Abstract
Immunological memory, defined as more efficient immune responses on antigen reexposure, can last for decades. The current paradigm is that memory is maintained by antigen-experienced “memory T cells” that can be long-lived quiescent or dividing. The contribution of T cell division to memory maintenance is poorly known and has important clinical implications. In this study, we directly addressed the role of dividing T cells in immunological memory maintenance by evaluating the consequences of their elimination. The specific ablation of dividing T cells was obtained by administration of ganciclovir to immune mice expressing the herpes simplex type 1 thymidine kinase suicide gene in T cells. We show that depletion of dividing T cells for 5 or 2 weeks suffices to abolish in vitro and in vivo memory responses against the male H-Y transplantation alloantigen or against lymphocytic choriomeningitis virus antigens, respectively. Similar results were obtained after the nonspecific elimination of all dividing cells by using hydroxyurea, a cytostatic toxic agent commonly used for cancer chemotherapy. This immune amnesia occurred in otherwise immunocompetent mice and despite the persistence of functional quiescent T cells displaying a “memory” phenotype. Thus, division of antigen-experienced T cells is an absolute requirement for immunological memory maintenance and the current concept of memory T cells is challenged.
Immunological memory is one of the main features of adaptative immunity. It is characterized by a more rapid and intense immune response on reexposure to an immunogen. In responses against pathogens, immunological memory can translate into infection protection that can last for decades. The population dynamics of lymphocytes that ensures long-term memory maintenance in vivo and the physiological consequences of this dynamics are poorly understood. In particular, the respective contributions of long-lived quiescent vs. dividing T cells for immunological memory maintenance remain to be investigated.
The current paradigm is that “memory T cells” support immunological memory. Tentative identification of these cells has been based on cell surface markers whose high (hi)orlow(lo) expression levels are modulated on activation (1, 2). Likewise in mice, T cells are commonly subdivided into naive (CD44lo-CD45RBhi-CD62Lhi), effector (CD44hi-CD45RBlo/hi-CD62Llo), and memory subsets (CD44hi-CD45RBlo-CD62Llo). However, this immunophenotypic classification is simplistic: (i) the memory T cell phenotype appears quite heterogeneous (3), different for CD4+ and CD8+ T cells, comprising at least two subsets referred to as “effector memory” and “central memory” T cells (4–6); (ii) phenotypic changes from naive to memory/effector type may be a stigmata of antigen (Ag) activation rather than a hallmark of memory/effector function; (iii) at least some of these phenotypic changes are reversible (3, 7); (iv) homeostatic proliferation can induce naive T cells to acquire the memory/effector phenotype in the absence of Ag (2, 8, 9); and (v) T cell immunophenotype does not always correlate with function (10). Despite these uncertainties in identifying memory T cells, previous studies investigating their lifespan could only rely on such immunophenotypic characterization (11–13) and did not question the in vivo relevance of their turnover and division rate.
The contribution of T cell division to memory maintenance can be investigated by disabling dividing cells in immunized individuals. This strategy, in contrast to previous approaches, circumvents the difficulties in properly identifying the T cells supporting memory. We took advantage of a transgenic mouse model allowing exclusive and conditional ablation of dividing T cells based on their specific expression of a suicide gene, the herpes simplex type 1 thymidine kinase (TK). Cells expressing TK can metabolize the nucleoside analog ganciclovir (GCV) into toxic triphosphated GCV that, by blocking DNA elongation, kills dividing cells. The fact that only dividing cells are killed by triphosphated GCV is a key property of this system. We generated transgenic mice specifically expressing the TK gene in both CD4+ and CD8+ T cells (TK+). Thus, in TK+ mice, although all T cells express TK, only the dividing ones are eliminated during GCV administration, whereas quiescent T cells and all other cells are spared. The efficiency of this system has been exemplified by its apt control of T cell-mediated pathologies, such as graft-versus-host disease or cardiac and skin allograft rejections (14–16). Because initiation time and duration of GCV treatment can be controlled, TK+ mice offer a unique possibility to explore the role of T cell division in immunological memory maintenance. Likewise, mice can be immunized to establish memory; 2 months later, dividing T cells can be specifically eliminated for various times by GCV treatment; and finally, mice can be assessed for secondary immune responses after GCV cessation.
We explored such anamnestic responses against male H-Y transplantation alloantigen and against lymphocytic choriomeningitis virus (LCMV) Ags, both in vitro and in vivo. In both models, we demonstrate that T cell division is an absolute requisite for maintaining immunological memory. Moreover, we show that the treatment of sensitized mice with hydroxyurea (HU), a toxic cytotoxic drug commonly used in cancer chemotherapy that kills all dividing cells, also induces immune amnesia while immunocompetency is preserved.
Materials and Methods
Mice. The TK transgenic B6 mice (EpCD4ΔTK line 2) were generated in our laboratory (17). The ΔTK transgene is under control of CD4 regulatory sequences comprising promoter and enhancer elements but lacking the silencer, ensuring expression in both CD4+ and CD8+ T cells (18). Limiting dilution analyses demonstrate that, in these mice, >98% of dividing TK+ T cells are ablated by GCV (data not shown). In H-2b P14 transgenic mice (line 318) 80% of CD8+ T cells express the TCR-Vα2/Vβ8 specific for the GP33–41 epitope from the LCMV envelope glycoprotein. Mice were bred under specific pathogen-free conditions, and manipulations were performed according to European Economic Community guidelines.
In Vivo Sensitization. H-Y immunization. B6 male tail skin grafts were performed on female B6 mice. Graft appearance was monitored at least three times a week and every day around the time of rejection (16).
GP33–41 immunization. P14xTK mice were immunized with 100 μg of GP33–41 peptide (Neosystem, Strasbourg, France) emulsified in an equal volume of incomplete Freund's adjuvant (Sigma-Aldrich) and injected s.c. at the base of the tail.
LCMV infection and follow-up. Mice were inoculated in the hind footpad with 30 μl of viral suspension containing 104.2 plaque-forming units of LCMV-WE. LCMV RNA was detected by RT-PCR as described (19).
GCV administration. Mice received GCV (Roche Diagnostics) i.p. at the dose of 60 mg·kg-1·day-1 during 2 weeks in GP33–41- or LCMV-sensitized mice and during 2 or 5 weeks in H-Y-sensitized B6 female mice. In 2-week treatment, GCV was administered by five daily consecutive injections per week followed by 2 washout days; 5-week treatment was done by one injection every 2 days during 5 days followed by 2 washout days. HU administration. HU (Hydrea, Bristol-Myers Squibb) was administered as cycle of 2 i.p. HU injections at 1 g·kg-1·day-1, 7 h apart. Mice received three cycles (every 2 days), five or six cycles (every 2 or 3 days), corresponding to 5-, 10-, or 12-day-long treatments, respectively.
Proliferation and Cytotoxicity Assays. Proliferation assays were done as described (16). Splenocytes (2.5 × 105) from female B6 mice were cocultured for 5 days with 105 25-Gy-irradiated male B6 spleen cells; splenocytes (5 × 105) from P14 mice were cultured for 2 days in the presence of GP33–41 peptide (0.5 μg/ml) in a 200-μl final volume in U-bottomed 96-well plates. Secondary effector cytolytic T lymphocytes (CTLs) were obtained by stimulating splenocytes (106 cells per ml) for 5 days at 37°C in the presence of either 0.5 μg/ml GP33–41 peptide or 25-Gy-irradiated male spleen cells (106 cells per ml). In adoptive transfer experiments, effector CTLs were obtained after culture by purification of donor CD8+ Thy1.2+ by using magnetic purification (Miltenyi Biotec, Auburn, CA). Target cells were either the H-2b mouse lymphoma EL4 cells (ATCC-TIB39) pulsed for 1 h with GP33–41 peptide (50 μM) or 48-h ConA-activated male B6 splenocytes, both labeled with Na-51Cr [100 μCi per 106 cells in 1 ml, Amersham Pharmacia (1 Ci = 37 GBq)]. Serial 3-fold dilutions of effector cells in triplicate were incubated with 104 target cells at ratios from 100:1 to 0.1:1. After 4 h at 37°C, 50-μl supernatants were incubated overnight on Lumaplates (Packard), and radioactivity was counted in a Betaplate counter (Wallac, Gaithersburg, MD). Percent of specific lysis was calculated as 100 × [(experimental release - spontaneous release)/(maximal release - spontaneous release)]. The percentage of spontaneous vs. total 51Cr release was <20%, and lysis of control syngeneic targets was <5%. Lytic units (LUs) were determined according to established procedures (20) from curves obtained for various effector/target ratios.
CTL Precursor Frequency. Spleen cells were plated in limiting dilution conditions (48 replicates per dilution) in flat-bottom 96-microwell plates containing 2 × 105 irradiated (25 Gy) syngeneic spleen feeder cells loaded with GP33–41 peptide (50 μM). After 5 days of culture, CTL activity was determined on GP33–41-pulsed EL4 targets labeled with Na-51Cr (see above). A well was considered positive when its value was >3 SD above the mean spontaneous 51Cr release obtained in presence of irradiated feeder cells. The frequency of responding cells was determined by using the LIMITING DILUTION ANALYSIS software (Oxford University Press, New York).
Immunostaining and Flow Cytometry. All isolated lymphoid cells were stained with fluorochrome or biotin-coupled antibodies directed against CD44 (IM7), CD45RB (C363.16A), TCR-Vα2 (B20.1) from BD Pharmingen and CD4 (CT-CD4), CD8 (53–6.7) from Caltag (South San Francisco, CA) and revealed by allophycocyanin (APC)-streptavidin (BD Pharmingen). GP33–41-specific cells were identified by binding to the H-2Db/GP33–41 dimer. The dimeric H-2Db:Ig protein (BD Pharmingen) was mixed with GP33–41 peptide at 40 M excess, in PBS at 37°C overnight. After cell surface Fc receptors blocking with anti-mouse CD16/CD32 Fc-RIII/II receptor (BD Pharmingen), 106 lymphocytes were incubated with 0.5 μg of H-2Db/GP33–41 dimer complex for 1 h at 4°C, followed by phycoerythrin-conjugated anti-mouse IgG1 incubation for 30 min at 4°C. Intracellular IFN-γ staining was performed by using the BD Pharmingen kit after cell activation in the presence of GP33–41 (0.1 μg/ml) and Golgi plug for 5 h at 37°C. Four-color analysis was performed with a FACSCalibur flow cytometer using CELL QUEST software (Becton Dickinson).
Results
Experimental Models. Immunological memory against the male H-Y transplantation alloantigen was generated by grafting male B6 skin onto B6 TK+ female recipients displaying a wild-type TCR repertoire. Memory against LCMV was generated by injection of live LCMV or its immunodominant peptide (GP33–41) in double TCR-transgenic P14xTK+ mice. Two months after the initial sensitizations, specific depletion of dividing T cells was realized by GCV treatment of the sensitized mice. Consequence on immunological memory maintenance was evaluated 3 days after GCV cessation. In all experiments, sensitized GCV-treated TK- mice served as controls for GCV-treated TK+ littermates.
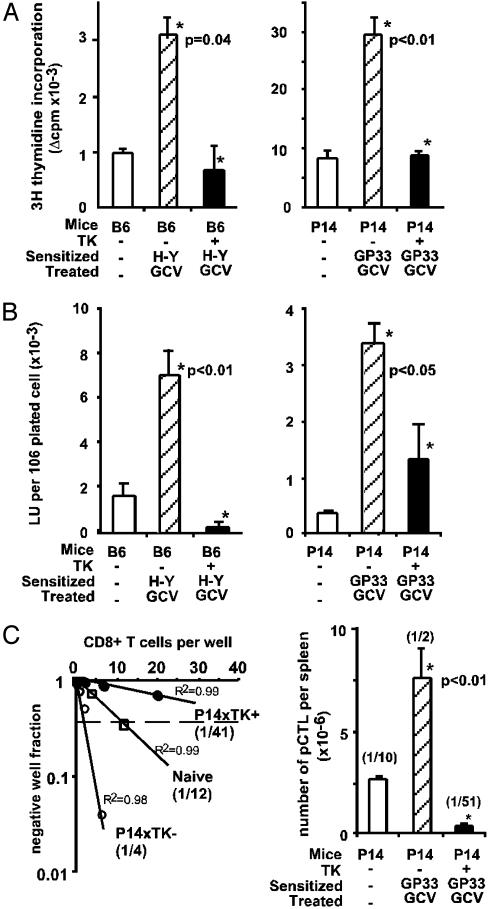
Abrogation of in Vitro Antigen-Specific Memory Responses After in Vivo Elimination of Dividing T Cells. We first tested memory by comparing the in vitro lymphocyte responses from naive or sensitized GCV-treated mice. GCV was administered during 2 and 5 weeks to GP33–41 and H-Y Ag-sensitized mice, respectively. Spleen T cells from in vivo sensitized GCV-treated TK- mice, restimulated in vitro with their sensitizing H-Y Ag or GP33–41 peptide, proliferated more rapidly (i.e., 48 h ahead; data not shown) and more intensely than those from naive mice (Fig. 1A). In contrast, this typical memory-type proliferative response was lost in equally treated TK+ mice (Fig. 1A), 3 days after the end of GCV treatment. When cytotoxic responses were assayed, strong specific secondary CTL activities were detected in splenocytes from H-Y or GP33–41 in vivo sensitized GCV-treated TK- mice. This response was abrogated by GCV in splenocytes from TK+ mice (Fig. 1B). Quantitative limiting dilution analyses of CTL precursors from GCV-treated P14xTK+ mice revealed a 96% reduction in GP33–41-specific CTL precursor frequency (Fig. 1C). Altogether, in vivo ablation of dividing T cells abrogates both proliferative and cytotoxic memory responses to GP33–41 and H-Y Ags.
Fig. 1.
In vitro evaluation of Ag-specific memory-type immune responses after in vivo elimination of dividing T cells. Two months after in vivo sensitization, TK+ mice or TK- littermates were treated with GCV, 5 weeks for H-Y male skin-grafted B6 mice and 2 weeks for GP33–41-sensitized P14xTK mice. Then, 3 days after GCV cessation, Ag-specific responses of splenocytes were evaluated in treated and in naive control mice. Results represent the mean ± SE (n = 2–7) and P values (Mann–Whitney test; *) comparing responses of TK+ and TK- mice. (A) Ag-specific proliferative responses. Δcpm values were calculated after [3H]thymidine incorporation by deducing syngeneic reactivity values. (B) Ag-specific secondary CTL responses, indicated as LU for 106 plated CD8+ CD44hi (Left) or CD8+ TCR Vα2 (Right) cells. One LU corresponds to the number of effector cells required to exhibit 20% or 30% of specific lysis in H-Y- or GP33–41-sensitized mice, respectively. Similar CTL responses were obtained in P14xTK mice sensitized through intraplantar LCMV infection (data not shown). (C) Determination of the frequency of GP33–41 CTL precursors (pCTL) in spleen of P14xTK mice by limiting dilution analysis (Left). pCTL frequencies among CD8+ T cells, calculated from the dilution leading to 37% of negative wells, are indicated in parentheses together with correlation coefficients for one representative mouse per group. (Right) Number ± SE and mean frequency (in parentheses) of GP33–41 pCTL in P14xTK mice determined by limiting dilution analysis among CD8+ T cells.
We performed similar studies after adoptive transfer of naive TCR-transgenic CD8+ cells. In this experimental situation, the adopted cells represent only a small proportion of the total T cell repertoire, thus making it more physiologically relevant. This experimental situation also allows the depletion of only Ag-specific donor CD8+ T cells but not other dividing T cells. In this model, loss of CD8+ memory responses was similar to that observed directly in transgenic P14 mice, as revealed by the reduced number of GP33–41-specific and CD44hi CD8+ donor T cells, and the CTL activity (see Fig. 6, which is published as supporting information on the PNAS web site).
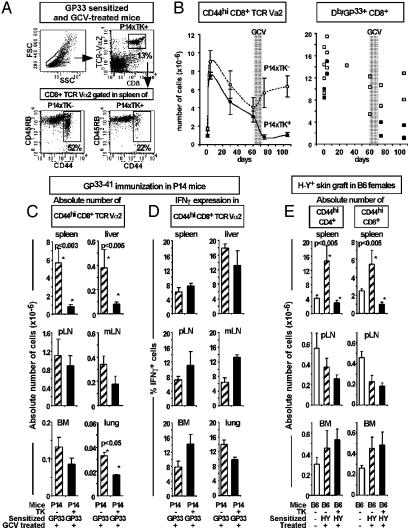
Phenotypic Analysis of T Cells After GCV-Mediated Depletion. We next aimed to determine whether such abrogation of in vitro memory-type responses was correlated with the disappearance of CD44hi T cells, which are commonly described as memory T cells (1, 3, 10). GP33–41 sensitization could be evidenced by a 4- to 5-fold increase of CD44hi CD8+ TCR-Vα2 T cell numbers by day 4 (Fig. 2B Left) and was followed by a contraction of the T cell pool during the next 60 days. After GCV treatment, the CD44hi CD8+ TCR-Vα2 cell numbers were significantly reduced in the spleens of GP33–41-sensitized P14xTK+ compared with P14xTK- mice (Fig. 2 A and B Left and 2C). These changes correlated well with the depletion of GP33–41-specific splenocytes binding the H-2Db/GP33–41 dimer complex in sensitized GCV-treated P14xTK+ mice, measured 3 or 30 days after the end of GCV treatment (Fig. 2B Right), and with the abrogation of the in vitro memory-type immune responses (Fig. 1). Reductions in the number of CD44hi T cells, which affected both CD45RBhi and CD45RBlo subpopulations (Fig. 2 A and data not shown), could be observed in all the lymphoid and nonlymphoid tissues tested, although to various extents. Significant reductions were observed in liver and lung (Fig. 2C). In contrast, significant numbers of CD44hi T cells persisted in peripheral and mesenteric lymph nodes, bone marrow (Fig. 2C), and blood (data not shown). Altogether, after 2 weeks of GCV treatment, the cumulative numbers of persisting, thus quiescent, CD44hi T cells from all tissues tested represented ≈40% of controls.
Fig. 2.
Cell surface phenotype and function of CD44hi T cells in different lymphoid compartments after elimination of dividing T cells. (A) Representative FACS dot plot obtained after cell surface staining, with antibodies to CD8, TCR-Vα2, CD44, and CD45RB, on spleen cells from GP33–41-sensitized and GCV-treated P14xTK- and P14xTK+ mice, 3 days after the end of GCV. (B) Evolution of CD44hi CD8+ TCR-Vα2 splenocyte numbers (mean ± SE, n = 3–11; Left), and dimer Db/GP33–41-specific CD8+ cell numbers (in individual mice; Right) after GP33–41 immunization (on day 0) and GCV treatment (on day 60 for 2 weeks, gray area). Spleen cells were collected on days 0, 4, 30, 60, 75, and 105 after sensitization in P14xTK- (open symbols) and P14xTK+ mice (filled symbols). Before GCV treatment, the percentages and numbers of CD8+ Vα2 T cells and dimer Db/GP33–41-specific CD8+ cell numbers in P14xTK- and P14xTK+ mice are not significantly different. (C–E) Flow cytometry analysis on lymphocytes obtained 3 days after GCV treatment from spleen, pooled inguinal, brachial, and axillary lymph nodes (pLN), mesenteric lymph nodes (mLN), bone marrow from femurs and tibias (BM), and liver and lung taken from the naive (open bars), sensitized, and GCV-treated TK- or TK+ mice studied in Fig. 1. Mean ± SE (n = 6–11) of absolute numbers of CD44hi CD8+ TCR-Vα2 cells in P14 mice (C) and CD44hi CD4+ or CD44hi CD8+ T cells in female B6 mice (E). (D) Percentage of IFN-γ-producing cells among CD44hi CD8+ TCR-Vα2 cells 3 days after GCV treatment. The percentage of IFN-γ+ cells in naive P14 mice was <2% in all organs except bone marrow (8%). In the absence of peptide stimulation, <0.8% of cells were IFN-γ+. (C–E) *, P values <0.05 comparing TK+ and TK- mice or given (Mann–Whitney test).
These observations were confirmed in the skin allograft model. Indeed, in H-Y-sensitized TK+ mice, GCV induced a significant reduction in the numbers of splenic CD44hi CD4+ and CD44hi CD8+ cells, which reached numbers observed in naive mice (Fig. 2E). As for the P14xTK+ mice, little or no depletion of the CD44hi T cells occurred in peripheral lymph nodes, bone marrow, and blood (Fig. 2E and data not shown).
Functionality of the Persisting Quiescent CD44hi T Cells. GCV treatment leads to a major decrease in the in vitro secondary immune responses, whereas significant numbers of CD44hi T cells persist. We thus investigated whether the quiescent CD44hi CD8+ TCR-Vα2 T cells from P14xTK+-sensitized mice were functional by evaluating the IFN-γ production triggered on GP33–41 Ag restimulation. IFN-γ production was measured 5 h after stimulation, a time point at which no production is detected in naive CD44lo T cells (data not shown). In all tissues studied, 8–19% of CD44hi T cells produced IFN-γ, and no apparent reduction was observed in GCV-treated P14xTK+ compared with P14xTK- mice (Fig. 2D). These results indicate that, at the single-cell level, quiescent T cells persisting after GCV treatment can be triggered by their specific Ag to produce IFN-γ with the kinetics expected from Ag-experienced T cells. However, the efficient IFN-γ production by Ag-experienced T cells persisting after GCV treatment contrasts with the loss of cytotoxic activity (Fig. 1).
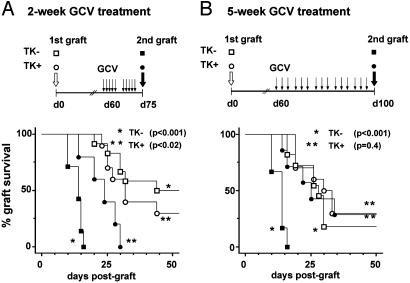
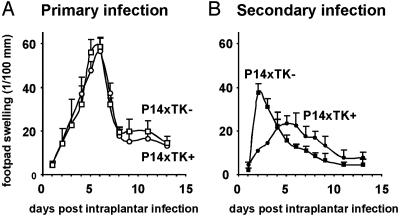
In Vivo Immune Amnesia After Specific Elimination of Dividing T Cells. The persistence in vivo of functional T cells with a memory-type phenotype (Fig. 2D) contrasts with the abolition of the Ag-specific memory-type responses in vitro (Fig. 1). Because immunological memory is a functional property of an entire organism and not of individual cells, the ultimate way to solve this issue was to test the memory response in vivo after depletion of dividing T cells. In vivo memory responses were investigated both against male skin grafts and LCMV infection. In TK- female mice, male skin secondary allograft rejection was accelerated compared with that of primary allografts (Fig. 3), exemplifying memory to H-Y Ag. In TK+ mice, this acceleration was reduced by a 2-week administration of GCV (Fig. 3A) and abolished by a 5-week treatment (Fig. 3B). Abrogation of memory was also observed after LCMV infection (Fig. 4). Mice were exposed to primary and secondary infections through intraplantar LCMV inoculation, and immune responses were then monitored by quantitative evaluation of delayed-type hypersensitivity (DTH) responses revealed by footpad swelling. Primary DTH responses in naive P14xTK- and P14xTK+ mice peaked on day 5 (Fig. 4A). Secondary responses in P14xTK- control mice were much accelerated, peaking on day 1 (Fig. 4B). In contrast, in GCV-treated P14xTK+ mice, the secondary DTH responses peaked on day 5, as for a primary response. It was also weaker, likely reflecting the reduction in CTL precursor compared with naive mice (Fig. 1C). Thus, elimination of dividing T cells in vivo results in immune amnesia.
Fig. 3.
In vivo evaluation of specific memory responses against male transplantation Ag after elimination of dividing T cells. B6 female TK+ mice or TK- littermates received a primary B6 male tail skin graft. Sixty days thereafter, mice were treated with GCV for 2 weeks (A) or 5 weeks (B). Three days after the end of GCV, a second male skin graft was implanted. Graft survival was monitored and cumulative survival Kaplan–Meier graphs are shown. P values were calculated with a log rank method comparing first- and second-graft survival in TK- (*, n = 10) and TK+ (**, n = 10) mice. GCV-treated TK+ mice also received a third-party fully allogeneic BALB/c skin graft (not shown) that was rejected with kinetics similar to naive mice; median survival time of BALB/c graft was 15 ± 1.5 days in B6 TK+ (n = 5) treated 5 weeks with GCV and 14 ± 0.5 days in naive B6 mice (n = 5); P = 0.29, log rank test.
Fig. 4.
In vivo evaluation of specific memory immune responses against LCMV after elimination of dividing T cells. P14xTK+ mice (n = 19, ○) or P14xTK- littermates (n = 24, □) were infected in the hind footpad with 104.2 plaque-forming units of LCMV-WE. DTH responses were monitored by measuring thickness differences between infected and noninfected footpads with a gauge caliper. Mean ± SE footpad swelling during primary infection (A) and during secondary infection (B) 3 days after a 2-week GCV treatment, initiated 60 days after the primary infection, are illustrated. In LCMV infection, we have previously shown that TK+ T cells are able to generate normal memory immune responses (14).
Effect of the Toxic Antimitotic HU on Immunological Memory Maintenance. The induction of immune amnesia by specific depletion of dividing T cells in our TK/GCV system led us to investigate whether antimitotic drugs could also affect immune memory maintenance. We used HU, a drug commonly used in humans for the treatment of proliferative syndromes, which is toxic for all dividing cells.
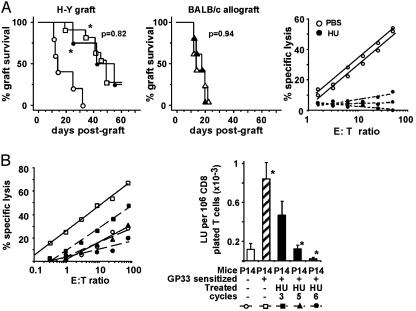
We first evaluated the effect of HU treatment on memory maintenance in H-Y-sensitized thymectomized B6 female mice. This treatment is designed to prevent naive T cell production in sensitized mice and thus to study the effect of HU on the established memory lymphoid compartment. In PBS-treated controls, the rejection of a secondary graft was accelerated compared with that of a primary graft (Fig. 5A Left). In contrast, after five cycles of HU injections, the secondary H-Y grafts were rejected with the same kinetics as primary grafts, demonstrating immune amnesia (Fig. 5A Left). In these mice, although the cumulative number of persisting CD44hi CD4+ and CD8+ T cells from all tissues tested represented 67% of immune thymectomized controls (data not shown), immune amnesia was observed and correlated with the disappearance of H-Y-specific secondary CTL activity (Fig. 5A Right). The rejection kinetic values of fully disparate BALB/c skin allografts in PBS- or HU-treated mice were similar, demonstrating that HU treatment did not induce a general immunodeficiency (Fig. 5A Center).
Fig. 5.
Effect of a toxic antimitotic HU treatment on immunological memory maintenance. (A) Effect of HU treatment on H-Y memory maintenance. Female thymectomized B6 mice were H-Y-sensitized with a primary B6 male skin graft (□). Sixty days later, mice were treated with five cycles of HU injections over 10 days or with PBS as control. Three days after the end of treatment, HU-treated (•, n = 5) and PBS-treated (○, n = 5) immune mice were grafted with a second male skin graft and the kinetics of graft rejection was monitored (Left). Cumulative survival Kaplan–Meier graphs are shown for one representative of two experiments and P was calculated with a log rank method comparing first- and second-graft survival in HU-treated mice. Immunocompetence of the treated mice was assessed by grafting, at the same time as the H-Y secondary graft, a fully allogeneic primary BALB/c skin allograft in HU-treated (▴)or PBS-treated (▵) H-Y-sensitized mice (Center). H-Y-specific CTL activity from splenocytes of H-Y-sensitized female thymectomized mice was tested 3 days after the end of HU or PBS treatment in individual mice (Right). (B) Effect of HU treatment on GP33–41 memory maintenance in P14 transgenic mice. P14 mice were sensitized with GP33–41 peptide and treated 60 days later with three, five, or six cycles of HU injections over 5, 10, or 12 days, respectively. Three days after the last injection, Ag-specific secondary CTL activity was determined in spleen cells: percent specific lysis, plotted against the lymphoid cell to target cell ratio is shown for one representative mouse per group (Left); mean ± SE of LU for 3 to 5 mice per group (Right). *, P values <0.05 comparing HU-treated and nontreated mice (Mann–Whitney test).
We also performed similar experiments in GP33–41-sensitized P14 mice. HU treatment resulted in the complete abolition of Ag-specific memory-type CTL activity after five to six cycles (10–12 days) of treatment (Fig. 5B). This effect was time–dose-dependent because three cycles (5 days) of treatment had only a partial effect. Abrogation of Ag-specific memory-type CTL responses was accompanied by a major loss (>75%) of CD44hi CD8+ TCR-Vα2 T cells in spleen, pooled inguinal, brachial, and axillary lymph nodes, and bone-marrow compartments (data not shown). However, the proliferative responses of ConA-activated splenocytes obtained from controls or 10-day HU-treated mice were comparable (12.3 ± 6.1 and 10.2 ± 2.6 × 103 cpm of incorporated [3H]thymidine, respectively). Altogether, these results demonstrate that HU treatment also induces immune amnesia in otherwise immunocompetent mice.
Discussion
Although it was initially thought that immunological memory is maintained by resting long-lived T cells, the pioneering work of Tough and Sprent (11, 12) led to the now-accepted paradigm that “memory T cells are dividing.” This conclusion relies on linked observations showing that (i) CD44hi T cells appear after a primary antigenic stimulation, (ii) these cells can transfer memory to naive mice, hence the term memory T cells, and (iii) some of these memory T cells incorporate BrdUrd. More recently, reduction of telomere length in memory-phenotype T cells (21, 22) or up-regulation of cell-cycle genes in Ag-experienced T cells (23) emphasize the notion that memory T cells are dividing (24). These experiments could not assess (i) whether T cell division is essential to memory maintenance and (ii) whether the so-called “memory” phenotype adequately characterizes the T cells supporting memory maintenance.
Our results demonstrate, based on in vivo functional assays, that T cell division is required for memory maintenance in vivo. Indeed, the sole ablation of dividing T cells suffices to abrogate immunological memory. The loss of immunological memory is progressive, increasing with the extended duration of dividing cell ablation. This time-dependence effect corroborates the requirement of T cell division for memory maintenance.
Immune amnesia was observed in fully immunocompetent mice still capable of driving normal primary immune responses. Indeed, in “amnesic” mice (i) secondary male H-Y allografts and third-party fully allogeneic skin grafts were rejected with kinetic values similar to naive mice (Figs. 3 and 5); (ii) LCMV clearance was effective on day 20 postinfection, as assessed by absence of LCMV mRNA amplification on urine samples by using RT-PCR (data not shown); (iii) polyclonal mitogen-induced proliferative responses were similar to those of naive mice; and (iv) DTH responses to virus occurred with primary-like response kinetics (Fig. 4).
The immune amnesia observed after GCV or HU treatments could have quantitative and/or qualitative causes, i.e., be due to (i) the fact that an actively dividing T cell subset carries the memory function of the entire Ag-specific pool or (ii) the significant reduction in the number of otherwise functional Ag-specific T cells. However, immune amnesia cannot be entirely explained by the elimination of CD44hi T cells that are commonly referred to as memory T cells and that are known to have a significant turnover based on telomere shortening (21, 22, 25) or BrdUrd incorporation (11–13, 26). Indeed, despite major losses of CD44hi T cells in tissues where the memory T cells localize after sensitization (27), overall, up to 31% (in B6 euthymic mice) and 67% (in B6 thymectomized mice; data not shown) of the CD44hi T cells persisted in mice with immune amnesia compared with control-sensitized mice. This finding indicates that such quiescent CD44hi T cells are not qualitatively or quantitatively sufficient to drive memory responses. The nature, homeostasis, and behavior of the persisting CD44hi T cells deserve further investigation.
These results, added to those of others, suggest that protective T cell memory is not due to long-lived memory T cells but rather to frequently dividing “activated effector T cells” (28, 29) or dividing effector memory T cells (25, 27, 30). Our results also indicate that ex vivo analysis of immunophenotype or IFN-γ production so far is not predictive of immunological memory. Hypothetically, bona fide memory T cells may exist, however, possibly encompassing a self-renewal capacity as described for B cells (31), but the phenotypic marker(s) allowing their characterization remains to be discovered. Cell-cycle-associated proteins (23) and telomerase expressions (22) now appear to be hallmarks of such cells.
Besides contributing to the long-term persistence of a constant T cell pool supporting immune memory, we speculate that a possible role of T cell division for immunological memory maintenance is to promote an activation state (32) that favors more rapid and thus more efficient responses on Ag reencounter (6, 33). In this line, we observed a high rate of BrdUrd incorporation in CD44hi T cells from extralymphoid tissues, such as liver and lung (unpublished results), where effector memory T cells ensure immediate secondary immune responses at local Ag entry ports (27, 34). Thus, whatever the lifespan of individual Ag-experienced T cells, division appears to be the essential feature that renders memory responses more efficient than primary responses, as demonstrated for B cells (35). This finding raises questions about the signals that drive this proliferation: notably, cytokines (36–38), regulatory T cells (39), interaction with MHC molecules (38, 40), and also the role of Ag persistence (28, 29). We are currently investigating whether the Ag nature and mode of presentation also influence T cell dynamics and, as a consequence, memory maintenance.
Finally, recognizing that cell division is an absolute requisite for maintaining immunological memory has several major clinical implications. In the field of vaccination, this should help in designing vaccines and vaccination schemes that, by better sustaining Ag-experienced T cell division, would lead to better and longer protection. In the field of cancer chemotherapy, the observation that HU treatment also induces immune amnesia suggests that cancer-associated immunodeficiency could be largely iatrogenic, because of antimitotic treatments, and thus could possibly be reduced by designing therapeutic schemes aimed at better preserving immunological memory.
Supplementary Material
Acknowledgments
We thank B. Clerc and M. Ripaux for technical assistance, the Nouvelle Animalerie Commune Pitié-Salpétrière and I. Raymond for animal care, members of our laboratory for helpful comments, and C. Frisén and R. Zinkernagel for critical reading of the manuscript. This work was supported in part by Université Pierre et Marie Curie, Centre National de la Recherche Scientifique, and Agence Nationale de Recherches sur le SIDA.
Abbreviations: Ag, antigen; TK, herpes simplex type 1 thymidine kinase; GCV, ganciclovir; LCMV, lymphocytic choriomeningitis virus; HU, hydroxyurea; CTL, cytolytic T lymphocyte; DTH, delayed-type hypersensitivity; LU, lytic unit.
References
- 1.Dutton, R. W., Bradley, L. M. & Swain, S. L. (1998) Annu. Rev. Immunol. 16, 201-223. [DOI] [PubMed] [Google Scholar]
- 2.Oehen, S. & Brduscha-Riem, K. (1998) J. Immunol. 161, 5338-5346. [PubMed] [Google Scholar]
- 3.Zimmermann, C., Brduscha-Riem, K., Blaser, C., Zinkernagel, R. & Pircher, H. (1996) J. Exp. Med. 183, 1367-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708-712. [DOI] [PubMed] [Google Scholar]
- 5.Geginat, J., Lanzavecchia, A. & Sallusto, F. (2003) Blood 6, 4260-4266. [DOI] [PubMed] [Google Scholar]
- 6.Wherry, E. J., Teichgraber, V., Becker, T. C., Masopust, D., Kaech, S. M., Antia, R., Von Andrian, U. H. & Ahmed, R. (2003) Nat. Immunol. 4, 225-234. [DOI] [PubMed] [Google Scholar]
- 7.Rogers, P. R., Dubey, C. & Swain, S. L. (2000) J. Immunol. 164, 2338-2346. [DOI] [PubMed] [Google Scholar]
- 8.Goldrath, A. W., Bogatzki, L. Y. & Bevan, M. J. (2000) J. Exp. Med. 192, 557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, B. K., Rao, V. P., Ge, Q., Eisen, H. N. & Chen, J. (2000) J. Exp. Med. 192, 549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murali-Krishna, K. & Ahmed, R. (2000) J. Immunol. 165, 1733-1737. [DOI] [PubMed] [Google Scholar]
- 11.Tough, D. F. & Sprent, J. (1994) J. Exp. Med. 179, 1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprent, J. & Tough, D. F. (1994) Science 265, 1395-1400. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, X., Fujii, H., Kishimoto, H., LeRoy, E., Surh, C. D. & Sprent, J. (2002) J. Exp. Med. 195, 283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, J. L., Saron, M. F., Boyer, O., Thomas-Vaslin, V., Bellier, B., Lejeune, L., Charlotte, F. & Klatzmann, D. (2000) Hum. Gene Ther. 11, 2473-2481. [DOI] [PubMed] [Google Scholar]
- 15.Braunberger, E., Cohen, J. L., Boyer, O., Pegaz-Fiornet, B., Raynal-Raschilas, N., Bruneval, P., Thomas-Vaslin, V., Bellier, B., Carpentier, A., Glotz, D. & Klatzmann, D. (2000) Mol. Ther. 2, 596-601. [DOI] [PubMed] [Google Scholar]
- 16.Thomas-Vaslin, V., Bellier, B., Cohen, J. L., Boyer, O., Raynal-Raschilas, N., Glotz, D. & Klatzmann, D. (2000) Transplantation 69, 2154-2161. [DOI] [PubMed] [Google Scholar]
- 17.Salomon, B., Maury, S., Loubière, L., Caruso, M., Onclercq, R. & Klatzmann, D. (1995) Mol. Cell. Biol. 15, 5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen, J. L., Boyer, O., Salomon, B., Onclerco, R., Depetris, D., Lejeune, L., Dubus-Bonnet, V., Bruel, S., Charlotte, F., Mattei, M. G. & Klatzmann, D. (1998) Transgenic Res. 7, 321-330. [DOI] [PubMed] [Google Scholar]
- 19.Park, J. Y., Peters, C. J., Rollin, P. E., Ksiazek, T. G., Gray, B., Waites, K. B. & Stephensen, C. B. (1997) J. Med. Virol. 51, 107-114. [PubMed] [Google Scholar]
- 20.Cerottini, J. C., Engers, H. D., Macdonald, H. R. & Brunner, T. (1974) J. Exp. Med. 140, 703-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns, J. B., Lobo, S. T. & Bartholomew, B. D. (2000) Eur. J. Immunol. 30, 1894-1901. [DOI] [PubMed] [Google Scholar]
- 22.Hathcock, K. S., Kaech, S. M., Ahmed, R. & Hodes, R. J. (2003) J. Immunol. 170, 147-152. [DOI] [PubMed] [Google Scholar]
- 23.Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. (2002) Cell 111, 837-851. [DOI] [PubMed] [Google Scholar]
- 24.Berard, M. & Tough, D. F. (2002) Immunology 106, 127-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto, F., Langenkamp, A., Geginat, J. & Lanzavecchia, A. (2000) Curr. Top. Microbiol. Immunol. 251, 167-171. [DOI] [PubMed] [Google Scholar]
- 26.Flynn, K. J., Riberdy, J. M., Christensen, J. P., Altman, J. D. & Doherty, P. C. (1999) Proc. Natl. Acad. Sci. USA 96, 8597-8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. (2001) Science 291, 2413-2417. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann, M., Kündig, T., Hentgartner, H. & Zinkernagel, R. M. (1997) Proc. Natl. Acad. Sci. USA 94, 640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinkernagel, R. M. (2002) Curr. Opin. Immunol. 14, 523-536. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadzadeh, M., Hussain, S. F. & Farber, D. L. (2001) J. Immunol. 166, 926-935. [DOI] [PubMed] [Google Scholar]
- 31.Fearon, D. T., Manders, P. & Wagner, S. D. (2001) Science 293, 248-250. [DOI] [PubMed] [Google Scholar]
- 32.Wells, A. D., Walsh, M. C., Sankaran, D. & Turka, L. A. (2000) J. Immunol. 165, 2432-2443. [DOI] [PubMed] [Google Scholar]
- 33.Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. (2000) Nat. Immunol. 1, 47-53. [DOI] [PubMed] [Google Scholar]
- 34.Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. (2001) Nature 410, 101-105. [DOI] [PubMed] [Google Scholar]
- 35.Tangye, S. G., Avery, D. T., Deenick, E. K. & Hodgkin, P. D. (2003) J. Immunol. 170, 686-694. [DOI] [PubMed] [Google Scholar]
- 36.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288, 675-678. [DOI] [PubMed] [Google Scholar]
- 37.Sprent, J. & Surh, C. D. (2002) Annu. Rev. Immunol. 20, 551-579. [DOI] [PubMed] [Google Scholar]
- 38.Kaech, S. M., Wherry, E. J. & Ahmed, R. (2002) Nat. Rev. 2, 251-262. [DOI] [PubMed] [Google Scholar]
- 39.Murakami, M., Sakamoto, A., Bender, J., Kappler, J. & Marrack, P. (2002) Proc. Natl. Acad. Sci. USA 99, 8832-8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kassiotis, G., Garcia, S., Simpson, E. & Stockinger, B. (2002) Nat. Immunol. 3, 244-250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.