Abstract
The purpose of this study was to measure the compliance of the carpal tunnel in candidate animal models of carpal tunnel syndrome, by measuring the resistance when passing a tapered metal rod through the carpal tunnel. Forepaws from ten dogs, ten rabbits and ten rats with intact carpal tunnels and ten fresh frozen human wrist cadavers were used. The slopes of the linear part of the force-displacement curve (a measure of stiffness), normal force and increasing area ratio (InAR) were significantly different among the four species (p<0.05). Post hoc analysis indicated that the mean slopes for the human carpal tunnel were the largest indicating the least compliance, while those of the rat were the least (p<0.05). The features of the compliance for the dog carpal tunnel were closest to the human. The development of animal models of CTS should consider the compliance of the carpal tunnel, as it will be more difficult to increase pressure in a more compliant tunnel.
Keywords: Carpal Tunnel Syndrome, Disease Models, Animals, Human, Biomechanics
INTRODUCTION
Carpal tunnel syndrome (CTS) is a common hand disease in which the median nerve is compressed at the wrist.1 Most cases are idiopathic.2 Although repetitive hand use is suspected in some cases,3-5 the correlation with activity in most cases is still unclear.6 The three pathological hallmarks of CTS are elevated carpal tunnel pressure7-12, fibrosis of the subsynovial connective tissue (SSCT),13-16 and abnormal nerve function.17 More recent studies have suggested that the neuropathy is also associated with changes in the gliding characteristics18,19 and permeability20 of the SSCT.
Clinical studies of CTS are limited by the difficulty of evaluating the early stages of its pathology. Thus, a validated animal model of CTS is an important need. A good animal model would have comparable anatomy: a carpal tunnel containing the median nerve, tendons, and SSCT, as the human carpal tunnel does. In addition, because CTS is characterized by increased carpal tunnel pressure, the animal carpal tunnel model should have mechanical characteristics similar to the human carpal tunnel. A model with a very complaint carpal tunnel might not be useful if the goal is to investigate changes in biology within the tunnel that might induce pressure elevation, such as gradual thickening of the SSCT.
Previous studies have reported animal models of CTS in the rabbit,21-24 rat,25,26 and dog.27 The rabbit model is commonly used to study CTS28,29. Lim et al.22 determined the dose-responsiveness for an acute pressure-induced median neuropathy model of human acute CTS in the rabbit, using saline infusion, showing that significant electrophysiological changes were caused by pressures greater than 30 mm Hg. Diao et al.21 also investigated the relationship between pressure and median nerve function in the carpal tunnel in the rabbit model. Angioplasty catheters were placed in the carpal tunnel to create pressures ranging from 50 to 80 mm Hg, with similar findings. These experimental models have some limitations, though. First, it is not clear how much increase in volume of the carpal tunnel contents is needed to increase carpal tunnel pressure over 30 mmHg in the rabbit model. Second, human CTS is rarely the result of an acute change in pressure caused by an expansion of canal contents over minutes or hours, as might occur for example after a traumatic hematoma. In most cases, human carpal tunnel syndrome develops gradually over months or years. This suggests that a relative rigidity of the carpal tunnel is a necessary precondition; otherwise the tunnel would simply expand, and the pressure would not rise. Thus, it is important to know if the compliance of animal carpal tunnels is comparable to that of the human carpal tunnel. The purpose of this study was to measure the compliance of the carpal tunnel in human and potential animal models by measuring the resistance when passing a tapered metal rod through the carpal tunnel. The null hypothesis was that the compliance of the carpal tunnels of the various species was not different.
METHODS
Forepaws from ten dogs (weight 22-28kg), ten rabbits (weight 3.6-5.2kg) and ten rats (approximately 0.5kg in weight) with intact carpal tunnels and ten fresh frozen human wrist cadavers were used for this study. The animal cadavers were obtained immediately after sacrifice for other Institutional Animal Care and Uses Committee (IACUC) approved studies, in which the paws were not involved. This study was approved by our Institutional Review Board.
Identification and Dissection of Carpal Tunnel
A skin incision was made longitudinally over the carpal tunnel region. The entire transverse carpal ligament was exposed and carefully dissected without damaging its attachment and edges. Human transverse carpal ligament is a dense band of fibers with clear distal and proximal edges, which runs between hamate and pisiform medially to scaphoid and trapezium laterally. The canine transverse carpal ligament is also a dense band of fibers, located beneath the flexor digitorum superficialis (FDS) tendon. Therefore, unlike the human carpal tunnel, the canine carpal tunnel does not include the FDS tendons. Instead, the canine carpal tunnel contains a single large common FDP tendon, along with the median nerve and its associated vessels. The rabbit transverse carpal ligament was identified by locating a palpable nodule of subcutaneous cartilage which forms part of the transverse carpal ligament. In the rat model, the transverse ligament was identified as running from the pisiform medially to the falciformis and navicular laterally. Therefore, the carpal tunnel contents in the human, rabbit, and rat models included the FDP and FDS tendons, the median nerve and its associated vessels, and the SSCT. After identification of the entire carpal tunnel, the skin, subcutaneous tissue, and all the tissues in the carpal tunnel were removed from the specimens, leaving the hollow carpal tunnel intact (Figure 1). The length and thickness of the transverse carpal ligaments were measured with an electronic digital caliper.
Figure 1.
Carpal tunnel ligaments in the (A) human, (B) dog, (C) rabbit and (D) rat with the each individual tapered testing rod.
Mechanical Evaluation of Carpal Tunnel Compliance
The prepared specimens were mounted on a custom-made testing device. The measurement system consisted of one mechanical actuator with a linear potentiometer, a 25 lb load cell (Transducer Techniques, Inc., Temecular, California, USA) and a tapered metal rod. The experiment set up is shown in Figure 2. Due to differences in size of the carpal tunnel, different sized rods were used for each species. An 80 mm long metal rod tapering from 10 to 26 mm diameter used for the human specimens, a 53.6 mm long metal rod tapering from 7.6 to 18.6 mm diameter used for the canine specimens, a 40 mm long metal rod tapering from 4.5 to 12.5 mm diameter used for the rabbit specimens, and a 22.6 mm long metal rod tapering from 1.2 to 3.2 mm diameter used for the rat specimens. Although the different sized rods were used for different species, the tangent of the angle of slope of the taper was the same (0.1) (Figure 3). The tip of the metal rod was connected to the actuator through Green Spot Dacron trolling line (Cortland Line Co., Inc., Cortland, New York, USA) which passed through the carpal tunnel. The actuator pulled the metal rod through the carpal tunnel from proximal to distal. The start position of the metal rod was set to contact as much as possible of the carpal tunnel entrance without any measurable load. The motor rate of the actuator was set at 1mm/sec and the sampling rate was set at 20 Hz. The displacement varied according to species to 35mm, 20mm, 15mm, and 12mm for humans, dogs, rabbits and rats, respectively. Throughout testing, the specimens were kept moist by spraying with phosphate-buffered saline. Each specimen was tested five times, and the means of the five tests were used for data analysis. The normal force on the wall of the carpal tunnel was deduced from the original force recorded from the load cell adjusted by the tangent angle of the tapered rod. The relationship between forces is shown in Figure 4.
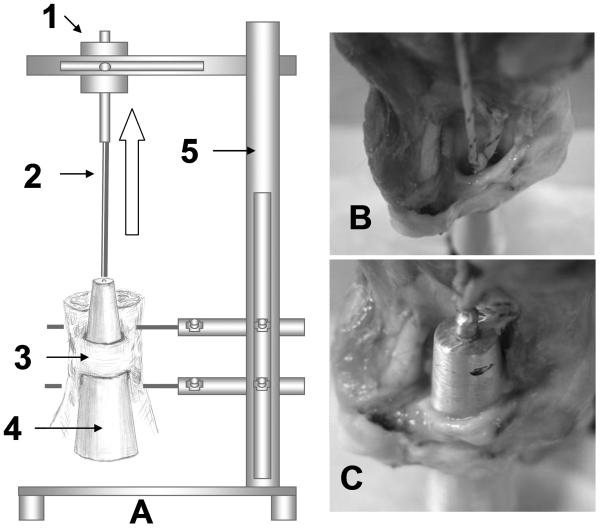
Figure 2.
The experimental set up for carpal tunnel compliance measurement. The device includes 1) mechanical actuator with linear potentiometer and load transducer; 2) cable between mechanical actuator and tapered rod; 3) specimen with transverse carpal tunnel ligament; 4) tapered metal rod; and 5) testing frame. The arrow showed the direction of the actuator pulled the metal rod through the carpal tunnel from proximal to distal. (B) The starting position of the metal rod. (C) The final position of the metal rod.
Figure 3.
The tapered metal rods for the four specimens. The tangent of the angle of slope θ of the taper was 0.1.
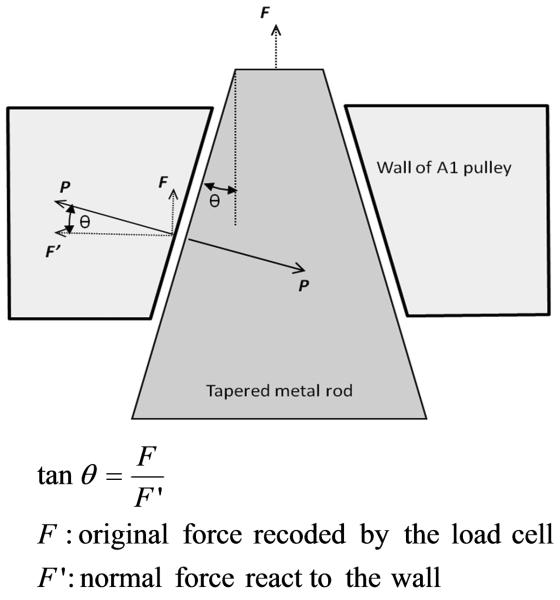
Figure 4.
Relationship between the original and normal forces on the wall of carpal tunnel.
Data Analyses
The increasing area ratio (InAR) was calculated from the absolute increasing cross sectional area of the tapered rod during the test divided by the original area of the rod at the start point. The formula of InAR was defined as following:
Where:
Afinal: final cross sectional area of the rod at the final point;
Aoriginal: original cross sectional area of the rod at the starting point.
Custom-made MATLAB programs were used to calculate the slopes of the linear part of the curves between the original force and displacement, normal force and InAR. Compliance was assessed as the inverse of the slopes of the original force/displacement and normal force/InAR curves, i.e. the higher the compliance the lower the slope.
Statistical Analyses
Statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA) software. The results were expressed as mean (±standard deviation, SD). Analysis of variance (ANOVA) test was used to compare the means of variables among humans, dogs, rabbits and rats, followed by post hoc analysis with least significant difference (LSD) for individual comparisons. Statistical significance was set at a level of p<0.05.
RESULTS
The results for rod radius at the starting point, and the length and thickness of the transverse carpal ligaments in the four species are shown in Table 1. Human carpal tunnels were the longest and thickest, while those of rats were the shortest and thinnest.
Table 1.
Means (SD) of initial radius of rod, length and thickness of transverse carpal ligaments in humans, dogs, rabbits and rats.
| Number of the Sample Size | Humans (N=10) |
Dogs (N=10) |
Rabbits (N=10) |
Rats (N=10) |
|---|---|---|---|---|
| Initial Rod Radius (mm) | 6.28 (0.48) | 4.38 (0.23) | 2.39 (0.10) | 0.69 (0.06) |
| Length of Ligament (mm) | 26.65 (3.27) | 13.03 (1.12) | 8.46 (1.39) | 2.46 (0.40) |
| Thickness of Ligament (mm) | 2.35 (0.10) | 1.31 (0.13) | 0.37 (0.05) | 0.22 (0.04) |
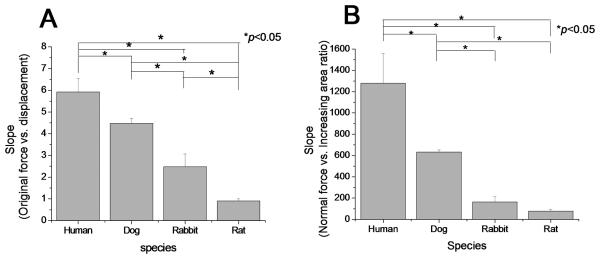
The mean slopes of the linear part of the original force/displacement curves of the four species were significantly different (p<0.05). Post hoc analysis indicated that the mean slope for the human carpal tunnels was the largest and the rat was the least among four species (p<0.05) (Figure 5A). The mean slopes of the normal force/InAR curves of the human model was significantly higher than that of dog, rabbit, and rat model (p<0.05). The mean slope of the normal force/InAR curves of dog model was significantly larger than that of rabbit and rat model (p < 0.05). However, there was no significant difference between rabbit and rat in the mean slope of the normal force/InAR curve (Figure 5B).
Figure 5.
Slopes of linear part of the curves from (A) original force vs. displacement of the rod; (B) normal force vs. InAR. (*p<0.05, significant difference)
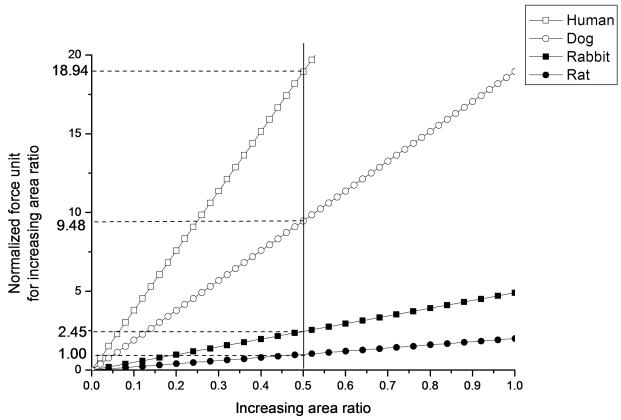
In order to quantify the difference of InAR across the four species, we chose one of the typical subjects in each group for comparison and defined the normal force for increasing rat carpal tunnel area by 50% as a unit, and normalized the normal force for the other three species. The comparison of InAR and normalized force across the four species are shown in Figure 6. The force needed to increase the carpal tunnel area by 50% in the human, dog and rabbit was 18.94, 9.48, 2.45 times of the normal force for the rat, respectively.
Figure 6.
Comparing the linear part of the curves among four species, showing the relationship between increasing area ratio and normalized force unit among the four species. Normalized force unit for increasing area ratio was defined as the normal force which increased the rat area by 50%.
DISCUSSION
While it is clear that carpal tunnel syndrome is associated with increased carpal tunnel pressure,30 the etiology of that pressure elevation is unclear.30 Studies of the etiology of human CTS have been restricted by the incapability to longitudinally assess the changes which occur prior to the onset of clinical symptoms. An animal model can overcome this limitation, and also since provides an opportunity to study the effect of gradually increasing tunnel pressure on nerve function and morphology. Since carpal tunnel pressure elevation requires a relatively rigid carpal tunnel, a proper animal model should have not only comparable anatomy but also mechanical characteristics similar to the human carpal tunnel. However, there is little literature to compare the mechanical properties among candidate animal models of CTS.
We have shown that the compliance of carpal tunnels in humans, dogs, rabbits and rats are significantly different. Humans have the largest tunnel, and the thickest and longest ligament. Human carpal tunnels are also the stiffest, while those of rats are least stiff. Both dog and rabbit were intermediate, with the dog carpal tunnel being most similar mechanically to the human. We do not know why these differences exist, or what causes them. It is possible that the material properties of the flexor retinaculum are different, or that it relates to differences in carpal bone anatomy of the strength of the intercarpal ligaments.
Although the carpal tunnels in all four species are composed of carpal bones dorsally and a transverse carpal ligament volarly, the anatomical structures differ substantially between species. In humans, the transverse carpal ligament is firmly attached to the hook of the hamate and pisiform bones on the ulnar side of the carpal tunnel and the tubercle of the trapezium and distal pole of the scaphoid on the radial side of the carpal tunnel. The connections between ligament and carpal bones are strong.31 The canine carpus includes seven small, irregular bones arranged into two rows. The proximal row contains three bones: intermedioradial carpal, ulnar carpal and accessory carpal. The accessory carpal bone is a small bone that serves as a lever arm for some of the flexor muscles of the carpus, similar to the human pisiform. The distal row contains four bones (I-IV). The canine transverse carpal ligament is mainly attached to the intermedioradial carpal bone medially and the accessory carpal laterally, and lies between the FDS and FDP tendons. In rabbits, the carpal bones consist of three proximal bones and a small accessory carpal bone, and, again, four distal carpal bones (I-IV). The rabbit transverse carpal ligament contains a triangular-shaped subcutaneous segment of fibrocartilage which can be palpated and used as a marker for identification of the ligament. This structure was not present in the other species. In rats, there are also two rows of carpal bones. The proximal row includes the pisiform, triangular, lunate, and navicular bones. The distal row contains the hamate, capitate and falciformis bones. The rat transverse ligament connects the pisiform medially to the falciformis and navicular laterally.
Of course, compliance of the carpal tunnel is just one factor contributing to the suitability of an animal model. The human median nerve is large, and there are in addition profundus and superficialis tendons and considerable tenosynovium and subsynovial connective tissue in the human carpal tunnel. Among candidate animal models, none of the non-primate models are ideal: dogs lack superficialis tendons inside the carpal tunnel, rabbits have relatively small nerves, and rats have very little synovium.28 Only humans and, to some extent, rats, use their forelimbs for grasping. And, of course, humans do not typically bear weight on their carpal tunnels, as is the case in the other species we studied.
Based on our findings, we believe that the relative rigidity of the human carpal tunnel, as compared to other species, may be one factor in understanding the human predilection for carpal tunnel syndrome. The relative laxity of the rat carpal tunnel, combined with a paucity of synovial tissue,28 suggests that the rat might not be an ideal model in which to study the development of CTS. While the rabbit is commonly used to study CTS, its carpal tunnel stiffness is also quite different from the human case. While the dog has not, to our knowledge, been used to study carpal tunnel syndrome, our data suggests that it might be a new model worth pursuing, notwithstanding the differences in tendon anatomy.
This study has several limitations. First, this is an in vitro study. Results in vivo, especially over time, could be different. Second, our model assumes that the carpal tunnel is round. However, none of the carpal tunnels we studied were perfectly round. We believe though that comparing the linear part of the curves, when contact was excellent circumferentially, decreased the effect of any errors in assumptions based on different shapes of the metal rods and carpal tunnels.
In conclusion, we have shown that there are significant differences in the stiffness of the carpal tunnel across species. We suggest that this factor be considered when choosing animal models of carpal tunnel syndrome, and that the results of this study may be helpful in developing more relevant animal models of CTS.
ACKNOWLEDGEMENT
This study was funded by grants from NIH (NIAMS AR49823) and Mayo Foundation.
REFERENCES
- 1.Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845–864. doi: 10.1016/s0095-4543(05)70180-9. [DOI] [PubMed] [Google Scholar]
- 2.Michelsen H, Posner MA. Medical history of carpal tunnel syndrome. Hand Clin. 2002;18:257–268. doi: 10.1016/s0749-0712(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 3.Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg M, Morgenstern H, Kelsh M. Impact of occupations and job tasks on the prevalence of carpal tunnel syndrome. Scand J Work Environ Health. 1992;18:337–345. doi: 10.5271/sjweh.1564. [DOI] [PubMed] [Google Scholar]
- 5.Nordstrom DL, Vierkant RA, DeStefano F, Layde PM. Risk factors for carpal tunnel syndrome in a general population. Occup Environ Med. 1997;54:734–740. doi: 10.1136/oem.54.10.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong TJ, Castelli WA, Evans FG, Diaz-Perez R. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984;26:197–201. [PubMed] [Google Scholar]
- 7.Cobb TK, Dalley BK, Posteraro RH, Lewis RC. The carpal tunnel as a compartment. An anatomic perspective. Orthop Rev. 1992;21:451–453. [PubMed] [Google Scholar]
- 8.Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–383. [PubMed] [Google Scholar]
- 9.Lowry WE, Jr., Follender AB. Interfascicular neurolysis in the severe carpal tunnel syndrome. A prospective, randomized, double-blind, controlled study. Clin Orthop Relat Res. 1988;227:251–254. [PubMed] [Google Scholar]
- 10.Mackinnon SE, McCabe S, Murray JF, et al. Internal neurolysis fails to improve the results of primary carpal tunnel decompression. J Hand Surg [Am] 1991;16:211–218. doi: 10.1016/s0363-5023(10)80099-1. [DOI] [PubMed] [Google Scholar]
- 11.Rojviroj S, Sirichativapee W, Kowsuwon W, et al. Pressures in the carpal tunnel. A comparison between patients with carpal tunnel syndrome and normal subjects. J Bone Joint Surg Br. 1990;72:516–518. doi: 10.1302/0301-620X.72B3.2187880. [DOI] [PubMed] [Google Scholar]
- 12.Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg [Am] 1989;14:624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- 13.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Jinrok O, Zhao C, Amadio PC, et al. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004;22:1310–1315. doi: 10.1016/j.orthres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Oh J, Zhao C, Amadio PC, et al. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005;23:1226–1231. doi: 10.1016/j.orthres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Oh J, Zhao C, Zobitz ME, et al. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg Am. 2006;88:824–831. doi: 10.2106/JBJS.E.00377. [DOI] [PubMed] [Google Scholar]
- 17.Silver MA, Gelberman RH, Gellman H, Rhoades CE. Carpal tunnel syndrome: associated abnormalities in ulnar nerve function and the effect of carpal tunnel release on these abnormalities. J Hand Surg [Am] 1985;10:710–713. doi: 10.1016/s0363-5023(85)80214-8. [DOI] [PubMed] [Google Scholar]
- 18.Oh S, Belohlavek M, Zhao C, et al. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color Doppler sonographic imaging. J Ultrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- 19.Osamura N, Zhao C, Zobitz ME, et al. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech (Bristol, Avon) 2007;22:999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osamura N, Zhao C, Zobitz ME, et al. Permeability of the subsynovial connective tissue in the human carpal tunnel: a cadaver study. Clin Biomech (Bristol, Avon) 2007;22:524–528. doi: 10.1016/j.clinbiomech.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Diao E, Shao F, Liebenberg E, et al. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–223. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Lim JY, Cho SH, Han TR, Paik NJ. Dose-responsiveness of electrophysiologic change in a new model of acute carpal tunnel syndrome. Clin Orthop Relat Res. 2004:120–126. doi: 10.1097/01.blo.0000142352.88039.33. [DOI] [PubMed] [Google Scholar]
- 23.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 24.Rosen HR, Ammer K, Mohr W, et al. Chemically-induced chronic nerve compression in rabbits--a new experimental model for the carpal tunnel syndrome. Langenbecks Arch Chir. 1992;377:216–221. doi: 10.1007/BF00210276. [DOI] [PubMed] [Google Scholar]
- 25.Clark BD, Barr AE, Safadi FF, et al. Median nerve trauma in a rat model of work-related musculoskeletal disorder. J Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta R, Rowshan K, Chao T, et al. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Rainer WG, Mayer J, Sadler TR, Jr., Dirks D. Effect of graded compression on nerve conduction velocity. An experimental study. Arch Surg. 1973;107:719–721. doi: 10.1001/archsurg.1973.01350230071014. [DOI] [PubMed] [Google Scholar]
- 28.Ettema AM, Zhao C, An KN, Amadio PC. Comparative Anatomy of the Subsynovial Connective Tissue in the Carpal Tunnel of the Rat, Rabbit, Dog, Baboon, and Human Hand. 2006;1(2):78–84. doi: 10.1007/s11552-006-9009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Osamura N, Zhao C, et al. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2007 doi: 10.1016/j.jbiomech.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt HM. Normal Anatomy and Variations of the Median Nerve in the Carpal Tunnel. In: Luchetti, Amadio, editors. Carpal Tunnel Syndrome. Springer; Berlin Heidelberg: 2007. pp. 13–20. [Google Scholar]