vivo in the early stage of simian immunodeficiency virus infection in rhesus monkeys (25). The reason for this discrepancy with our reported results is unclear at present; however, it should be pointed out that all these data were obtained by using freshly isolated, unfractionated PBMCs, and purified NK cells were not used to evaluate cytolytic function. In contrast, our functional experiments were performed after culturing purified NK cells for 6 days in the absence of virus and in the presence of rIL-2. In this way, we were able to measure the true capacity of purified NK cells, when stimulated by a classical cytokine, to trigger killing of NK-sensitive targets such as K562. Of note is that spontaneous lysis of K562 cells by NK cells was abnormally low in viremic patients, and the abnormal population of CD56-/CD16+ NK cells was highly represented in viremic patients but was not seen in HIV-infected patients whose viremia was suppressed by HAART or in healthy donors.
We sought to determine whether a correlation existed between abnormal phenotype of NK cells and cytolytic dysfunction. The redirected killing assay clearly demonstrated that the KIRs analyzed and LIR1/ILT2 that were expressed either normally or in an elevated fashion on NK cells of viremic patients were responsible for a greater receptor-specific inhibition of cytolytic function. It should be pointed out that of the iNKRs that we examined, only NKG2A was down-regulated, and this down-regulation was associated with the least inhibitory function in the redirected killing assay. The reason for this selective decrease in expression under conditions in which other iNKRs are up-regulated is presently unclear.
In a similar manner, the functional capabilities of the NCRs as determined in the redirected killing assay reflected the phenotypic abnormalities that were observed. In this regard, NK cytolytic function mediated through NKp46, NKp30, and NKp44, whose expression was markedly decreased among viremic individuals, was strongly impaired. Furthermore, NK cytolytic function mediated through NKG2D, whose expression was not significantly diminished in viremic patients, was only modestly impaired. As with the iNKRs, NK functional capability related to NCRs and NKG2D was normal in HIV-infected individuals who were rendered aviremic while receiving HAART.
It has been reported that levels of TGF-β are elevated in the plasma of HIV-infected individuals (26), and TGF-β has recently been shown to down-regulate NKp30 and NKG2D in vitro (27). Thus, we investigated whether there were any differences in the capability of NK cells from viremic versus aviremic HIV-infected individuals versus healthy donors to secrete TGF-β in culture after 6 h (freshly isolated) and 6 days in culture with rIL-2 (activated). We did not find any differences in secretion of TGF-β in the supernatant of NK cultures among the three cohorts of subjects. Thus, it is unlikely that the decrement in NKp30 expression that was observed in HIV-infected patients is attributable to a TGF-β-dependent autocrine mechanism. However, it is possible that the decrement may be caused by high levels of TGF-β that are produced by cells other than NK cells.
We also demonstrated that direct infection of NK cells by HIV-1 was not responsible for the abnormal expression and activity of iNKR, NCRs, or NKG2D, because freshly isolated and activated NK cells from HIV-1 positive donors did not harbor HIV. Finally, we demonstrated that the impaired spontaneous cytolytic function of freshly isolated NK cells among viremic individuals was associated with an impaired secretion of INF-γ as has been reported with cloned NK cells (28). Only since the availability of HAART, which has been shown to successfully suppress plasma viremia to below detectable levels in a significant proportion of HIV-infected patients, have investigators been able to carefully examine the precise effects of viremia on various immune cell functions (29, 30). In this regard, in comparisons of viremic and aviremic HIV-infected individuals, we have recently demonstrated that viremia is associated with an abnormal pattern of gene expression in resting CD4+ T cells (31) as well as abnormal phenotype and functional capability of B cells (32, 33). This study demonstrates striking differences in the phenotype and function of NK cells in viremic individuals. Longitudinal studies of viremic patients are necessary to evaluate recovery time of NK cell function in HIV-1-infected viremic donors who subsequently become aviremic on HAART. The dichotomy of expression of KIRs, NKG2A, and NCRs, together with the functional correlates, provides insight into the mechanisms of impairment of NK function in viremic HIV-infected patients and serves as a potentially important model for the relevance of the physiologic balance of NK receptor expression in the integrity of the innate immune system.
Acknowledgments
We thank the patients for their cooperation and generosity in participating in these studies, the National Institute of Allergy and Infectious Diseases study coordinators for recruiting patients, and the National Institutes of Health Department of Transfusion Medicine for recruiting the healthy donors.
Abbreviations: NK, natural killer; iNKR, inhibitory NK receptor; NCR, natural cytotoxicity receptor; MHC-I, MHC class I-positive; TCR, T cell receptor; KIR, killer Ig-like receptor; HAART, highly active antiretroviral therapy; PBMC, peripheral blood mononuclear cell; HLA, human leukocyte antigen; rIL-2, recombinant IL-2; PE, phycoerythrin; E/T, effector/target; TGF-β, transforming growth factor β.
References
- 1.Kärre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. (1986) Nature 319, 675-678. [DOI] [PubMed] [Google Scholar]
- 2.Moretta, A., Bottino, C., Vitale, M., Pende, D., Biassoni, R., Mingari, M. C. & Moretta, L. (1996) Annu. Rev. Immunol. 14, 619-648. [DOI] [PubMed] [Google Scholar]
- 3.Moretta, A., Bottino, C., Vitale, M., Pende, D., Cantoni, C., Mingari, M. C., Biassoni, R. & Moretta, L. (2001) Annu. Rev. Immunol. 19, 197-223. [DOI] [PubMed] [Google Scholar]
- 4.Lanier, L. L. (1998) Annu. Rev. Immunol. 16, 359-393. [DOI] [PubMed] [Google Scholar]
- 5.Moretta, A., Bottino, C., Mingari, M. C., Biassoni, R. & Moretta, L. (2002) Nat. Immunol. 3, 6-8. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, S., Groh, V., Wu, J., Steinle, A., Phillips, J. H., Lanier, L. L. & Spies, T. (1999) Science 285, 727-730. [DOI] [PubMed] [Google Scholar]
- 7.Oliva, A., Kinter, A. L., Vaccarezza, M., Rubbert, A., Catanzaro, A., Moir, S., Monaco, J., Ehler, L., Mizell, S., Jackson, R., et al. (1998) J. Clin. Invest. 102, 223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehniger, T. A., Herbein, G., Yu, H., Para, M. I., Bernstein, Z. P., O'Brien, W. A. & Caligiuri, M. A. (1998) J. Immunol. 161, 6433-6438. [PubMed] [Google Scholar]
- 9.Kottilil, S., Chun, T. W., Moir, S., Liu, S., McLaughlin, M., Hallahan, C. W., Maldarelli, F., Corey, L. & Fauci, A. S. (2003) J. Infect. Dis. 187, 1038-1045. [DOI] [PubMed] [Google Scholar]
- 10.Tarazona, R., Casado, J. G., Delarosa, O., Torre-Cisneros, J., Villanueva, J. L., Sanchez, B., Galiani, M. D., Gonzalez, R., Solana, R. & Pena, J. (2002) J. Clin. Immunol. 22, 176-183. [DOI] [PubMed] [Google Scholar]
- 11.Scott-Algara, D. & Paul, P. (2002) Curr. Mol. Med. 2, 757-768. [DOI] [PubMed] [Google Scholar]
- 12.Chun, T. W., Engel, D., Mizell, S. B., Hallahan, C. W., Fischette, M., Park, S., Davey, R. T., Jr., Dybul, M., Kovacs, J. A., Metcalf, J. A., et al. (1999) Nat. Med. 5, 651-655. [DOI] [PubMed] [Google Scholar]
- 13.Sivori, S., Pende, D., Bottino, C., Marcenaro, E., Pessino, A., Biassoni, R., Moretta, L. & Moretta, A. (1999) Eur. J. Immunol. 29, 1656-1666. [DOI] [PubMed] [Google Scholar]
- 14.Chun, T. W., Carruth, L., Finzi, D., Shen, X., DiGiuseppe, J. A., Taylor, H., Hermankova, M., Chadwick, K., Margolick, J., Quinn, T. C., et al. (1997) Nature 387, 183-188. [DOI] [PubMed] [Google Scholar]
- 15.Lazetic, S., Chang, C., Houchins, J. P., Lanier, L. L. & Phillips, J. H. (1996) J. Immunol. 157, 4741-4745. [PubMed] [Google Scholar]
- 16.Kinter, A., Arthos, J., Cicala, C. & Fauci, A. S. (2000) Immunol. Rev. 177, 88-98. [DOI] [PubMed] [Google Scholar]
- 17.Valentin, A., Rosati, M., Patenaude, D. J., Hatzakis, A., Kostrikis, L. G., Lazanas, M., Wyvill, K. M., Yarchoan, R. & Pavlakis, G. N. (2002) Proc. Natl. Acad. Sci. USA 99, 7015-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson, M. J. (2002) J. Leukocyte Biol. 71, 173-183. [PubMed] [Google Scholar]
- 19.Shalekoff, S., Pendle, S., Johnson, D., Martin, D. J. & Tiemessen, C. T. (2001) J. Clin. Immunol. 21, 390-401. [DOI] [PubMed] [Google Scholar]
- 20.Bonaparte, M. I. & Barker, E. (2003) AIDS 17, 487-494. [DOI] [PubMed] [Google Scholar]
- 21.Sirianni, M. C., Vincenzi, L., Topino, S., Giovannetti, A., Mazzetta, F., Libi, F., Scaramuzzi, D., Andreoni, M., Pinter, E., Baccarini, S., et al. (2002) Eur. J. Immunol. 32, 2711-2720. [DOI] [PubMed] [Google Scholar]
- 22.Brenner, B. G., Gryllis, C., Gornitsky, M. & Wainberg, M. A. (1993) Clin. Exp. Immunol. 93, 142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad, R., Sindhu, S. T., Tran, P., Toma, E., Morisset, R., Menezes, J. & Ahmad, A. (2001) J. Med. Virol. 65, 431-440. [PubMed] [Google Scholar]
- 24.Parato, K. G., Kumar, A., Badley, A. D., Sanchez-Dardon, J. L., Chambers, K. A., Young, C. D., Lim, W. T., Kravcik, S., Cameron, D. W. & Angel, J. B. (2002) AIDS 16, 1251-1256. [DOI] [PubMed] [Google Scholar]
- 25.Giavedoni, L. D., Velasquillo, M. C., Parodi, L. M., Hubbard, G. B. & Hodara, V. L. (2000) J. Virol. 74, 1648-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotz, M. & Seth, P. (1993) Ann. N.Y. Acad. Sci. 685, 501-511. [DOI] [PubMed] [Google Scholar]
- 27.Castriconi, R., Cantoni, C., Della Chiesa, M., Vitale, M., Marcenaro, E., Conte, R., Biassoni, R., Bottino, C., Moretta, L. & Moretta, A. (2003) Proc. Natl. Acad. Sci. USA 100, 4120-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott-Algara, D., Vuillier, F., Cayota, A. & Dighiero, G. (1992) Clin. Exp. Immunol. 90, 181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdez, H. (2002) AIDS Rev. 4, 157-164. [PubMed] [Google Scholar]
- 30.Saag, M. S. (2001) AIDS 15, 4-10. [Google Scholar]
- 31.Chun, T. W., Justement, J. S., Lempicki, R. A., Yang, J., Dennis, G., Jr., Hallahan, C. W., Sanford, C., Pandya, P., Liu, S., McLaughlin, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 1908-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moir, S., Malaspina, A., Ogwaro, K. M., Donoghue, E. T., Hallahan, C. W., Ehler, L. A., Liu, S., Adelsberger, J., Lapointe, R., Hwu, P., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malaspina, A., Moir, S., Kottilil, S., Hallahan, C. W., Ehler, L. A., Liu, S., Planta, M. A., Chun, T. W. & Fauci, A. S. (2003) J. Immunol. 170, 5965-5972. [DOI] [PubMed] [Google Scholar]