Abstract
Recent years have witnessed progress in our understanding of coronary vasospasm (CVS). It is evident that this is not only an East Asian but also a global disease associated with significant symptoms and possible lethal sequelae for afflicted individuals. A correct diagnosis depends on the understanding of pathogenesis and symptomatology of CVS. With the correct diagnosis, we can manage CVS patients effectively and promptly, providing optimal patient safety. Advances in our understanding of interactions between inflammation, endothelium, and smooth muscle cells have led to substantial progress in understanding the pathogenesis of symptoms in CVS and have provided some insights into the basic etiology of this disorder in some patient subpopulations. We look forward to a time when therapy will address pathophysiology and perhaps, even the primary etiology.
Keywords: Coronary vasospasm, Endothelial nitric oxide synthase, Inflammation, Nitric oxide, Rho-kinase
INTRODUCTION
Coronary vasospasm (CVS) with transient ST-segment elevation can occur in diseased coronary arteries as Prinzmetal’s variant angina[1]; it may also occur in angiographically normal coronary arteries as so-called ‘variant of the variant’ angina[2]. Subsequently, many investigators found that most CVS are associated with ST-segment depression rather than ST-segment elevation on electrocardiography (ECG)[3-5]. Therefore, variant angina is only one aspect of the spectrum of coronary vasospastic myocardial ischemia[6]. CVS plays an important role in the pathogenesis not only of variant angina, but also of ischemic heart disease, including effort angina, unstable angina, acute myocardial infarction, and sudden death[7-11]. Therefore, angina caused by CVS is now usually called ‘coronary vasospastic angina’. The name ‘variant angina’ is less often used and is usually denoted as angina with transient ST-segment elevation.
CASE PRESENTATION
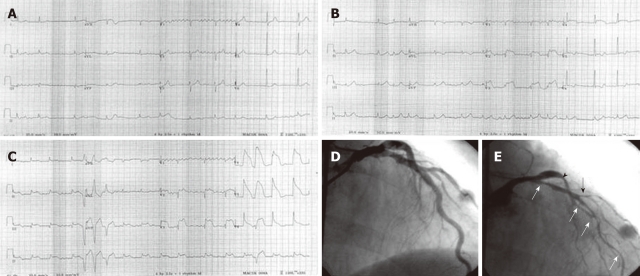
A 67-year-old man was admitted at midnight (0:30 am) to the emergency department due to sudden onset ischemic chest pain associated with cold sweating and palpitation. He was a heavy smoker who had experienced several of these episodes during the past 15 years in which most episodes occurred in the early morning hours. The baseline ECG (Figure 1A) showed no evidence of myocardial ischemia on admission to the emergency department. A few hours later, the follow-up ECG due to recurrent chest pain (Figure 1B) showed ST-segment elevation in the V2-4 leads. In the following days, serial ECGs due to chest pain showed dynamic ST-segment elevation (Figure 1C) in the anterolateral and inferior leads associated with multiform premature ventricular contractions. Cardiac troponin I was normal in two successive tests 6 h apart. The high-sensitivity C-reactive protein was also normal (0.65 mg/L). Baseline coronary angiography during admission showed no evidence of significant fixed coronary artery stenosis (Figure 1D). Diffuse spasm in the proximal to distal portion (white arrows) and diagonal branch (black arrow) of the left anterior descending artery and in the proximal portion of the left circumflex artery (arrowhead) were noted following intracoronary methylergonovine administration (Figure 1E). The diagnosis of coronary vasospastic angina was made. The patient responded well to two long-acting calcium antagonists (nifedipine and verapamil) and nicorandil. He had an uneventful follow-up period of 2 years.
Figure 1.
Baseline electrocardiography (ECG) of a patient with variant angina. A: The ECG showed no evidence of myocardial ischemia on admission to the emergency department; B: A few hours later, the follow-up ECG, due to chest pain, showed ST-segment elevation in the V2-4 leads. C: During the following days, serial ECGs due to chest pain showed dynamic ST-segment elevation in the anterior and inferior leads. D: The patient’s ECG was normal when he was not having chest pain. Baseline coronary angiography showed no evidence of significant fixed coronary artery stenosis; E: Diffuse spasm in the proximal to distal portion (white arrows) and diagonal branch (black arrow) of the left anterior descending artery and in the proximal portion of the left circumflex artery (arrowhead) were noted following intracoronary methylergonovine administration.
DIAGNOSIS OF CVS: MOST CVS ARE ASSOCIATED WITH ST-SEGMENT DEPRESSION
The diagnosis of CVS is not necessarily easy. In contrast to stable effort angina, which is reproducibly induced by exercise testing, CVS is usually not induced by exercise, particularly in the afternoon; it occurs usually at rest, particularly from midnight to early morning. The attack is transient, often lasts only a few minutes, and is unpredictable. Thus, ambulatory monitoring of ECG is important to detect the attack. However, even during ambulatory ECG monitoring, an attack may not be apparent, especially when attacks are infrequent. Furthermore, most CVS are associated with ST-segment depression rather than ST-segment elevation, which may not attract the cardiologists’ attention[12]. Therefore, provocation tests for CVS were developed to make a diagnosis of CVS-related ischemic heart disease. An important issue is the indication of provocation testing performed. The pharmacologic provocation testing of CVS is recommended in patients with recurrent episodes of apparent ischemic chest pain at rest who have normal or mildly abnormal coronary angiograms, with no clinical observations substantiating the diagnosis of variant angina, i.e. ST-segment elevation during pain[13].
Several provocative tests for CVS are available. Of these, the ergonovine and acetylcholine tests are most commonly used. Ergonovine is an ergot alkaloid that stimulates both α-adrenergic and serotonergic receptors, and intracoronary administration of doses ranging from 10-80 μg in total are most commonly used. Intracoronary nitroglycerin 50-200 μg is administered subsequently if the luminal diameter is decreased more than 70% after intracoronary ergonovine, in association with clinical symptoms and/or electrocardiographic changes[14]. There is no standard definition for a positive intracoronary provocation test. Yasue et al[15] defined CVS as an abnormal contraction of an epicardial coronary artery resulting in myocardial ischemia. With this definition, there are no limits to the degree of lumen reduction required to diagnose CVS, since ischemia must accompany the changes in vessel size. The American College of Cardiology/American Heart Association guidelines for coronary angiography suggest that CVS is present when a reduction in lumen diameter of > 50% occurs during a provocative test[13]. In early 1990, coronary provocation testing using methylergonovine was developed[16]. The intracoronary route of administration of methylergonovine for provocation of CVS is safe, sensitive, and specific. This route is preferable in hypertensive patients and affords the opportunity to evaluate the left and right coronary circulations separately. Auch-Schwelk et al[17] reported that the contractions to ergonovine are not dependent on nitric oxide release, but are synergistically augmented by thromboxane. However, methylergonovine causes similar effects on vascular smooth muscle, but contractions are inhibited by the release of nitric oxide from the endothelium.
Intracoronary acetylcholine administration in doses of 10-100 μg is also used for CVS provocation[18]. The duration of the action of acetylcholine is very short and the induced CVS usually disappears spontaneously within 2-3 min, without the need for intracoronary nitroglycerin administration. Sinus node and conduction system inhibition is a major side effect of the acetylcholine test in which temporary pacing is needed, especially when intracoronary acetylcholine is administered into the right coronary artery. Acetylcholine provocation is also commonly used to assess coronary endothelial function as it stimulates the release of endothelium-derived nitric oxide with subsequent vasorelaxation of the vascular smooth muscle cells. However, with increasing doses of acetylcholine application, the vasoconstrictor effect on the vascular smooth cells may override the endothelial effect and a vasoconstrictor response may result. Thus, in the normal setting, there is an endothelium-induced coronary vasodilation in response to acetylcholine stimulation, while in the presence of a dysfunctional endothelium the vasoconstrictor effects of acetylcholine prevail and cause a vasoconstriction. Overall, it may be difficult to differentiate between coronary endothelial dysfunction and a CVS, unless the vasoconstrictor response is distinct or > 50% of the vessel diameter. The vasomotion can result in as much as a < 50% change in vessel diameter in patients without CVS[19]. This can also be applied for the cold pressor test.
CVS can also be induced by hyperventilation, which causes respiratory alkalosis[20]. Its sensitivity is 65% and the specificity is 100%. It may be safe when CVS is induced by hyperventilation. Nonetheless, it may be dangerous to use this method to induce multivessel CVS. Histamine, epinephrine, dopamine, dobutamine, serotonin, exercise in the morning, and the cold pressor test all induce CVS with a lower sensitivity than ergonovine or acetylcholine[21].
Angiographically normal coronary arteries occur in 25% of patients with acute coronary syndrome[4,5,10]. The CVS can be induced in 50%-60% of these patients[5,10]. Since variant angina is a presentation of transient ST-elevation acute coronary syndrome, the diagnosis and initial management procedures must adhere to the guidelines proposed by the American Heart Association in 2005[22]. Initial general therapies for acute coronary syndrome include immediate oxygen therapy, continuous cardiac monitoring, establishment of intravenous access, and medications of aspirin, nitroglycerin, and/or morphine. Morphine is indicated only for refractory chest pain after nitroglycerin use. If a normalized ST-segment is noted after the above general therapies, a diagnosis of variant angina is most likely and reperfusion therapy is unnecessary. If ST-segment elevation persists, then reperfusion therapies are necessary, according to the facilities available in the emergency room.
The importance of the differential diagnosis between persistence and transience of elevated ST-segments lies in the follow-up ECG and patient monitoring. The next diagnostic step for transient ST-segment elevation is coronary angiography, as this is the only certain method to distinguish between patients who have severe fixed multivessel disease or only angiographically normal or near-normal coronary arteries. This differential diagnosis is important because the treatment strategies proposed for variant angina with severe, fixed multivessel disease (e.g. aspirin, clopidogrel, nitrates, angiotensin-converting enzyme inhibitor, and/or percutaneous coronary intervention) or only angiographically normal or near-normal coronary arteries (e.g. calcium antagonists and/or nitrates) are different. Because there are some patients with CVS who are refractory to the conventional medications and who may suffer from life-threatening arrhythmias[10] or sudden death[23], and because percutaneous coronary intervention is not the correct management for CVS[24], it is important for every emergency room, ward doctor, and cardiologist to be alert to the presence of CVS, a type of dynamic coronary artery stenosis, which may be silent and lethal.
In 1991, Dote et al[25] first reported 5 cases of multivessel CVS and transient myocardial stunning. Thereafter, the term transient left ventricular apical ballooning or Takotsubo cardiomyopathy was used to describe transient myocardial stunning by many investigators. After recent investigations, Takotsubo cardiomyopathy is recognized as a form of myocardial stunning following a stressful event that is presumably induced by intense CVS[26,27], microvascular dysfunction[28,29], or a marked catecholamine response[30,31]. Clinically, Takotsubo cardiomyopathy is characterized by (1) acute onset (usually following a stressful or emotional event, especially in older women); (2) variable severity of clinical (mainly, dyspnea and chest pain) and electrocardiographic manifestations of acute myocardial ischemia (typically involving territories larger than a single coronary branch); (3) mild cardiac enzyme elevation; (4) absence of obstructive, fixed coronary lesions on early angiographic images; (5) apical, anteroapical, and inferoapical hypo- or dyskinesia, with preserved basal-segment contractility, producing an ampulla-like systolic deformity of the left ventricular silhouette; (6) spontaneous resolution of all features in 1-4 wk, including normalized left ventricular function; and (7) a generally favorable late prognosis and rare recurrence rate. There are some different features between Takotsubo cardiomyopathy and CVS: (1) more postmenopausal female patients have Takotsubo cardiomyopathy; (2) older patients have Takotsubo cardiomyopathy; and (3) more daytime attacks of Takotsubo cardiomyopathy. Recent prospective studies suggest that the incidence of Takotsubo cardiomyopathy in acute coronary syndromes is 0%-2% on early coronary angiography[32]. Another study in an Italian population revealed that 12% of female patients with suspected anterior acute myocardial infarction had Takotsubo cardiomyopathy[33]. Although it is important to differentiate CVS from Takotsubo cardiomyopathy, there are some overlaps of these 2 entities. In a recent case series study of Takotsubo cardiomyopathy, the author described an experimental reproduction of transient apical ballooning in the catheterization laboratory during acetylcholine testing[34]. The author suggested that coronary vasospastic angina is caused by localized, long-term neurohormonal dysfunction of one coronary artery, making patients susceptible to localized spastic episodes under transient influences of physiologic stimuli, such as emotions. On the other hand, onset of Takotsubo cardiomyopathy appears to represent the superimposition of transient diffuse endothelial dysfunction (probably lasting a finite period) and of an adrenergic surge episode. Persistent apical ballooning after the early stages appears to represent residual, secondary myocardial stunning. As a newly recognized disorder, much remains unknown about Takotsubo cardiomyopathy, especially its etiology. Many aspects are also puzzling. Nevertheless, delayed (5-30 d) acetylcholine testing accompanied by echocardiographic monitoring should be routinely pursued to identify the mechanism of Takotsubo cardiomyopathy, to better characterize individual prognoses, and to tailor treatments[34].
NEW UNDERSTANDING OF THE MECHANISMS OF CVS
Autonomic system dysfunction
In the 1980s, researchers demonstrated that autonomic nervous system dysfunction was one of the possible mechanisms involved in the development of CVS[18,35]. Pathologic findings of degeneration and fibrotic changes in the perivascular nerves of vasospastic coronary arteries support the hypothesis of autonomic nervous system dysfunction in CVS[36].
Endothelial dysfunction, oxidative stress, and genetic susceptibility
In the 1990s, deficiency of nitric oxide activity due to endothelial dysfunction and oxidative stress were identified as other possible mechanisms for CVS[37-40]. Plasma levels of vitamin E and another antioxidant were also found to be low in patients with CVS[40,41]. Subsequently, Japanese investigators showed that polymorphisms of Glu298Asp in exon 7 and T-786C in the 5’-flanking region of the endothelial nitric oxide synthase (eNOS) gene and paraoxonase gene Gln192Arg (Q192R) polymorphism were significantly associated with CVS[42-44]. Paraoxonase I gene has an antioxidant effect and CVS occurs more often in cigarette smokers[45]. Of CVS, endothelial function is impaired both in coronary and brachial arteries and is improved by vitamin C infusion in smokers[46]. Cigarette smoke extract suppresses the acetylcholine-induced endothelium dependent vasorelaxation and the suppression is prevented by antioxidants in isolated arteries[47,48]. Thus, cigarette smoking degrades nitric oxide through oxygen radicals. These findings suggest that decreased nitric oxide activity in CVS patients is partly due to increased nitric oxide degradation by oxygen radicals. However, eNOS polymorphisms are found in only one-third of CVS patients and therefore, other genes or factors may also be involved in the pathogenesis of CVS. Murase et al[49] showed that while genetic risk and gene environment in both genders were involved with CVS, eNOS gene polymorphism was associated with CVS only in women[50]. Type A personality, severe anxiety, and panic disorders were factors associated with CVS, even without significant obstructive coronary artery disease. Although the oxidized form of low-density lipoprotein impairs production of nitric oxide due to down-regulation of eNOS and the oxidative inactivation of nitric oxide by oxygen free radicals[51,52], hypercholesterolemia is not a risk factor for CVS[45,53]. Nakagawa et al[12] found that CVS preferentially occurs at branch points and nonplaque sites, whereas the atherosclerotic lesion is predominantly localized at the nonbranch points of the curved proximal segments. This indicates that CVS may be a manifestation of coronary artery disease distinctly different from coronary atherosclerosis which is associated with hypercholesterolemia.
Smooth muscle hypercontraction
The classical pathway of vascular smooth muscle contraction through which stimuli induce myosin light chain phosphorylation is an increase of the intracellular Ca2+ concentration. However, Ca2+-independent regulation also occurs through the inhibition of myosin light chain phosphatase, and the level of myosin light chain phosphorylation is determined by a balance between myosin light chain phosphorylation by myosin light chain kinase and dephosphorylation by myosin light chain phosphatase[54]. Some investigators found that small GTPase RhoA and its downstream effector, ROCK/Rho-kinase, inhibit myosin light chain phosphatase resulting in accentuation of myosin light chain phosphorylation and Ca2+ sensitization in response to vasoconstrictor stimuli[55]. In the late 1990s, researchers showed that RhoA/ROCK activity was enhanced in rat arteries with hypertension and vasospasm[56,57]. Shimokawa et al[58] and Kandabashi et al[59] developed swine models of CVS and showed that ROCK activity is enhanced in coronary artery smooth muscle after wrapping the coronary artery with interleukin-1 beads. They subsequently showed that the ROCK inhibitor, fasudil, relieved CVS in humans[60]. Thus, enhanced vascular smooth muscle contraction through the Rho/ROCK pathway plays an important role in the development of CVS. Recent studies show that decreased endothelial nitric oxide activity increases RhoA/ROCK activity in coronary arteries[61,62]. Fluvastatin, which blocks the RhoA/ROCK pathway was also found to suppress CVS[63]. These findings connect the activity of RhoA/ROCK to endothelial nitric oxide and are in agreement with the clinical observations that spastic arteries are supersensitive to both vasoconstrictor agonists and nitrates[64].
Inflammation
In 1978, Lewis et al[65] first reported a case of variant angina and localized pericarditis. They postulated that there was a link between inflammation and CVS. In the mid and late 2000s, we and others showed that chronic inflammation was associated with CVS, as evidenced by elevated peripheral blood monocyte counts, high-sensitivity C-reactive protein, interleukin-6, and adhesion molecules[66-75]. Cigarette smoking, a major risk factor for CVS, is associated with low-grade inflammation[76]. An interaction between smoking and high-sensitivity C-reactive protein was recently reported by our group[77]. Based on several studies, inflammation exists in patients with CVS. However, the mechanism remains elusive.
Magnesium deficiency, insulin resistance, and KATP channel dysfunction
In the 1980s to 1990s, magnesium deficiency was also considered as a possible factor contributing to the genesis of CVS[78,79]. Furthermore, it has been reported that infusion of magnesium reduced coronary spasm attacks in patients with CVS[80,81]. The plausible mechanism might be the calcium channel blocking effect of magnesium ions at the level of vascular smooth muscle cells. Extracellular magnesium inhibits capacitative calcium ion entry in vascular smooth muscle cells[82]. Magnesium-induced coronary dilatation may also be mediated via intracellular cyclic adenosine 3’,5’-monophosphate. Previous studies have shown that adenosine 3’,5’-monophosphate elevations contribute to coronary dilatation[83]. Magnesium infusion may cause an increase in adenosine 3’,5’-monophosphate within coronary smooth muscle cells, leading to the dilatation of coronary arteries[84]. In 1995, Shinozaki et al[85] found that insulin resistance associated with compensatory hyperinsulinemia is an independent factor for CVS. They postulated that hyperinsulinemia causes vascular endothelial dysfunction and CVS, and subsequently amplifies atherosclerotic lesion formation. However, CVS does not always precede obstructive atherosclerotic coronary artery disease. The mechanisms between insulin resistance and CVS have not been definitely defined. In 2006, Kakkar et al[86] found that spontaneous CVS occurs in KATP mutant mice, which arises from a smooth muscle-extrinsic process. They postulated that endothelial dysfunction with loss of KATP channels and decreased nitric oxide production and/or bioavailability promotes smooth muscle hypercontractility. Another possibility includes the sympathetic neurons, where opening of presynaptic KATP channels decreases norepinephrine release enhancing smooth muscle relaxation to dilate coronary arteries. A defect in these channels decreasing the threshold for norepinephrine release might be associated with CVS.
Summary
CVS provoked by ergonovine results in altered vascular muscle function rather than a disturbance in endothelial nitric oxide release[17]. This is in line with clinical studies demonstrating endothelial dysfunction in many patients without evidence of CVS. Dysfunctional endothelium could play an additional role in the pathogenesis of CVS, because contractions due to the endogenous ligand serotonin are markedly augmented after inhibition of nitric oxide synthase[17]. The interactions between the autonomic nervous system, inflammation, nitric oxide availability, eNOS regulation, Rho/ROCK activity of vascular smooth muscle cells, and KATP channels in smooth muscle cells provide some insights towards the basic etiology of this disorder in some subpopulations.
MANAGEMENT
In the event of an acute CVS attack, chest pain can usually be relieved by sublingual nitroglycerin. With occasional refractory CVS, intravenous or intracoronary administration of nitroglycerin may be necessary. Since the durations of action for nitroglycerin and nitrates are short, i.e. 1 h or less, the long-acting calcium antagonists are necessary to prevent recurrence. The effect of the calcium antagonists is often dramatic. Of particular importance, the calcium antagonist should be given before going to bed at night as CVS attacks usually occur from midnight into the early morning. It may require 2 calcium antagonists (dihydropyridine and non-dihydropyridine) to relieve CVS-related angina. Calcium antagonists should not be withdrawn even if symptomatic attacks occur rarely because of long-term spasticity-related silent myocardial ischemia[45,87] and sudden death from life-threatening cardiac arrhythmias[10]. Long-acting nitrates are also useful, but their potency is reduced by their tolerance. Combinations of different classes of calcium antagonists with nitrates may be necessary for patients with refractory CVS. β-blockers are not effective in suppressing CVS-related chest pain, especially in patients with angiographically normal or near-normal coronary arteries[88]. Recent clinical research shows that magnesium[81], statins[63], antioxidants[39,40], and the Rho-kinase inhibitor fasudil[60] are also beneficial for the treatment of CVS. In addition to the use of effective anti-CVS medications, CVS inducers must be avoided. These inducers include cigarette smoking, catecholamines, muscarinic agonists, ergot alkaloids, prostaglandins, alcohol, emotional stress, and propranolol[21].
The role of coronary intervention in patients with refractory CVS and organic stenosis is limited[89]. In CVS patients who did not respond to conventional treatments, internal mammary artery revascularization with angiographically normal coronary arteries was reported[90]. An implantable cardioverter defibrillator with aggressive medical therapy for CVS was reported to be effective in patients who had a previous syncopal event, documented ventricular tachycardia, or surviving out of hospital cardiac arrest[91-93] (Table 1).
Table 1.
Therapeutic strategies for CVS
| Quit smoking | Obligatory |
| Long-acting Calcium antagonists | Use before going to bed at night |
| Long-acting Nitrates | Decreased potency by tolerance |
| Magnesium | Evidence by intravenous infusion |
| RhoA/ROCK inhibitor | Fasudil |
| Statins | Evidence by fluvastatin |
| Coronary bypass graft | Controversial |
| Implantable cardioverter defibrillator | For life-threatening ventricular arrhythmias |
CVS: Coronary vasospasm.
PROGNOSIS
The natural history of CVS-related ischemic heart disease is generally good as long as patients avoid cigarette smoking and have good compliance with adequate calcium antagonists therapy[45,94-98]. Cardiac events are likely to occur during the first 3-6 mo. Patients without a stenosis of 70% or more have a 94% 1-year MI-free survival rate, while patients with multivessel atherosclerotic coronary artery disease and variant angina only have a 83% 1-year MI-free survival rate[94]. A recurrent angina rate of 11% was noted in patients with CVS without significant fixed coronary artery disease during a 4-year follow-up period[45]. Nonfatal acute myocardial infarction is a possible complication of variant angina with a incidence of 5%-10%[94,99]. Seventy-five percent of nonfatal myocardial infarction occurs during the first 3 mo[94]. The extent and severity of underlying fixed obstructive coronary artery disease is the prognostic factor which predicts survival within 3-6 mo after the diagnosis of CVS[94]. Significant arrhythmic events during or following attacks of variant angina occur in 20%-50% of patients, among a large series of mostly untreated patients[90]. The risk of sudden death for patients with coronary vasospastic angina is approximately 2% and is most common in patients with multivessel CVS and prior significant arrhythmias during angina occurrence[99]. From another point of view, Wakabayashi et al[100] tested consecutive Japanese patients for CVS 10-20 d after acute myocardial infarction treated by percutaneous coronary intervention. They found that provoked CVS occurs in 70% of infarct-related arteries and about 50% of noninfarct-related arteries. Provoked CVS was an independent predictor of adverse outcome.
CONCLUSION
Advances in our understanding of interactions between inflammation, the vascular endothelium, and smooth muscle cells have led to substantial progress in our understanding of CVS-pathogenesis and symptomatology, and have provided some insights towards the basic etiology of this disorder in some patient subpopulations. The absence of significant obstructive coronary artery disease should not lead the physician to conclude that the patient does not have ischemic heart disease. Since most patients with CVS present with ST-segment depression rather than ST-segment elevation, a high index of suspicion for CVS-related ischemic chest pain is important, because the treatment of choice varies accordingly. Many young cardiologists are now much interested in coronary intervention and, therefore, are not familiar with CVS-related angina. Since CVS may be complicated by lethal cardiac arrhythmias, sudden death, and incorrect management, it is very important for every physician to be alert to the presence of CVS, which is a dynamic type of coronary artery stenosis[101,102]. With the correct diagnosis, we can manage CVS patients effectively and promptly, providing for optimal patient safety.
Footnotes
Peer reviewers: Dr. Thomas Hellmut Schindler, PhD, Department for Cardiology, University Hospitals of Geneva, Geneva 1211, Switzerland; Shinji Satoh, MD, PhD, Department of Cardiology and Clinical Research Institute, National Hospital Organization Kyushu Medical Center, 1-8-1 Jigyohama, Chuo-ku, Fukuoka 810-8563, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–388. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- 2.Cheng TO, Bashour T, Kelser GA Jr, Weiss L, Bacos J. Variant angina of Prinzmetal with normal coronary arteriograms. A variant of the variant. Circulation. 1973;47:476–485. doi: 10.1161/01.cir.47.3.476. [DOI] [PubMed] [Google Scholar]
- 3.Harding MB, Leithe ME, Mark DB, Nelson CL, Harrison JK, Hermiller JB, Davidson CJ, Pryor DB, Bashore TM. Ergonovine maleate testing during cardiac catheterization: a 10-year perspective in 3,447 patients without significant coronary artery disease or Prinzmetal's variant angina. J Am Coll Cardiol. 1992;20:107–111. doi: 10.1016/0735-1097(92)90145-d. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Park EH, Yang DK, Park TH, Kim SG, Yoon JH, Cha KS, Kum DS, Kim HJ, Kim JS. Role of vasospasm in acute coronary syndrome: insights from ergonovine stress echocardiography. Circ J. 2005;69:39–43. doi: 10.1253/circj.69.39. [DOI] [PubMed] [Google Scholar]
- 5.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–527. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Maseri A, Severi S, Nes MD, L'Abbate A, Chierchia S, Marzilli M, Ballestra AM, Parodi O, Biagini A, Distante A. "Variant" angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol. 1978;42:1019–1035. doi: 10.1016/0002-9149(78)90691-4. [DOI] [PubMed] [Google Scholar]
- 7.Maseri A, L'Abbate A, Baroldi G, Chierchia S, Marzilli M, Ballestra AM, Severi S, Parodi O, Biagini A, Distante A, et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of "preinfarction" angina. N Engl J Med. 1978;299:1271–1277. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- 8.Yasue H, Kugiyama K. Coronary artery spasm: Japanese view. Coron Artery Dis. 1990;1:668–674. [Google Scholar]
- 9.Hung MJ, Cherng WJ. Comparison of white blood cell counts in acute myocardial infarction patients with significant versus insignificant coronary artery disease. Am J Cardiol. 2003;91:1339–1342. doi: 10.1016/s0002-9149(03)00325-4. [DOI] [PubMed] [Google Scholar]
- 10.Hung MJ, Cheng CW, Yang NI, Hung MY, Cherng WJ. Coronary vasospasm-induced acute coronary syndrome complicated by life-threatening cardiac arrhythmias in patients without hemodynamically significant coronary artery disease. Int J Cardiol. 2007;117:37–44. doi: 10.1016/j.ijcard.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CW, Yang NI, Lin KJ, Hung MJ, Cherng WJ. Role of coronary spasm for a positive noninvasive stress test result in angina pectoris patients without hemodynamically significant coronary artery disease. Am J Med Sci. 2008;335:354–362. doi: 10.1097/MAJ.0b013e31815681b2. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa H, Morikawa Y, Mizuno Y, Harada E, Ito T, Matsui K, Saito Y, Yasue H. Coronary spasm preferentially occurs at branch points: an angiographic comparison with atherosclerotic plaque. Circ Cardiovasc Interv. 2009;2:97–104. doi: 10.1161/CIRCINTERVENTIONS.108.803767. [DOI] [PubMed] [Google Scholar]
- 13.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 14.Baim DS. Coronary angiography. In: Baim DS, editor. Grossman’s cardiac catheterization, angiography, and intervention. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 215. [Google Scholar]
- 15.Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760–765. doi: 10.2169/internalmedicine.36.760. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi Y, Yamazoe M, Shibata A. Effect of direct intracoronary administration of methylergonovine in patients with and without variant angina. Am Heart J. 1991;121:1094–1100. doi: 10.1016/0002-8703(91)90667-7. [DOI] [PubMed] [Google Scholar]
- 17.Auch-Schwelk W, Paetsch I, Krackhardt F, Gräfe M, Hetzer R, Fleck E. Modulation of contractions to ergonovine and methylergonovine by nitric oxide and thromboxane A2 in the human coronary artery. J Cardiovasc Pharmacol. 2000;36:631–639. doi: 10.1097/00005344-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Yasue H, Horio Y, Nakamura N, Fujii H, Imoto N, Sonoda R, Kugiyama K, Obata K, Morikami Y, Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- 19.Hackett D, Larkin S, Chierchia S, Davies G, Kaski JC, Maseri A. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation. 1987;75:577–582. doi: 10.1161/01.cir.75.3.577. [DOI] [PubMed] [Google Scholar]
- 20.Nakao K, Ohgushi M, Yoshimura M, Morooka K, Okumura K, Ogawa H, Kugiyama K, Oike Y, Fujimoto K, Yasue H. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80:545–549. doi: 10.1016/s0002-9149(97)00419-0. [DOI] [PubMed] [Google Scholar]
- 21.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WC, Curry RC Jr, Isner JM, Waller BF, McManus BM, Mariani-Constantini R, Ross AM. Sudden death in Prinzmetal's angina with coronary spasm documented by angiography. Analysis of three necropsy patients. Am J Cardiol. 1982;50:203–210. doi: 10.1016/0002-9149(82)90030-3. [DOI] [PubMed] [Google Scholar]
- 24.Corcos T, David PR, Bourassa MG, Val PG, Robert J, Mata LA, Waters DD. Percutaneous transluminal coronary angioplasty for the treatment of variant angina. J Am Coll Cardiol. 1985;5:1046–1054. doi: 10.1016/s0735-1097(85)80004-8. [DOI] [PubMed] [Google Scholar]
- 25.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases] J Cardiol. 1991;21:203–214. [PubMed] [Google Scholar]
- 26.Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB. Coronary vasoconstriction induced by mental stress (simulated public speaking) Am J Cardiol. 1995;75:503–505. doi: 10.1016/s0002-9149(99)80590-6. [DOI] [PubMed] [Google Scholar]
- 27.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Kono Y, Umemura T, Nakamura S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 28.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 29.Sadamatsu K, Tashiro H, Maehira N, Yamamoto K. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J. 2000;64:789–792. doi: 10.1253/jcj.64.789. [DOI] [PubMed] [Google Scholar]
- 30.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 31.Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K. 123I-MIBG myocardial scintigraphy in patients with "takotsubo" cardiomyopathy. J Nucl Med. 2004;45:1121–1127. [PubMed] [Google Scholar]
- 32.Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV Jr, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–647. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Parodi G, Del Pace S, Carrabba N, Salvadori C, Memisha G, Simonetti I, Antoniucci D, Gensini GF. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99:182–185. doi: 10.1016/j.amjcard.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 34.Angelini P. Transient left ventricular apical ballooning: A unifying pathophysiologic theory at the edge of Prinzmetal angina. Catheter Cardiovasc Interv. 2008;71:342–352. doi: 10.1002/ccd.21338. [DOI] [PubMed] [Google Scholar]
- 35.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 36.Jougasaki M, Yasue H, Takahashi K. Perivascular nerve lesion of the coronary artery involved in spasm in a patient with variant angina. Pathology. 1989;21:304–307. doi: 10.3109/00313028909061079. [DOI] [PubMed] [Google Scholar]
- 37.Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, Yoshimura M, Motoyama T, Inobe Y, Kawano H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–271. doi: 10.1161/01.cir.94.3.266. [DOI] [PubMed] [Google Scholar]
- 38.Kugiyama K, Ohgushi M, Sugiyama S, Motoyama T, Kawano H, Hirashima O, Yasue H. Supersensitive dilator response to nitroglycerin but not to atrial natriuretic peptide in spastic coronary arteries in coronary spastic angina. Am J Cardiol. 1997;79:606–610. doi: 10.1016/s0002-9149(96)00824-7. [DOI] [PubMed] [Google Scholar]
- 39.Kugiyama K, Motoyama T, Hirashima O, Ohgushi M, Soejima H, Misumi K, Kawano H, Miyao Y, Yoshimura M, Ogawa H, et al. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:103–109. doi: 10.1016/s0735-1097(98)00185-5. [DOI] [PubMed] [Google Scholar]
- 40.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Tsunoda R, Moriyama Y, Miyao Y, Yoshimura M, Ogawa H, et al. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:1672–1679. doi: 10.1016/s0735-1097(98)00447-1. [DOI] [PubMed] [Google Scholar]
- 41.Miwa K, Miyagi Y, Igawa A, Nakagawa K, Inoue H. Vitamin E deficiency in variant angina. Circulation. 1996;94:14–18. doi: 10.1161/01.cir.94.1.14. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura M, Yasue H, Nakayama M, Shimasaki Y, Sumida H, Sugiyama S, Kugiyama K, Ogawa H, Ogawa Y, Saito Y, et al. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet. 1998;103:65–69. doi: 10.1007/s004390050785. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, Motoyama T, Saito Y, Ogawa Y, Miyamoto Y, et al. T-786-->C mutation in the 5'-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999;99:2864–2870. doi: 10.1161/01.cir.99.22.2864. [DOI] [PubMed] [Google Scholar]
- 44.Ito T, Yasue H, Yoshimura M, Nakamura S, Nakayama M, Shimasaki Y, Harada E, Mizuno Y, Kawano H, Ogawa H. Paraoxonase gene Gln192Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum Genet. 2002;110:89–94. doi: 10.1007/s00439-001-0654-6. [DOI] [PubMed] [Google Scholar]
- 45.Hung MJ, Hung MY, Cheng CW, Yang NI, Cherng WJ. Comparison of clinical characteristics and prognosis in Taiwanese patients with coronary vasospastic angina pectoris without significant fixed coronary artery disease versus patients with significant fixed coronary artery disease and either stable angina pectoris or acute coronary syndromes. Am J Med Sci. 2007;334:160–167. doi: 10.1097/MAJ.0b013e3181405b30. [DOI] [PubMed] [Google Scholar]
- 46.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol. 1997;273:H1644–H1650. doi: 10.1152/ajpheart.1997.273.4.H1644. [DOI] [PubMed] [Google Scholar]
- 47.Ota Y, Kugiyama K, Sugiyama S, Ohgushi M, Matsumura T, Doi H, Ogata N, Oka H, Yasue H. Impairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract--role of free radicals and attenuation by captopril. Atherosclerosis. 1997;131:195–202. doi: 10.1016/s0021-9150(97)06106-6. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama S, Kugiyama K, Ohgushi M, Matsumura T, Ota Y, Doi H, Ogata N, Oka H, Yasue H. Supersensitivity of atherosclerotic artery to constrictor effect of cigarette smoke extract. Cardiovasc Res. 1998;38:508–515. doi: 10.1016/s0008-6363(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 49.Murase Y, Yamada Y, Hirashiki A, Ichihara S, Kanda H, Watarai M, Takatsu F, Murohara T, Yokota M. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J. 2004;25:970–977. doi: 10.1016/j.ehj.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S, Yoshimura M, Nakayama M, Abe K, Yamamuro M, Nagayoshi Y, Kojima S, Kaikita K, Sugiyama S, Yasue H, et al. A novel genetic marker for coronary spasm in women from a genome-wide single nucleotide polymorphism analysis. Pharmacogenet Genomics. 2007;17:919–930. doi: 10.1097/FPC.0b013e328136bd35. [DOI] [PubMed] [Google Scholar]
- 51.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 52.Shimokawa H, Tomoike H, Nabeyama S, Yamamoto H, Ishii Y, Tanaka K, Nakamura M. Coronary artery spasm induced in miniature swine: angiographic evidence and relation to coronary atherosclerosis. Am Heart J. 1985;110:300–310. doi: 10.1016/0002-8703(85)90148-6. [DOI] [PubMed] [Google Scholar]
- 53.Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation. 1993;87:76–79. doi: 10.1161/01.cir.87.1.76. [DOI] [PubMed] [Google Scholar]
- 54.Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- 55.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 56.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 57.Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- 58.Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, et al. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 59.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S, Kaibuchi K, et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation. 2000;101:1319–1323. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- 60.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 61.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 62.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasue H, Mizuno Y, Harada E, Itoh T, Nakagawa H, Nakayama M, Ogawa H, Tayama S, Honda T, Hokimoto S, et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J Am Coll Cardiol. 2008;51:1742–1748. doi: 10.1016/j.jacc.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 64.Okumura K, Yasue H, Matsuyama K, Ogawa H, Kugiyama K, Ishizaka H, Sumida H, Fujii H, Matsunaga T, Tsunoda R. Diffuse disorder of coronary artery vasomotility in patients with coronary spastic angina. Hyperreactivity to the constrictor effects of acetylcholine and the dilator effects of nitroglycerin. J Am Coll Cardiol. 1996;27:45–52. doi: 10.1016/0735-1097(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 65.Lewis JR, Kisilevsky R, Armstrong PW. Prinzmetal's angina, normal coronary arteries and pericarditis. Can Med Assoc J. 1978;119:36–39. [PMC free article] [PubMed] [Google Scholar]
- 66.Hung MJ, Kuo LT, Cheng CW, Chang CP, Cherng WJ. Comparison of peripheral monocyte counts in patients with and without coronary spasm and without fixed coronary narrowing. Am J Cardiol. 2004;93:620–624. doi: 10.1016/j.amjcard.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 67.Hung MJ, Cherng WJ, Cheng CW, Yang NI. Effect of antispastic agents (calcium antagonists and/or isosorbide dinitrate) on high-sensitivity C-reactive protein in patients with coronary vasospastic angina pectoris and no hemodynamically significant coronary artery disease. Am J Cardiol. 2005;95:84–87. doi: 10.1016/j.amjcard.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 68.Rich MW. Is vasospastic angina an inflammatory disease? Am J Cardiol. 2005;96:1612. doi: 10.1016/j.amjcard.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 69.Rich MW. An association between prinzmetal's angina pectoris and obstructive lung disease. Am J Cardiol. 2005;96:1612–1613. doi: 10.1016/j.amjcard.2005.03.105. [DOI] [PubMed] [Google Scholar]
- 70.Itoh T, Mizuno Y, Harada E, Yoshimura M, Ogawa H, Yasue H. Coronary spasm is associated with chronic low-grade inflammation. Circ J. 2007;71:1074–1078. doi: 10.1253/circj.71.1074. [DOI] [PubMed] [Google Scholar]
- 71.Li JJ, Zhang YP, Yang P, Zeng HS, Qian XW, Zhang CY, Zhu CG, Li J, Nan JL. Increased peripheral circulating inflammatory cells and plasma inflammatory markers in patients with variant angina. Coron Artery Dis. 2008;19:293–297. doi: 10.1097/MCA.0b013e3282fd5c4e. [DOI] [PubMed] [Google Scholar]
- 72.Cho SH, Park IH, Jeong MH, Hwang SH, Yun NS, Hong SN, Lee SR, Kim KH, Moon Y, Hong YJ, et al. Increased inflammatory markers and endothelial dysfunction are associated with variant angina. Korean Circ J. 2007;37:27–32. [Google Scholar]
- 73.Cho SH, Jeong MH, Park IH, Choi JS, Yoon HJ, Kim KH, Hong YJ, Park HW, Kim JH, Ahn Y, et al. Endothelial dysfunction, increased carotid artery intima-media thickness and pulse wave velocity, and increased level of inflammatory markers are associated with variant angina. J Cardiol. 2009;54:183–191. doi: 10.1016/j.jjcc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Hung MJ, Cherng WJ, Yang NI, Cheng CW, Li LF. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. Am J Cardiol. 2005;96:1484–1490. doi: 10.1016/j.amjcard.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 75.Hung MJ, Cherng WJ, Cheng CW, Li LF. Comparison of serum levels of inflammatory markers in patients with coronary vasospasm without significant fixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with significant fixed coronary artery disease. Am J Cardiol. 2006;97:1429–1434. doi: 10.1016/j.amjcard.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 76.Yasue H, Hirai N, Mizuno Y, Harada E, Itoh T, Yoshimura M, Kugiyama K, Ogawa H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ J. 2006;70:8–13. doi: 10.1253/circj.70.8. [DOI] [PubMed] [Google Scholar]
- 77.Hung MY, Hsu KH, Hung MJ, Cheng CW, Kuo LT, Cherng WJ. Interaction between cigarette smoking and high-sensitivity C-reactive protein in the development of coronary vasospasm in patients without hemodynamically significant coronary artery disease. Am J Med Sci. 2009;338:440–446. doi: 10.1097/MAJ.0b013e3181b9147f. [DOI] [PubMed] [Google Scholar]
- 78.Turlapaty PD, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 79.Goto K, Yasue H, Okumura K, Matsuyama K, Kugiyama K, Miyagi H, Higashi T. Magnesium deficiency detected by intravenous loading test in variant angina pectoris. Am J Cardiol. 1990;65:709–712. doi: 10.1016/0002-9149(90)91375-g. [DOI] [PubMed] [Google Scholar]
- 80.Miyagi H, Yasue H, Okumura K, Ogawa H, Goto K, Oshima S. Effect of magnesium on anginal attack induced by hyperventilation in patients with variant angina. Circulation. 1989;79:597–602. doi: 10.1161/01.cir.79.3.597. [DOI] [PubMed] [Google Scholar]
- 81.Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest. 2000;118:1690–1695. doi: 10.1378/chest.118.6.1690. [DOI] [PubMed] [Google Scholar]
- 82.Yoshimura M, Oshima T, Matsuura H, Ishida T, Kambe M, Kajiyama G. Extracellular Mg2+ inhibits capacitative Ca2+ entry in vascular smooth muscle cells. Circulation. 1997;95:2567–2572. doi: 10.1161/01.cir.95.11.2567. [DOI] [PubMed] [Google Scholar]
- 83.Mallet RT, Sun J, Fan WL, Kang YH, Bünger R. Magnesium activated adenosine formation in intact perfused heart: predominance of ecto 5'-nucleotidase during hypermagnesemia. Biochim Biophys Acta. 1996;1290:165–176. doi: 10.1016/0304-4165(96)00016-5. [DOI] [PubMed] [Google Scholar]
- 84.Sasaguri T, Itoh T, Hirata M, Kitamura K, Kuriyama H. Regulation of coronary artery tone in relation to the activation of signal transductors that regulate calcium homeostasis. J Am Coll Cardiol. 1987;9:1167–1175. doi: 10.1016/s0735-1097(87)80322-4. [DOI] [PubMed] [Google Scholar]
- 85.Shinozaki K, Suzuki M, Ikebuchi M, Takaki H, Hara Y, Tsushima M, Harano Y. Insulin resistance associated with compensatory hyperinsulinemia as an independent risk factor for vasospastic angina. Circulation. 1995;92:1749–1757. doi: 10.1161/01.cir.92.7.1749. [DOI] [PubMed] [Google Scholar]
- 86.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 87.Ueda O, Kohchi K, Kishi Y, Numano F. Long lasting spasticity in controlled vasospastic angina. Heart. 1999;81:528–532. doi: 10.1136/hrt.81.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kugiyama K, Yasue H, Horio Y, Morikami Y, Fujii H, Koga Y, Kojima A, Takahashi M. Effects of propranolol and nifedipine on exercise-induced attack in patients variant angina: assessment by exercise thallium-201 myocardial scintigraphy with quantitative rotational tomography. Circulation. 1986;74:374–380. doi: 10.1161/01.cir.74.2.374. [DOI] [PubMed] [Google Scholar]
- 89.Tanabe Y, Itoh E, Suzuki K, Ito M, Hosaka Y, Nakagawa I, Kumakura M. Limited role of coronary angioplasty and stenting in coronary spastic angina with organic stenosis. J Am Coll Cardiol. 2002;39:1120–1126. doi: 10.1016/s0735-1097(02)01746-1. [DOI] [PubMed] [Google Scholar]
- 90.Ono T, Ohashi T, Asakura T, Shin T. Internal mammary revascularization in patients with variant angina and normal coronary arteries. Interact Cardiovasc Thorac Surg. 2005;4:426–428. doi: 10.1510/icvts.2005.107128. [DOI] [PubMed] [Google Scholar]
- 91.Meisel SR, Mazur A, Chetboun I, Epshtein M, Canetti M, Gallimidi J, Katz A, Strasberg B, Peled B. Usefulness of implantable cardioverter-defibrillators in refractory variant angina pectoris complicated by ventricular fibrillation in patients with angiographically normal coronary arteries. Am J Cardiol. 2002;89:1114–1116. doi: 10.1016/s0002-9149(02)02283-x. [DOI] [PubMed] [Google Scholar]
- 92.Letsas KP, Filippatos GS, Efremidis M, Sideris A, Kardaras F. Secondary prevention of sudden cardiac death in coronary artery spasm: is implantable cardioverter defibrillator always efficient? Int J Cardiol. 2007;117:141–143. doi: 10.1016/j.ijcard.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 93.Takagi Y, Yasuda S, Takahashi J, Takeda M, Nakayama M, Ito K, Hirose M, Wakayama Y, Fukuda K, Shimokawa H. Importance of dual induction tests for coronary vasospasm and ventricular fibrillation in patients surviving out-of-hospital cardiac arrest. Circ J. 2009;73:767–769. doi: 10.1253/circj.cj-09-0061. [DOI] [PubMed] [Google Scholar]
- 94.Shimokawa H, Nagasawa K, Irie T, Egashira S, Egashira K, Sagara T, Kikuchi Y, Nakamura M. Clinical characteristics and long-term prognosis of patients with variant angina. A comparative study between western and Japanese populations. Int J Cardiol. 1988;18:331–349. doi: 10.1016/0167-5273(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 95.Waters DD, Miller DD, Szlachcic J, Bouchard A, Méthé M, Kreeft J, Théroux P. Factors influencing the long-term prognosis of treated patients with variant angina. Circulation. 1983;68:258–265. doi: 10.1161/01.cir.68.2.258. [DOI] [PubMed] [Google Scholar]
- 96.Yasue H, Takizawa A, Nagao M, Nishida S, Horie M, Kubota J, Omote S, Takaoka K, Okumura K. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. doi: 10.1161/01.cir.78.1.1. [DOI] [PubMed] [Google Scholar]
- 97.Bory M, Pierron F, Panagides D, Bonnet JL, Yvorra S, Desfossez L. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur Heart J. 1996;17:1015–1021. doi: 10.1093/oxfordjournals.eurheartj.a014996. [DOI] [PubMed] [Google Scholar]
- 98.Kodama S, Inoue Y, Mihara H, Sumi S, Kudo K, Okamura K, Ando C, Niimura H, Tsuchiya Y, Yamanouchi Y, et al. Prognostic factors for the long-term survival in patients with vasospastic angina Analysis of effects of patients' characteristics and therapeutic drugs. J Cardiol. 2009;54:10–20. doi: 10.1016/j.jjcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura M, Takeshita A, Nose Y. Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina. Circulation. 1987;75:1110–1116. doi: 10.1161/01.cir.75.6.1110. [DOI] [PubMed] [Google Scholar]
- 100.Wakabayashi K, Suzuki H, Honda Y, Wakatsuki D, Kawachi K, Ota K, Koba S, Shimizu N, Asano F, Sato T, et al. Provoked coronary spasm predicts adverse outcome in patients with acute myocardial infarction: a novel predictor of prognosis after acute myocardial infarction. J Am Coll Cardiol. 2008;52:518–522. doi: 10.1016/j.jacc.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 101.Shimokawa H, Yasuda S. Myocardial ischemia: Current concepts and future perspectives. J Cardiol. 2008;52:67–78. doi: 10.1016/j.jjcc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Maseri A, Beltrame JF, Shimokawa H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ J. 2009;73:394–403. doi: 10.1253/circj.cj-09-0033. [DOI] [PubMed] [Google Scholar]