Abstract
Gastrointestinal stromal tumor (GIST) is one of the most common malignant mesenchymal tumors of the stomach. Prognosis of this disease is related to tumor size and mitotic activity and early diagnosis is the only way to improve it. Diagnosis of GIST always requires histological and immunohistochemical confirmation as no imaging modalities can diagnose it conclusively. Endoscopic forceps biopsy results are frequently negative. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a technique which allows tissue samples to be obtained with minimal risks and is accurate in the diagnosis of GIST. From the point of view of the endoscopist, aggressive use of EUS-FNA is the only promising way to allow early diagnosis and early treatment of this disease.
Keywords: Gastrointestinal stromal tumor, Endoscopic ultrasound, Fine needle aspiration, Gastrointestinal endoscopy, Algorithm
INTRODUCTION
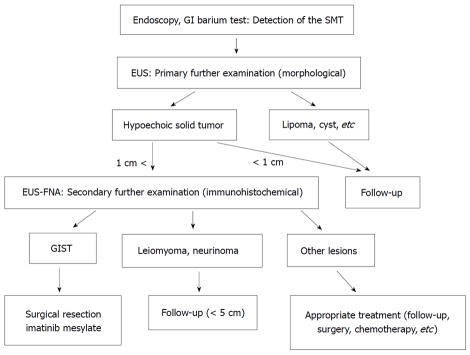
Gastrointestinal stromal tumor (GIST) is one of the most common malignant mesenchymal tumors of the gastrointestinal tract, and is pathologically defined by positive immunostaining for c-kit or CD34[1-6]. Every GIST is now considered to be potentially malignant and all GISTs without metastasis need to be resected[7]. Miettinen reported that small gastric GISTs less than 2 cm have a 100% cure rate after complete surgical resection[6]. So, early diagnosis and early surgical resection while the tumor is still small are important to improve the prognosis of this disease. However, since not all intramural lesions of the stomach are GIST, a preoperative pathological diagnosis should be obtained. The mucosal surface of a GIST is usually normal, and endoscopic forceps biopsy results are frequently negative. Therefore, most cases are preoperatively diagnosed as suspected GIST using imaging modalities only [esophagogastroduodenoscopy (EGD), endoscopic ultrasound (EUS), and computed tomography (CT), etc], and definitive diagnosis is then made by immunohistochemical analysis after surgery[1,3,4]. These clinical conditions make it difficult to diagnose GIST at an early stage. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is recognized as the only accurate diagnostic modality for the diagnosis of GIST[8-12]. At present, management algorithms for GIST remain controversial from the point of view of the endoscopist, especially for small lesions[8,13-15]. Furthermore, diagnosis of GIST using EUS-FNA has not spread globally[8-13]. This editorial outlines the clinical usefulness of our institutional management algorithm for GIST using EUS-FNA for early diagnosis and early treatment of GIST (Figure 1)[8,14,15].
Figure 1.
Diagnostic and therapeutic algorithm of gastrointestinal stromal tumor using endoscopic ultrasound-guided fine needle aspiration. Quoted and modified from reference [8,14]. GIST: gastrointestinal stromal tumor; GI: gastrointestinal; SMT: submucosal tumor; EUS: endoscopic ultrasound; EUS-FNA: endoscopic ultrasound-guided fine needle aspiration.
CLINICAL CHARACTERISTICS OF THE GASTRIC GIST
GIST is the most common mesenchymal tumor of the digestive tract[1]. It originate from the interstitial cell of Cajal located in the proper muscle layer and is characterized by over-expression of the tyrosine kinase receptor KIT[1,2]. Pathologically, the diagnosis of GIST relies on morphology and immunohistochemistry (c-kit is generally positive) (Figure 2)[16]. Incidence of GIST is estimated at 1.5/100 000/year[7]. GIST predominantly occurs in middle-aged and older persons (5th to 7th decade), with no significant difference in distribution between males and females[17,18]. Most GISTs arise in the stomach (approximately 60%) and small intestine (approximately 30%) and infrequently in other organs[19,20]. The symptoms, which depend on tumor size and location, are usually nonspecific[21]. Small GISTs are usually asymptomatic and are detected either during investigations or surgical procedures for unrelated disease. The commonest presentation of GIST is bleeding related mucosal erosion (delle)[21]. There are no recognized specific imaging examinations for GIST diagnosis. Barium contrast studies and endoscopy may provide useful data on the localization of GIST. CT scan is usually performed for staging of GIST. Endoscopic biopsy for the tumor is difficult without ulceration. Reported diagnostic accuracy for submucosal tumor (SMT) is about 40%[22]. GIST usually, and primarily, metastasizes to the liver and peritoneal cavity, while pleural, lung, or lymph node metastases are rare[19]. For localized tumors, wedge resection of the stomach is considered to be adequate treatment since GISTs tend to be exophytic and do not involve regional lymph nodes[3,4,7]. For unresectable and/or metastatic disease, treatment with imatinib is the first choice. The overall 5-year survival rate for patients with primary gastric GISTs who underwent complete resection, ranges from 20% to 63%, with a recurrence rate of 17% to 76%[19,23]. Predicting the postoperative metastatic risk (malignant potential) of GIST is often difficult, and various histopathological criteria have been proposed based on tumor size and tumor cell proliferating activity[3,4]. However, it has been recognized that a subset of small GISTs with low mitotic activity occasionally metastasize; thus, no GIST can be definitely labeled as benign. Since every GIST is now considered as potentially malignant, all GISTs may need to be resected, even small intramural lesions of the gastrointestinal tract[3-6]. Clinical imaging modalities (endoscopy, EUS, etc) can provide only tumor size as a predictor of metastatic potential. Miettinen reported that with small gastric GIST (< 2 cm) there occurred no metastasis in 1 765 cases (Table 1)[6]. In our previous study and experience, postoperative hepatic metastasis occurred in one case of 2.5 cm gastric GIST (Figures 3 and 4)[15], and in all GISTs less than 2 cm there was no postoperative relapse[8]. In other words, complete surgical resection of gastric GIST smaller than 2 cm has a 100% cure rate. So, early diagnosis and early surgical resection while the tumor is still small is a promising way for improving the prognosis of this disease.
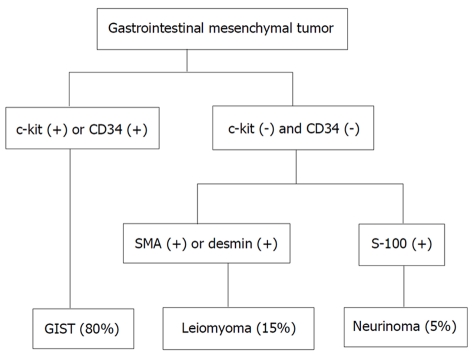
Figure 2.
Flow chart of diagnosis for gastrointestinal mesenchymal tumors using immunohistochemical analysis. Quoted and modified from reference [16]. GIST: gastrointestinal stromal tumor; SMT: submucosal tumor.
Table 1.
Reported risk of progressive disease (%)1 of primary gastric gastrointestinal stromal tumor by mitotic index and size
|
Tumor size |
||||
| Mitotic index | 0-2 cm | 2-5 cm | 5-10 cm | > 10 cm |
| < 5 per 50 hpf | 0% | 1.9% | 3.6% | 10% |
| > 5 per 50 hpf | 0% | 16% | 55% | 86% |
Adapted from reference [6], 2005. Data are based on long-term follow-up of 1765 gastric gastrointestinal stromal tumors. 1Defined as metastasis or tumor-related death.
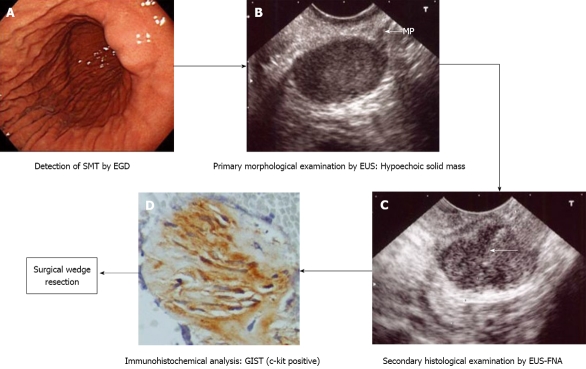
Figure 3.
Management process of gastric submucosal tumor (in a case of gastrointestinal stromal tumor) according to our institutional algorithm (Figure 1). Quoted and modified from reference [15]. A: Esophagogastroduodenoscopy (EGD) shows submucosal tumor in the lower body of the stomach; B: Endoscopic ultrasound (EUS) reveals 2.5 cm subepithelial hypoechoic solid tumor with continuity to proper muscle layer (arrow-mp); C: Puncture of the small gastrointestinal stromal tumor (GIST) under EUS guidance. Arrow: tip of needle; D: The immunohistochemical fnding of endoscopic ultrasound-guided fne needle aspiration (EUS-FNA) specimen of GIST. The tumor is diffusely positive for c-kit.
Figure 4.
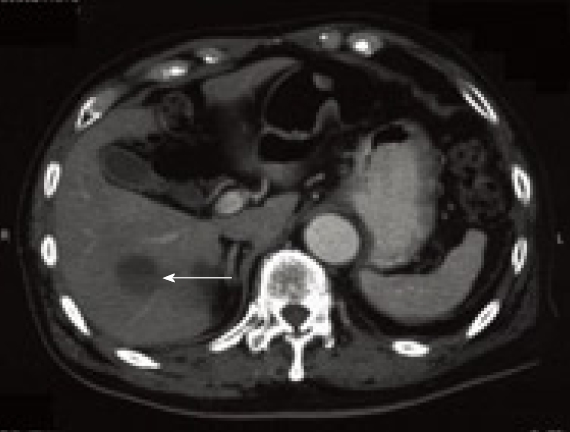
Computed tomography at 2 years after surgery showing hepatic metastasis (arrow). Quoted from reference [15].
EUS AND EUS-FNA DIAGNOSIS
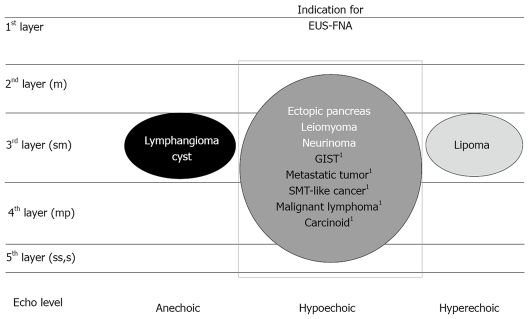
EUS allows clear imaging of the gastrointestinal wall layers and precise evaluation of the submucosal tumor (Figure 5)[15] whether from extrinsic compression or from the layer in which the intramural lesion originates[8,11,14,15]. Usually, GIST is imaged by EUS as a hypoechoic solid tumor continuous with the proper muscle[8,11,14,15]. A large group of submucosal lesions such as lipomas, cysts, and submucosal varices have typical features that allow accurate diagnosis based solely on the data gathered from endoscopy and EUS imaging[8,11,14,15]. However, an important subset of submucosal lesions such as GISTs, leiomyoma, neurinoma, carcinoid tumors, ectopic pancreas, lymphoid mass tissues, SMT-like cancers, and metastases may have overlapping echo (hypoechoic solid mass) and endoscopic features and cannot be accurately determined without a biopsy sample. In the diagnostic process of GIST, immunohistochemical analysis of tissue sample such as c-kit is vital for confirmation of this disease[1-4]. Therefore, EUS-FNA should be performed for all hypoechoic solid tumors imaged by EUS (Figure 1).
Figure 5.
Differential diagnosis of gastric submucosal tumor by endoscopic ultrasound. Quoted and modified from reference [15]. GIST: gastrointestinal stromal tumor; SMT: submucosal tumor; EUS-FNA: endoscopic ultrasound-guided fine needle aspiration. 1Malignant tumor.
Observations to date indicate that EUS-FNA is a safe and accurate procedure[8-12,14,15]. The reported accuracy of preoperative diagnosis of EUS-FNA using immunohistochemical analysis for surgically resected GIST cases ranges from 91% to 100%[8,10-12]. The diagnostic accuracy of EUS-FNA using immunohistochemical analysis is excellent. The reported diagnostic rate for tumors less than 2 cm, 2 cm to 4cm, and 4cm or more was 71%, 86%, and 100%, respectively[8]. This accurate preoperative histological proof of GIST using EUS-FNA facilitates the surgeon’s and oncologist’s decision, making for early local resection and early start of imatinib treatment [8,10-12].
MANAGEMENT ALGORITHMS FOR GIST FROM THE POINT OF VIEW OF THE ENDOSCOPIST
Some management algorithms for GIST are available[1,8,13-15,24]. However, few algorithms exist for using EUS-FNA in early diagnosis and early treatment of GIST[8,13-15]. In our hospital, we designed an algorithm for early diagnosis of GIST using EUS-FNA, and have performed decision-making for GIST according to this algorithm in the daily clinical setting (Figure 1)[14,15]. In the authors’ experience and in discussions with surgeons at our institution, crucial preoperative planning and management are facilitated by the histological diagnoses provided with EUS-FNA. Operative planning, including decisions on the type of surgery to be conducted, varies dramatically in relation to the histological diagnosis[8,11,13-15]. For example, a patient with localized GIST can be cured with a wedge resection, or if the GIST is extensive, the patient can receive imatinib. However, a patient with SMT-like advanced gastric cancer would undergo gastrectomy with lymph-node dissection and might need postoperative chemotherapy. A patient with benign SMT, such as ectopic pancreas, could avoid surgery completely following EUS-FNA confirmation of histologic benignancy. In addition, a definitive histological diagnosis by EUS-FNA is routinely requested by oncologists before initiating any chemotherapy, radiotherapy, or palliative treatment[25]. Thus, EUS-FNA evidently has a significant positive impact on clinical management of patients by providing a definitive histological diagnosis[8]. We believe that aggressive use of EUS-FNA in the management algorithm for GISTs is a key factor for improving the prognosis of this disease. However, it is difficult to obtain histological samples from a small tumor (especially less than 1 cm) using current EUS-FNA. Furthermore, EUS-FNA for small GIST theoretically has a risk of seeding due to penetration of the tumor by the needle. Having regards to the technical problems of EUS-FNA and the extremely rare metastatic risk in small GIST less than 1 cm, we recommend aggressive use of EUS-FNA for all GI tract submucosal hypoechoic tumors larger than 1 cm.
PATIENT MANAGEMENT ACCORDING TO THE ALGORITHM: REPRESENTATIVE CASES
A case of GIST
Representative EGD, EUS and EUS-FNA findings in a patient with a small gastric GIST (2.5 cm) are shown in Figure 3[15]. The results of immunohistochemical analysis of the tumor showed positive reaction for c-kit and CD34, and negative reaction for muscle actin and S-100. The tumor was diagnosed as GIST, and the patient underwent local resection. The immunohistochemical staining pattern in the surgically resected lesion had similar results (diagnosed as GIST). However, this tumor had high mitotic activity (> 5/50 HPF), and was classified as of intermediate risk of aggressive behavior of GIST. CT at 2 years after surgery revealed hepatic metastasis (Figure 4). The patient was then treated with imatinib mesylate.
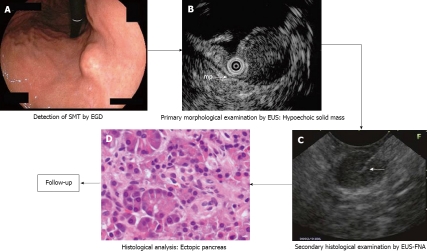
A case of ectopic pancreas
Figure 6[15] shows EGD, EUS and EUS-FNA findings in a patient with a small non-GIST (1.5 cm). EUS revealed a small hypoechoic tumor, suspected as GIST, with continuity to the proper muscle layer. The tumor thereafter was diagnosed as an ectopic pancreas by EUS-FNA, and follow-up was then performed on the patient.
Figure 6.
Management process of gastric submucosal tumor (in a case of ectopic pancreas) according to our institutional algorithm (Figure 2). Quoted and modified from reference [15]. A: Esophagogastroduodenoscopy (EGD) showing submucosal tumor in the middle body of the stomach; B: Endoscopic ultrasound (EUS) revealing 1.5 cm subepithelial hypoechoic solid tumor with continuity to proper muscle layer (arrow-mp); C: Puncture of the small submucosal nodule under EUS guidance. Arrow: tip of needle; D: Histology of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) specimen reveals pancreatic acinar cells.
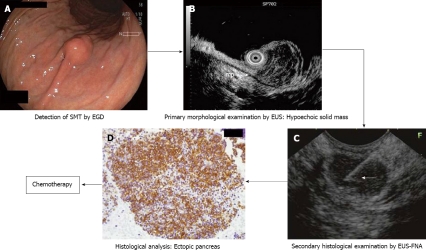
A case of B-cell lymphoma
Figure 7[15] shows EGD, EUS and EUS-FNA findings in a patient with small non-GIST (2 cm). EUS revealed small hypoechoic tumor within the submucosal layer suspected as carcinoid tumor, lymphoma, or metastatic tumor, etc. The tumor was diagnosed as B-cell lymphoma (positive reaction to CD20.) by the following EUS-FNA, and then chemotherapy was performed on the patient.
Figure 7.
Management process of gastric submucosal tumor (in a case of B-cell lymphoma) according to our institutional algorithm (Figure 2). Quoted and modified from reference [15]. A: Esophagogastroduodenoscopy (EGD) showing submucosal tumor in the middle body of the stomach; B; Endoscopic ultrasound (EUS) reveals 1.5 cm subepithelial hypoechoic solid tumor within submucosal layer. Proper muscle layer (arrow-mp) is intact; C: Puncture of the small gastric malignant lymphoma under EUS guidance. Arrow: tip of needle; D: The immunohistochemical findings of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) specimen reveals CD-20 positive diffuse large B cell lymphoma.
CONCLUSION
EUS-FNA is a safe and accurate test in the diagnosis of GIST. At present, aggressive use of EUS-FNA is the only viable way of allowing early diagnosis and early treatment of this disease from the point of view of the endoscopist.
Footnotes
Peer reviewer: Sherman M Chamberlain, MD, FACP, FACG, AGAF, Associate Professor of Medicine, Section of Gastroenterology, BBR-2538, Medical College of Georgia, Augusta, GA 30912, United States
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
References
- 1.Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004;134:145–153. doi: 10.4414/smw.2004.10530. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1–S29; quiz S30. [PubMed] [Google Scholar]
- 4.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 7.Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:64–67. doi: 10.1093/annonc/mdp131. [DOI] [PubMed] [Google Scholar]
- 8.Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawaki A, Mizuno N, Takahashi K, Nakamura T, Tajika M, Kawai H, Isaka T, Imaoka H, Okamoto Y, Aoki M, et al. Long-term follow up of patients with small gastrointestinal stromal tumors in the stomach using endoscopic ultrasonography-guided fine-needle aspiration biopsy. Dig Endosc. 2006;18:40–44. [Google Scholar]
- 10.Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 11.Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747–753. doi: 10.1007/s00535-004-1383-0. [DOI] [PubMed] [Google Scholar]
- 12.Chatzipantelis P, Salla C, Karoumpalis I, Apessou D, Sakellariou S, Doumani I, Papaliodi E, Konstantinou P. Endoscopic ultrasound-guided fine needle aspiration biopsy in the diagnosis of gastrointestinal stromal tumors of the stomach. A study of 17 cases. J Gastrointestin Liver Dis. 2008;17:15–20. [PubMed] [Google Scholar]
- 13.Kubota T. Gastrointestinal stromal tumor (GIST) and imatinib. Int J Clin Oncol. 2006;11:184–189. doi: 10.1007/s10147-006-0579-0. [DOI] [PubMed] [Google Scholar]
- 14.Akahoshi K, Endoscopic ultrasonography in the stomach. In: Tajiri H, Oyama T, editors, Knack and pitfall of gastrointestinal endoscopy in diagnosis of esophageal, gastric and duodenal diseases. Tokyo: Yodosya; 2009. pp. 149–156. [Google Scholar]
- 15.Akahoshi K, Matsui N, Sumida Y, Kubokawa M, Motomura Y, Oya M, Matono H, Sakamoto M, Miyazaki M, Maekawa R, et al. Diagnosis of the gastric Submucosal tumors by endoscopic ultrasonography-guided fine needle aspiration. Endoscopia Digestiva. 2009;21:1709–1717. [Google Scholar]
- 16.Ohashi T, Hirota S. Immunohistochemical diagnosis of GIST (gastrointestinal stromal tumor) Rhinshogeka. 2004;59:129–135. [Google Scholar]
- 17.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 18.Rossi CR, Mocellin S, Mencarelli R, Foletto M, Pilati P, Nitti D, Lise M. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer. 2003;107:171–176. doi: 10.1002/ijc.11374. [DOI] [PubMed] [Google Scholar]
- 19.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumours. Ann Chir Gynaecol. 1998;87:278–281. [PubMed] [Google Scholar]
- 21.Ludwig DJ, Traverso LW. Gut stromal tumors and their clinical behavior. Am J Surg. 1997;173:390–394. doi: 10.1016/S0002-9610(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 22.Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–862. [PubMed] [Google Scholar]
- 23.Lehnert T. Gastrointestinal sarcoma (GIST)--a review of surgical management. Ann Chir Gynaecol. 1998;87:297–305. [PubMed] [Google Scholar]
- 24.Pisters PW, Patel SR. Gastrointestinal stromal tumors: Current management. J Surg Oncol. 2010:Epub ahead of print. doi: 10.1002/jso.21460. [DOI] [PubMed] [Google Scholar]
- 25.Wu PC, Langerman A, Ryan CW, Hart J, Swiger S, Posner MC. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery. 2003;134:656–665; discussion 665-666. doi: 10.1016/s0039-6060(03)00314-3. [DOI] [PubMed] [Google Scholar]