Abstract
Endoscopic submucosal dissection (ESD) has allowed the achievement of histologically curative en bloc resection of gastrointestinal neoplasms regardless of size, permitting the resection of previously non-resectable tumors. The ESD technique for treatment of early gastric cancer has spread rapidly in Japan and a few other Asian countries due to its excellent eradication rate compared to endoscopic mucosal resection. Although numerous electrosurgical knives have been developed for ESD, technical difficulties and high complication rates (bleeding and perforation) have limited their use worldwide. We developed the grasping type scissor forceps (GSF) to resolve such ESD-related problems. Our animal and preliminary clinical studies showed that ESD using GSF is a safe (no intraoperative complication) and technically efficient (curative en bloc resection rate 92%) method for dissection of early gastrointestinal tumors. The use of GSF is a promising option for performing ESD on early stage GI tract tumors both safely and effectively.
Keywords: Endoscopic submucosal dissection, Novel device, Grasping type scissor forceps, Early gastrointestinal tract neoplasms, Endoscopic therapy
INTRODUCTION
The Endoscopic submucosal dissection (ESD) technique introduced by Hirao et al[1] has subsequently been modified by several investigators, allowing curative en bloc resection of broad superficial tumors with the use of special cutting knives. It has been reported that ESD improves the rate of successful en bloc resection in early stage gastrointestinal neoplasm as compared with endoscopic mucosal resection (EMR)[2-7]. However, ESD using knives is technically difficult and carries a high risk of perforation and bleeding. Complication rates with knives for the dissection of gastric tumor are reported to be 0% to 70%[4,8-12]. Incision using knife devices merely provides contact between the knife and the tissue and cuts using an electrosurgical current. These cutting processes do not fix the device to the targeted tissue, making it difficult to accurately place the knife during electrosurgical incision because of bodily motions such as cardiac or respiratory movement[13]. Lack of fixation and compression effects can cause unexpected incision and insufficient coagulation and result in major complications such as perforation and bleeding. We believe that the most effective and simplest way of avoiding such complications is to grasp and incise the targeted tissue using an electrosurgical current. We have therefore developed a new grasping type scissor forceps (GSF) which can grasp and incise the targeted tissue using an electrosurgical current[13-18]. In this article we describe the concepts of development, clinical outcomes in ESD and future potential of this new device.
CLINICAL OUTCOMES OF ESD USING CONVENTIONAL KNIFE DEVICES
Recent results of en bloc resection rate of ESD for neoplasms in the esophagus, stomach and colorectum are 95%-100%, 76%-96% and 77%-98.6% respectively[4]. On the other hand, reported bleeding and perforation rate of ESD for neoplasms in the esophagus, stomach and colorectum are 0% and 6.9%, 0%-40% and 0%-50%, and 0%-12% and 1.4%-10% respectively[4,8,12,19-21]. Current ESD using knife devices shows high tumor eradication rates but substantial risks during the procedure.
THEORETICAL PROBLEMS OF ESD USING CONVENTIONAL KNIFE DEVICES
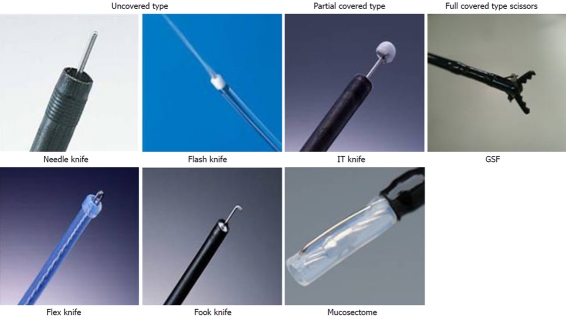
At present, numerous electrosurgical knives such as the diathermic needle knife[3,22], insulation-tipped electrosurgical knife (IT)[2], hook knife[23], flex knife[10], triangle-tipped knife[24], flush knife[25], fork knife[19], and mucosectome[20] are available for ESD (Figure 1). During ESD using conventional knife devices, problems may be encountered i.e. unintentional incision due to bodily motions, perforation due to thin gut wall and bleeding. Our proposed measures for each problem are shown in Table 1. We consider that the shortcomings of current knives are deficiency of fixing and lift up, compression to the targeted tissue and outside insulation. Therefore we developed a grasping type scissor forceps (Figures 1 and 2) to overcome the shortcomings of conventional knife devices[13-18].
Figure 1.
Several endoscopic cutting devices have been developed in Japan. They are divided into 3 broad categories as above: Uncovered type knife, partial-covered type knife, and full-covered type scissors.
Table 1.
Problems during ESD using conventional knives and proposed measures
| Problems | Proposed measures |
| Unintentional incision | Device fixation to the targeted tissue |
| Outside insulation | |
| Perforation | Sufficient submucosal injection |
| Outside insulation | |
| Lift up the targeted tissue by device | |
| Good visualization of the targeted area | |
| Bleeding | Compression of the blood vessel by device |
ESD: Endoscopic submucosal dissection.
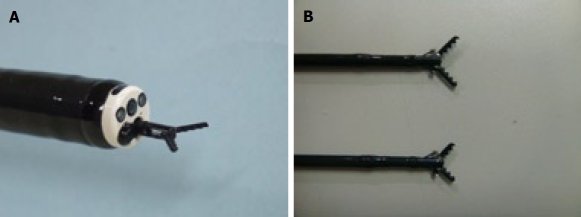
Figure 2.
Grasping type scissor forceps (GSF). A: Distal tip of the long type GSF. The outer side of the forceps is insulated so that electrosurgical current energy is concentrated at the blade to avoid burning to the surrounding tissue (closed discharge). B: Long type (upper side image: 6mm) and short type (lower side image: 4 mm) GSF.
NEWLY DEVELOPED GRASPING TYPE SCISSOR FORCEPS
The grasping type scissor forceps (GSF) (XDP2618DT, Fujifilm Corporation, Saitama, Japan) (Figure 2)[13-18] can grasp and cut a piece of tissue with an electrosurgical current. It has a 0.4 mm wide and 4 mm or 6 mm long serrated cutting edge to facilitate grasping the tissue. The outer side of the forceps is insulated so that electrosurgical current energy is concentrated at the blade to avoid burning the surrounding tissue. Furthermore, the forceps can be rotated to the desired orientation. The diameter of the forceps is 2.7 mm. The GSF is available for a standard endoscope with working channel width of 2.8 mm or over. This device is disposable and not reusable and is used for circumferential marginal incision, submucosal dissection and hemostatic treatment. The autocut mode (ICC 200; Erbe, Tübingen, Germany) 120W (effect 3) is used for cutting (circumferential incision and submucosal excision) and the soft coagulation mode 70W (effect 3) is used for hemostatic coagulation.
In our previous animal study (porcine stomach)[13], we resected three specimens safely and easily with no unintentional incision by ESD using the GSF. The histological analysis of the ulcers due to the procedure showed shallow submucosal ulcers without perforation and excessive burning. All clinical studies[14-18] described in this article were reviewed and approved by the ethics committee of Aso Iizuka Hospital.
TECHNIQUES OF ESD USING GSF
ESD technique using GSF is as follows (Figure 3). Circumferential markings are made by using a hook knife (KD-620LR, Olympus) with coagulation current 20-40W created by an electrosurgical generator (ICC 200; Erbe, Tübingen, Germany) (Figure 4A). Next, 10% glycerin with 0.9% NaCl and 5% fructose (Glyceol; Chugai Pharmaceutical Co., Tokyo, Japan) or hyaluronic acid solution (MucoUp: Johnson and Johnson Co., Tokyo, Japan) is injected into the submucosal layer to lift up the lesion. The lesion is separated from the surrounding normal mucosa following complete incision around the lesion using the GSF (Figure 4B and C), A piece of submucosal tissue is grasped, lifted up and cut with the GSF using electrosurgical current to achieve submucosal excision (Figure 4D). Finally, the lesion is completely resected (en bloc resection) by GSF (Figure 4E and F). Treatment of bleeding during the procedure is done by coagulation with the GSF (Soft coagulation mode 70W, effect 3). Prophylactic coagulation of visible vessels is carried out using GSF (Soft coagulation mode 70W, effect 3).
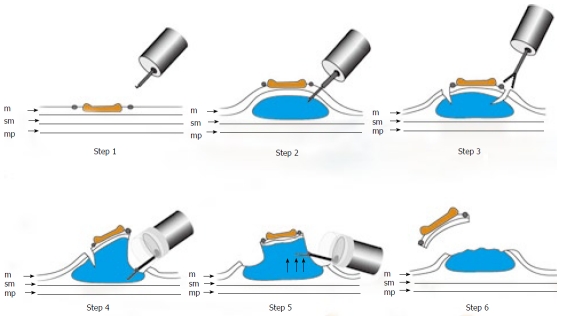
Figure 3.
Schematic shows ESD using GSF. Step 1: Marking dots are made on the circumference of the lesion to outline the incision line; Step 2: A concentrated glycerin solution mixed with a small volume of epinephrine and indigo carmine dye is injected into the submucosal layer around the target lesion to lift the entire lesion; Step 3: The lesion is separated from the surrounding normal mucosa by complete incision around the lesion using the GSF; Step 4: A piece of submucosal tissue is grasped by GSF; Step5: A grasped tissue is lifted up and cut with the GSF using electrosurgical current to effect submucosal exfoliation; Step 6: The lesion is resected in one piece; m: Mucosa; sm: Submucosa; mp: Muscularis propria.
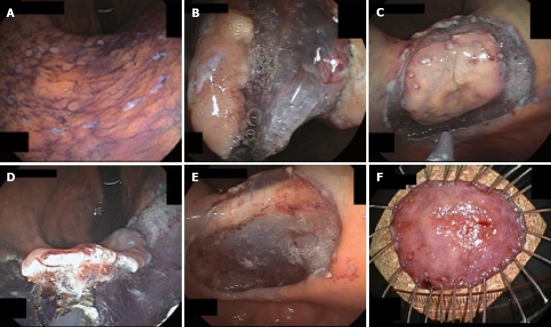
Figure 4.
Endoscopic view of the procedure of ESD using GSF. A: Marks are made at several points along the outline of the lesion with a coagulation current; B: The mucosa is incised outside the marker dots to separate the lesion from the surrounding non-neoplastic mucosa using GSF; C: Completion of the GSF cutting around the lesion with a safe lateral margin; D: The submucosal connective tissue beneath the lesion is grasped and lifted up and excised using GSF from the underlying muscle layer; E: The lesion is cut completely from the muscle layer; F: The resected specimen showing en bloc resection of the lesion.
CLINICAL OUTCOMES OF ESD USING GSF IN OUR PRELIMINARY STUDIES
Clinicopathological characteristics of the lesions treated by ESD using GSF[17,18] are summarized in Table 2. All lesions were treated simply and safely with no unexpected incision by a beginner endoscopist in ESD. The grasping and lift up steps before cutting the targeted tissue provided good visualization of the interest and allowed the use of sufficient pre-cut coagulation. The technical outcome[17,18] is summarized in Table 3. The mean size of the epithelial tumors and resected specimens was 16.6 ± 9.0 mm and 33.0 ± 10.5 mm respectively. The curative en bloc resection rates according to tumor location were 100% (3/3) in the esophagus, 97% (34/35) in the stomach[17], 80% (8/10) in the colon and rectum[18] and 92% (45/49) overall. All lesions were resected in en bloc fashion endoscopically. Four cases (8%) were judged as histologically positive margin of the tumor cells due to burning effect. The mean operating time according to tumor location was 74.7 ± 26.3 min in the esophagus, 104.1 ± 54.4 min in the stomach[17], 154.9 ± 83.6 min in the colon and rectum[18] and 114.0 ± 63.4 min overall.
Table 2.
Clinicopathological characteristics (n = 49)
| Gender, male/female | 32/17 | ||
| Mean age years (range) | 69 (31-87) | ||
| Location, n | Esophagus | 3 | |
| Middle | 1 | ||
| Lower | 2 | ||
| Stomach[17] | 35 | ||
| Upper | 9 | ||
| Middle | 8 | ||
| Lower | 18 | ||
| Duodenum | Bulbus | 1 | |
| Colorectum[18] | 10 | ||
| Cecum | 2 | ||
| Ascending | 1 | ||
| Transverse | 2 | ||
| Descending | 2 | ||
| Rectum | 3 | ||
| Histologic type and depth of invasion, n | |||
| Carcinoma | 27 | ||
| Mucosa | 24 | ||
| Superficial submucosa | 2 | ||
| Deep submucosa | 1 | ||
| Adenoma | Mucosa | 20 | |
| Carcinoid tumor | Deep submucosa | 1 | |
| Granular cell tumor | Deep submucosa | 1 |
Table 3.
Technical results of ESD using GSF (n = 49)
| Curative en-bloc resection rate, Total | 92% (45/49) |
| Esophagus | 100% (3/3) |
| Stomach[17] | 97% (34/35) |
| Duodenum | (0/1) |
| Colon and rectum[18] | 80% (8/10) |
| Mean ± SD operating time, minutes (Range), Total | 114.0 ± 63.4 (33-337) |
| Esophagus | 74.7 ± 26.3 (59-105) |
| Stomach[17] | 104.1 ± 54.4 (33-264) |
| Duodenum | 172 |
| Colon and rectum[18] | 154.9 ± 83.6 (70-337) |
| Mean ± SD size of tumor size, mm (Range) | 16.6 ± 9.0 (5-43) |
| Mean ± SD size of resected specimen, mm (Range) | 33.0 ± 10.5 (15-60) |
| Intra-operative perforation rate | 0/49 (0%) |
| Intra-operative major bleeding rate | 0/49 (0%) |
| Postoperative bleeding rate | 1/49 (2%) |
| Postoperative perforation rate | 0/49 (0%) |
GSF: Grasping type scissor forceps.
No intra-operative severe complications (perforation or uncontrollable bleeding) occurred and post-operative bleeding was seen in 2% (1 of 49) of cases.
THEORETICAL ADVANTAGES OF GSF AS COMPARED TO CONVENTIONAL KNIFE DEVICES
Safety
GSF can grasp the targeted tissue. The grasping step has three safe effects: (1) fixation, (2) lift-up effect and (3) compression effect (Table 4, Figure 5). (1) The grasping step prior to electrosurgical incision provides fixation of the device to the targeted tissue to avoid unintentional incision[13-18]. GSF has a thin serrated cutting edge and an insulated coating of the outer side of the forceps. These characteristics facilitate grasping the targeted tissue and concentrate electrosurgical current energy at the blade to avoid burning the surrounding tissue (closed discharge). (2) It also has a lift up effect on the targeted tissue which can sufficiently separate grasped tissue from the underlying proper muscle layer before incision and contributes to preventing perforation. The grasping and lift up steps before cutting the targeted tissue provides good visualization of the interest and allows the use of sufficient pre-cut coagulation[13-18]. (3) GSF has a compression effect which is effective for pre-cut coagulation and hemostatic treatment of post-cut hemorrhage. GSF can be used to grasp the targeted tissue again if the grasped site is inadequate before electrosurgical cutting[13-18]. We think that these merits reduce the chance of unintentional incisions, bleeding and other severe complications.
Table 4.
Theoretical advantages of GSF during ESD
| Advantageous mechanism | Expected physical effects | Technical outcome |
| Grasp | Fix | (1) No unintentional incision |
| Lift-up | (2) Good visualization | |
| (3) Sufficient separation from the underlying proper muscle layer to prevent perforation | ||
| Compress | (4) Hemostatic effect to prevent and treat bleeding | |
| Outside insulation | Closed discharge | (5) Minimizing damage to the surrounding tissue to prevent perforation |
| Rotatable | Change the direction of grasping | (6) Facilitating accurate targeting |
Figure 5.
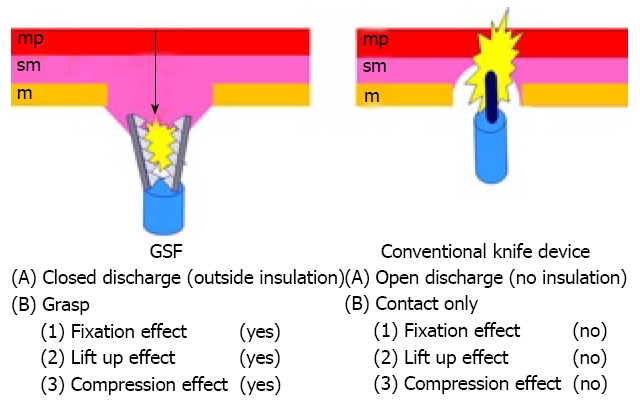
Mechanical differences between GSF and conventional knife devices. sm: Submucosa; mp: Muscularis propria.
Simplicity
Each step of ESD (circumferential incision, submucosal excision, hemostatic treatment) can be achieved by the following three operations: (1) grasping the targeted tissue (fixation), (2) lifting up the grasped tissue (separation of the grasped tissue from the underlying proper muscle layer) and (3) cutting the grasped tissue (or coagulating the blood vessel) using an electrosurgical current. These operations are simple and as easy as a bite biopsy technique.
Facility for training of ESD
ESD using GSF is safe and simple. In our preliminary studies[17,18] all cases were safely and effectively resected using this method by a beginner endoscopist in ESD. Furthermore, in the grasping confirmation step we could sufficiently discuss the adequacy of the grasped tissue with the attending endoscopist prior to incision using electrosurgical current. This precut confirmation step is the greatest advantage of our method and is useful for safety and for training in ESD.
CURRENT DISADVANTAGES OF ESD USING GSF
The procedure time of ESD using GSF was longer than the reported times using knife devices[4,26-30]. The main reasons were (1) these clinical trials were performed by a beginner in ESD (The endoscopist was the inventor of GSF with prior experience of only seven cases of conventional ESD) and (2) the frequent difficulties in rotating the GSF to the desired orientation during a retroflex approach. A significant learning curve is associated with achieving proficiency in ESD[31]. Probst et al[32] showed a learning curve resulting in a decreasing procedural duration and an increasing rate of complete en bloc resection over time. Procedure time will be shortened in the near future by the learning curve in ESD using GSF and a solution to the problem of rotating the GSF by further mechanical refinement.
FUTURE POTENTIAL
From recent studies, ESD shows a higher tumor eradication rate than conventional EMR irrespective of tumor size[4,33-37]. However, ESD using conventional knife devices requires highly skilled endoscopists and a sufficient training program is required for permeation of this technique[31,32]. At present because of this requirement, the merits of ESD can be obtained in only a few advanced institutions in Japan and several other Asian countries[4,12,21,26]. Because of its safety and simplicity, we believe that ESD using GSF will become widely accepted for use throughout the world[17,18]. We hope to expand the application of this procedure to decrease the complications associated with ESD. However, further refinements and experiences of this method are needed in order to more easily achieve the curative en bloc resection of early GI tract tumor. Furthermore, extensive controlled, randomized studies in controlling ESD-related complications e.g. vs IT knife, vs needle knife or vs other conventional devices are necessary to fully evaluate the usefulness of our new device.
Footnotes
Peer reviewer: Shotaro Enomoto, MD, PhD, Second Department of Internal Medicine, Wakayama Medical University, 811-1, Kimiidera, Wakayama 641-0012, Japan
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507–512. doi: 10.1067/mge.2002.128108. [DOI] [PubMed] [Google Scholar]
- 4.Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962–2967. doi: 10.3748/wjg.14.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980–986. doi: 10.1055/s-2006-944809. [DOI] [PubMed] [Google Scholar]
- 6.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 7.Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 8.Neuhaus H, Costamagna G, Devière J, Fockens P, Ponchon T, Rösch T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”) Endoscopy. 2006;38:1016–1023. doi: 10.1055/s-2006-944830. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 10.Yahagi N, Fujishiro M, Kakushima K, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type) Dig Endosc. 2004;16:34–38. [Google Scholar]
- 11.Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Ikeda F, Taniguchi H, Yumoto E, Suzuki S. Treatment of over 20 mm gastric cancer by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2007;13:3981–3944. doi: 10.3748/wjg.v13.i29.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus H. Endoscopic submucosal dissection in the upper gastrointestinal tract: present and future view of Europe. Dig Endosc. 2009;21 Suppl 1:S4–S6. doi: 10.1111/j.1443-1661.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 13.Akahoshi K, Akahane H, Murata A, Akiba H, Oya M. Endoscopic submucosal dissection using a novel grasping type scissors forceps. Endoscopy. 2007;39:1103–1105. doi: 10.1055/s-2007-966842. [DOI] [PubMed] [Google Scholar]
- 14.Akahoshi K, Honda K, Akahane H, Akiba H, Matsui N, Motomura Y, Kubokawa M, Endo S, Higuchi N, Oya M. Endoscopic submucosal dissection by using a grasping-type scissors forceps: a preliminary clinical study (with video) Gastrointest Endosc. 2008;67:1128–1133. doi: 10.1016/j.gie.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Akahoshi K, Motomura Y, Kubokawa M, Matsui N, Oda M, Okamoto R, Endo S, Higuchi N, Kashiwabara Y, Oya M, et al. Endoscopic submucosal dissection of a rectal carcinoid tumor using grasping type scissors forceps. World J Gastroenterol. 2009;15:2162–2165. doi: 10.3748/wjg.15.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akahoshi K, Honda K, Kubokawa M, Motomura Y, Matsui N, Endo S, Higuchi N, Taki K, Oya M, Akahane H, et al. Endoscopic resection of a large pedunculated duodenal polyp using a grasping type scissors forceps. Endoscopy. 2008;40 Suppl 2:E74–E75. doi: 10.1055/s-2007-995524. [DOI] [PubMed] [Google Scholar]
- 17.Akahoshi K, Honda K, Motomura Y, Kubokawa M, Okamoto R, Osoegawa T Nakama N, Kashiwabara Y, Higuchi N, Tanaka Y, Oya M, et al. Endoscopic Submucosal Dissection Using a Grasping Type Scissors Forceps for Early Gastric Cancers and Adenomas. Dig Endosc. 2010:in press. doi: 10.1111/j.1443-1661.2010.01037.x. [DOI] [PubMed] [Google Scholar]
- 18.Akahoshi K, Okamoto R, Akahane H, Motomura Y, Kubokawa M, Osoegawa T, Nakama N, Chaen T, Oya M, Nakamura K. Endoscopic Submucosal Dissection of Early Colorectal Tumors Using a Grasping Type Scissors Forceps: A Preliminary Clinical Study - a case series. Endoscopy. 2010:in press. doi: 10.1055/s-0029-1243973. [DOI] [PubMed] [Google Scholar]
- 19.Kim HG, Cho JY, Bok GH, Cho WY, Kim WJ, Hong SJ, Ko BM, Kim JO, Lee JS, Lee MS, et al. A novel device for endoscopic submucosal dissection, the Fork knife. World J Gastroenterol. 2008;14:6726–6732. doi: 10.3748/wjg.14.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara Y, Takenaka R, Okada H. Risk management to prevent perforation during endoscopic submucosal dissection. Dig Endosc. 2007;19:S9–S13. [Google Scholar]
- 21.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690–694. doi: 10.1055/s-2003-41516. [DOI] [PubMed] [Google Scholar]
- 23.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Kudo S. A novel procedure of en bloc EMR using triangle-tipped knife (abstract) Gastrointest Endosc. 2003;57:AB86. [Google Scholar]
- 25.Toyonaga T, Nishino E, Hirooka T, Dozaiku T, Sujiyama T, Iwata Y, Ono W, Ueda C, Tomita H, Hirooka T, et al. Use of short needle knife for esophageal endoscopic submucosal dissection. Dig Endosc. 2005;17:246–252. [Google Scholar]
- 26.Chang CC, Lee IL, Chen PJ, Wang HP, Hou MC, Lee CT, Chen YY, Cho YP, Lin JT. Endoscopic submucosal dissection for gastric epithelial tumors: a multicenter study in Taiwan. J Formos Med Assoc. 2009;108:38–44. doi: 10.1016/S0929-6646(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 27.Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J Gastroenterol. 2008;14:4289–4295. doi: 10.3748/wjg.14.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678–683; quiz 645. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–107. doi: 10.1016/j.gie.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866–867. doi: 10.1016/j.gie.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 32.Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic submucosal dissection of early gastric cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin. Gastroenterol. Hepatol. 2009;7:149–155. doi: 10.1016/j.cgh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418–422. doi: 10.1055/s-2007-966427. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video) Gastrointest Endosc. 2007;66:966–973. doi: 10.1016/j.gie.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 35.Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, Mori M, Itoh H. Endoscopic submucosal dissection for rectal tumors. Endoscopy. 2007;39:423–427. doi: 10.1055/s-2007-966237. [DOI] [PubMed] [Google Scholar]
- 36.Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc. 2009;23:1546–1551. doi: 10.1007/s00464-009-0395-5. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]