Abstract
Neuropathological, neuropsychological, and neuroimaging studies of human alcoholism provide evidence for degradation of frontal, pontine, thalamic, and cerebellar brain sites and disturbed associated functions. Current studies using neuroimaging combined with examination of executive functions, traditionally considered the sole purview of the frontal lobes, have identified a role for the cerebellum serving as a compensatory processing adjunct to enable normal performance on challenging tasks tapping executive functions. This overview proposes that disruption of an executive frontocerebellar network is a major contributor to characteristic behaviors of alcoholism that, on the one hand, enable alcohol use disorders, and on the other hand, lead to compensation for so-called frontally-based dysfunctions in alcoholism.
A salient behavioral characteristic defining alcohol use disorders is the continued use of alcohol despite physiological or psychological problems (http://rethinkingdrinking.niaaa.nih.gov/). Other clinically observed features describing alcoholic behavior include impaired judgment, blunted affect, poor insight, social withdrawal, reduced motivation, distractibility, cognitive rigidity, inattention, and perseveration (Oscar-Berman, 2000; Sullivan et al., 2000a). This constellation of higher-order, “executive” dysfunction has classically been ascribed to degradation of frontal lobe integrity (Cummings, 1993; Fuster, 1999). Application of specialized and detailed neuropsychological tests, however, has demonstrated that individuals with lesions limited to the cerebellum can be impaired in functions previously considered the exclusive purview of the frontal lobes (Schmahmann, 1997, 2000).
Brain structural damage in response to chronic alcohol exposure, although widespread (Pfefferbaum et al., 1992; Sullivan et al., 1998), also targets specific brain systems leaving others relatively intact (for review, Chanraud et al., 2010a). The cerebellum (Victor et al., 1959) and prefrontal cortex (Courville, 1955; Harper et al., 2003), although spatially disparate, are particularly compromised in the brains of alcoholics. The cerebellum is strongly interconnected to the cerebral cortex via feedforward/afferent loops through the pons and feedback/efferent loops through the thalamus. Convergent findings from primate viral tracing studies (Schmahmann and Pandya, 2008; Strick et al., 2009), human case studies of patients with cerebellar lesions (Fitzpatrick et al., 2008; Schmahmann and Pandya, 2008; Leggio et al., 2009), and human functional imaging studies (Habas et al., 2009; Krienen and Buckner, 2009) have revealed the presence of multiple cerebellar-based cortical systems.
Dissociable functions of these loops are related to the sites of termination in the cerebral cortex of specific projections from the cerebellum (Kelly and Strick, 2003a). Examples of these divergent but parallel loops include the motor and executive loops (Figure 1): 1) the motor network, involving motor lobules of the cerebellar vermis (e.g., IV, V, VI) and motor cerebral cortices (Biswal et al., 1995) affecting functions of gait and balance (Sullivan et al., 2006a; Sullivan et al., 2010a); and 2) the executive network, involving the cerebellar neocortex (e.g., lobule VII, lobule VIII, Crus I, and Crus II) and prefrontal cortical sites (e.g., BA9 and 46) contributing to cognitive functions, such as verbal (Desmond et al., 1997; Desmond et al., 2003) and spatial (Pfefferbaum et al., 2001) working memory and set shifting (Seeley et al., 2007; see in this issue Marvel and Desmond, 2010)(Figure 2). Such cerebellar-based systems have recently been examined using structure (magnetic resonance imaging (MRI)) / function (working memory tasks) paradigms in alcoholics and controls (Chanraud et al., 2010b). In controls, the best predictors of performance on the spatial working memory task with spatial tracking interference were volumes of the right middle frontal gyrus and right cerebellar Crus I. By contrast, in alcoholics, the best predictors of performance on the spatial working memory task with arithmetic problem solving interference were volumes of the left thalamus and left cerebellar Crus I. These brain structure-function correlations suggest that although the specific regions recruited by the alcoholics and controls were different, performance on the cognitive task by both groups relied on the integrity of cerebellar-based systems.
Figure 1.
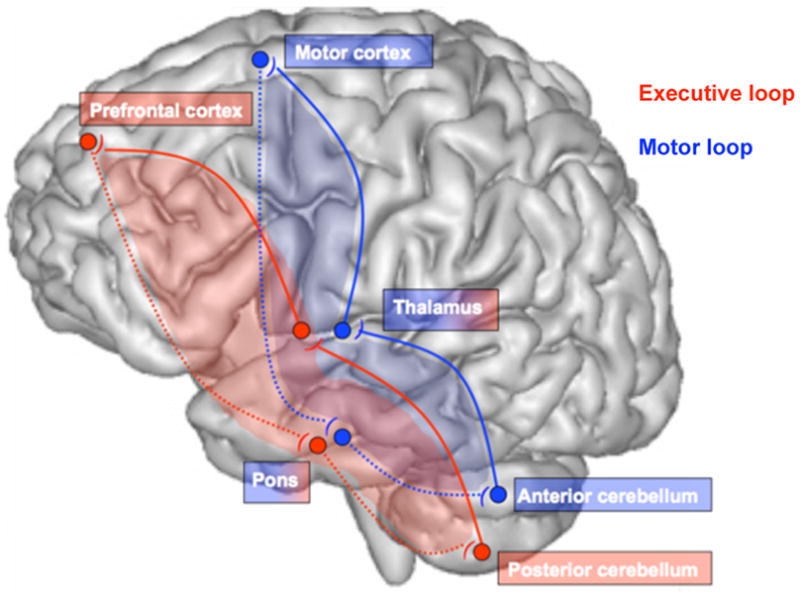
The corticocerebellar circuit: two dissociable but associated loops, the motor loop connecting motor cortex, thalamus, and anterior cerebellum and the executive loop connecting prefrontal-pontine-posterior cerebellar sites (see Kelly and Strick, 2003b).
Figure 2.
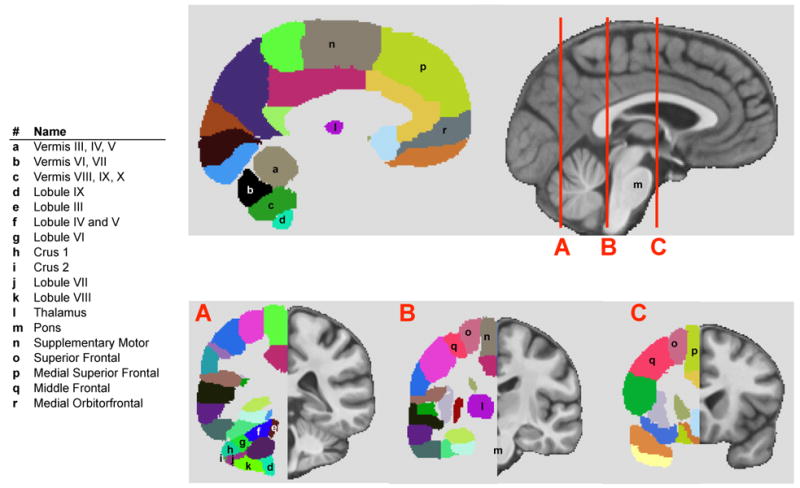
Parcellated regions (left) of the SRI24 brain (Rohlfing et al., 2010) (right). Principal lobules of the cerebellum are parcellated and numbered; several have been identified to subserve dissociable cognitive and motor functions. The cerebrum is also parcellated into regions defined by gyral markings and roughly corresponding to regional structures, many with known functions. Note the distance traversed by frontothalamopontocerebellar circuitry.
Here, we propose that disruption of the executive frontocerebellar network is a major contributor to characteristic behaviors of alcoholism that, on the one hand, enable alcohol use disorders, and on the other hand, lead to compensation for so-called frontally based dysfunctions in alcoholism. Specifically considered is the possibility that in alcoholism, compromise of the executive loop contributes to dysfunction affecting impulse control (Nixon et al., 2002; Fein et al., 2010), conflict processing (De Rosa et al., 2004), and disinhibition (Hada et al., 2000; Fein and Di Sclafani, 2004) that enable maintenance of addictive behavior. On the compensatory side, evidence indicates that recruitment of intact cerebellar loops can compensate for the alcoholic's otherwise impaired performance on tasks requiring, for example, visuospatial or working memory skills (cf., Sullivan and Pfefferbaum, 2005).
Brain Structures and Systems Affected in Individuals with Alcohol Use Disorders
The following sections review the literature regarding brains of alcoholics from a neuropathological perspective and from the standpoint of results from MRI modalities including structural MRI, diffusion tensor imaging, MR spectroscopy, and functional MRI. The findings presented are not comprehensive but have been selected to indicate compromise of frontocerebellar circuitry and its nodes in alcoholism. For comprehensive reviews of the brain regions targeted by chronic alcoholism and associated neurological deficits, see (cf., Oscar-Berman et al., 1997; Mann et al., 2001; Chanraud et al., 2010a; Sullivan et al., 2010b).
Neuropathology
Classical postmortem neuropathology studies identified damage to nodes of the frontocerebellar circuit in cases of chronic alcoholism. On the gross pathology level, the frontal cortex sustains notable shrinkage, due in part to cell loss, in contrast to the relatively spared motor cortex (Kril and Harper, 1989; Harper, 1998). Atrophy of the cerebellar vermis has been reported in alcoholics and even more frequently in alcoholics with exceptionally high levels of alcohol consumption (Karhunen et al., 1994) or thiamine deficiency (35–50% Victor et al., 1959). On the cellular level, Purkinje cells (Pentney, 1993) and cells in the granular and molecular layers of the cerebellar cortex (Phillips et al., 1987) are particularly affected, especially in alcoholics with a history of thiamine deficiency (Baker et al., 1999). Whether caused by neurotoxic effects of alcohol per se or to secondary events such as dietary deficiencies, excessive alcohol consumption adversely affects the pons (Adams et al., 1959) and thalamus (Kril and Butterworth, 1997; Harding et al., 2000). The pons sustains reduction of numbers of serotonergic (Halliday et al., 1993) and noradrenergic neurons (Arango et al., 1994). Although not always forthcoming with in vivo MRI (Shear et al., 1994), neuropathologically, the thalamus has been seen to be abnormally small in alcoholics (Kril and Butterworth, 1997) in terms of the size of thalamic nuclei and the number and size of neurons in the thalamic nuclei (Belzunegui et al., 1995; Harding et al., 2000).
In addition to volume shrinkage of the major gray matter nodes of the frontocerebellar circuit, neuropathological studies have consistently reported compromised white matter integrity indicating that chronic alcoholism may disrupt the white matter fiber bundles linking the nodes (Harper et al., 1985; De la Monte, 1988; Badsberg-Jensen and Pakkenberg, 1993). Postmortem analysis has revealed that white matter in the chronic alcoholic brain is subject to volume shrinkage (Harper and Kril, 1985; De la Monte, 1988), especially in the frontal cortex of alcoholics with Wernicke-Korsakoff syndrome (WKS, Kril and Butterworth, 1997). Indeed, in alcoholics with WKS, white matter impairment is negatively correlated with maximum daily alcohol consumption (Kril and Butterworth, 1997). The volume of cerebellar white matter is also reduced in alcoholics (Phillips et al., 1987), and loss of vermal white matter is reported in alcoholics with ataxia (Baker et al., 1999). However, there are no obvious microscopic white matter lesions in the cerebral hemispheres of uncomplicated alcoholics, and studies of lipid profiles have revealed only minor alterations (Harper and Kril, 1991; Olsson et al., 1996). An increase in the water content of frontal lobe white matter (Harper, 1998) suggests that the white matter shrinkage in this brain region may reflect demyelination. Consistent with this interpretation, expression of three genes encoding myelin proteins that are required for the highly ordered and compact structure of myelin and are specifically involved in stabilization and compaction of the myelin sheath was lower in the superior frontal cortex of human alcoholic subjects than controls (Lewohl et al., 2005). According to Harper (2009), neural loss may also result in axonal (Wallerian) degeneration and a permanent reduction in white matter volume (Harper et al., 1988). Thus, the pathophysiology of white matter disruption in alcoholics may involve changes in both myelination and axonal integrity (Harper et al., 2005).
Structural Neuroimaging
Comporting with postmortem findings, computed tomography (CT) and MRI studies reveal brain volume deficits specific to the prefrontal cortex and cerebellum (for reviews Oscar-Berman and Marinkovic, 2007; Chanraud et al., 2010a) even in chronic alcohol dependent subjects without obvious complications from nutritional deficiencies (e.g., thiamine deficiency) or hepatic disorders (Hayakawa and Kumagai, 1992; Wang et al., 1993; Shear et al., 1994; Chanraud et al., 2007). MR volumetric studies have also revealed thalamic (but see, Shear et al., 1992; Sullivan, 2003; Benegal et al., 2007; Cardenas et al., 2007; Chanraud et al., 2007) and pontine (Sullivan et al., 2010a) volume deficits in alcoholics. The pons is composed of a complex arrangement of nuclei (Schmahmann and Pandya, 1989) and extensive white matter fiber systems. The pons can be affected by central pontine myelinolysis (CPM), a complication associated with alcoholism (Victor, 1987). CPM, a relatively rare and serious condition that can result in quadriplegia and curtailed longevity (Adams et al., 1959), is neuroradiologically defined on T2- weighted images as a hyperintense, triangular-shaped lesion in the middle of the pons (Kleinschmidt-DeMasters et al., 1997). Even in uncomplicated and asymptomatic alcoholics, prolonged T2 relaxation times indicative of excessive local interstitial fluid can be observed in the pons of older alcoholics, although more regularly in alcoholics with WKS (Sullivan and Pfefferbaum, 2001). Also consistent with the neuropathological literature is the MRI observation of white matter volume shrinkage in the cerebellum and pons of alcoholics (Sullivan et al., 1998; Sullivan, 2000; Sullivan and Pfefferbaum, 2001; Sullivan, 2003; Sullivan et al., 2003; Chanraud et al., 2007).
The existence of a structural scaffolding for the frontocerebellar circuitry in alcoholism has been supported by correlational analysis. In alcoholics, volume deficits quantified with MRI in the pons co-occur with volume deficits in the white matter of the anterior superior cerebellar vermis and white and gray matter of the cerebellar hemispheres. By contrast, volume deficits in the thalamus co-occur with volume deficits in the gray matter of the posterior inferior vermis, cerebellar hemispheres, and parietal cortex (Sullivan, 2003). A lack of correlation between pontine and thalamic volumes suggests their independence in the afferent and efferent loops of the frontocerebellar network.
Diffusion Tensor Imaging (DTI)
Whereas structural MRI provides measurement of regional tissue expressed as a volume over multiple image slices and voxels, MR diffusion tensor imaging (DTI) provides a qualitative assessment of the microstructure of tissue, typically white matter, within voxels (Basser and Pierpaoli, 1996). DTI image acquisition and data analysis are complex, and details of these methods are available in numerous reviews (e.g., Le Bihan, 2003; Jones, 2010). In short, white matter fiber integrity is commonly measured in terms of fractional anisotropy (FA), which is usually higher in fibers with a homogeneous or linear structure, such as healthy white matter, and bulk mean diffusivity (MD), for which higher values, commonly due to larger presence of mobile water molecules in a tissue sample (Pierpaoli et al., 2001; Pfefferbaum et al., 2003; Pfefferbaum and Sullivan, 2003), reflect diminished fiber integrity. MD can be decomposed into two components: axial (longitudinal) diffusivity (λL), which can be altered with disruption of axonal integrity and axonal deletion; and radial (transverse) diffusivity (λT), which increases selectively with decline in myelin integrity (Song et al., 2002; Song et al., 2005; Sun et al., 2006b; Sun et al., 2006a). DTI has been further extended to provide visual depictions of white matter fiber systems (Stieltjes et al., 2001; Xu et al., 2002; Lehericy et al., 2004) and quantification of the integrity of specific fiber tracks (Gerig et al., 2005; Sullivan et al., 2006b).
Studies using DTI have detected untoward effects of alcoholism on the microstructure of white matter. In some cases, DTI has been shown to be more sensitive than conventional volumetric MRI in identifying disordered tissue (Pfefferbaum et al., 2000; Pfefferbaum and Sullivan, 2002). One pattern of spared and affected tissue that has emerged over a series of studies using quantitative fiber tracking is that frontal and superior fiber bundles show greater abnormalities than posterior and inferior fiber bundles in alcoholics relative to controls (Pfefferbaum et al., 2009; Pfefferbaum et al., 2010). Using Tract-Based Spatial Statistics (Smith et al., 2006) for DTI analysis, Meyerhoff and colleagues reported lower FA and higher diffusivity, indicative of tissue degradation, in dorsomedial and dorsolateral prefrontal cortical and cerebellar regions (Yeh et al., 2009). Another quantitative fiber tracking study revealed fewer white matter fibers per unit volume running between the midbrain and the pons in alcoholics than controls (Chanraud et al., 2009). Together, these DTI studies indicate a frontal selectivity to white matter damage in the context of widespread microstructural degradation of white matter systems (Pfefferbaum et al., 2006) and initial evidence that white matter tracts of the corticopontine pathway are also compromised. A caveat is the observation from one study showing relative preservation of corticocerebellar fiber systems in alcoholics (Pfefferbaum et al., 2009). Such preservation may be an avenue to enable invoking cerebellar systems in compensatory efforts, as observed in functional imaging studies (cf., Sullivan and Pfefferbaum, 2005).
Magnetic Resonance Spectroscopy (MRS)
Magnetic resonance spectroscopy (MRS) is a powerful noninvasive approach for the identification, visualization, and quantification of specific brain biochemicals (metabolites and neurotransmitters), thus enabling the direct assessment of the neurochemical status of discrete brain structures. Whereas MRI detects the spatial distribution and tissue density of hydrogen nuclei (1H) in water and fat, MRS measures 1H of typically carbon-containing compounds that are in sufficiently high concentrations to be detected (van der Graaf, 2010). A predominant MRS signal in the healthy human brain is N-acetylaspartate (NAA), found almost exclusively in neurons (Urenjak et al., 1993; Petroff et al., 1995) and thus considered a marker of neuronal integrity. Choline-containing (Cho) compounds, including free Cho, phosphocholine, and glycerophosphocholine, are associated with cell membrane synthesis, turnover, and metabolism (Stoll et al., 1995). The signal from total creatine (tCr), often used as a referent for other metabolites, is influenced by the state of high-energy phosphate metabolism (Tedeschi et al., 1995).
Studies of recently detoxified alcoholics (1 – 6 weeks) show abnormally low levels of NAA, inferred from ratios to tCr or amount of underlying tissue, in frontal white matter (Schweinsburg et al., 2001; Schweinsburg et al., 2003; Meyerhoff et al., 2004; Bartsch et al., 2007), frontal gray matter (Jagannathan et al., 1996; Bendszus et al., 2001; Durazzo et al., 2004), thalamus (Jagannathan et al., 1996; Murata et al., 2001), and cerebellum (Jagannathan et al., 1996; Bendszus et al., 2001; Murata et al., 2001; Parks et al., 2002). Likewise, Cho, whether expressed as a ratio to tCr or tissue water, is lower in recently detoxified alcoholics than controls in thalamus (Murata et al., 2001; Durazzo et al., 2004) and cerebellum (Martin et al., 1995; Jagannathan et al., 1996; Bendszus et al., 2001; Murata et al., 2001; Ende et al., 2005; Bartsch et al., 2007). Such changes in the biochemical status of discrete brain regions are reinforced by findings of correlations with performance on various behavioral tasks. For example, low NAA in the cerebellar vermis was related to poor performance on tasks of visuospatial learning and memory (Durazzo et al., 2004). In another study, an increase in NAA/tCr with continued abstinence for approximately one month was related to improved performance on the auditory-verbal-learning test (Bendszus et al., 2001). These selective relations demonstrate the functional impact of metabolites changes in these nodes of the frontocerebellar circuitry.
When metabolites were evaluated as a function of the likelihood of relapse, only patients that relapsed within 3 weeks of detoxification revealed reduced cerebellar NAA concentrations (Parks et al., 2002). Similarly, in individuals that relapsed relative to those that remained abstinent, baseline compared to one-year follow-up levels of NAA and tCr were lower in the dorsolateral prefrontal cortex and cerebellar vermis (Durazzo et al.). These longitudinal MRS studies thus also provide evidence for the role of a dysfunctional frontocerebellar circuit in the maintenance of addiction to alcohol.
Functional neuroimaging
Functional magnetic resonance imaging (fMRI) provides assessment of the utilization of blood oxygen (i.e., the blood oxygen-level dependent [BOLD] effect) measureable during performance of specific cognitive, sensory, or motor tasks. Several fMRI studies reveal that alcoholics activate either a different neural network (Pfefferbaum et al., 2001; Tapert et al., 2001; Tapert et al., 2004) or activate appropriate regions but more widely (Desmond et al., 2003; Parks et al., 2003) to perform behaviorally (e.g., in terms of accuracy or reaction time) on par with controls. For example, self-paced finger-tapping activated frontocerebellar networks in controls (e.g., anterior cingulate, anterior lobe and vermis of the cerebellum) but only the parietal precuneus in alcoholics (Parks et al., 2010). This finding suggests compensatory alterations of frontocerebellar circuits whereby alcoholics must recruit higher ordering planning regions such as the parietal lobe in order to perform equivalently to controls.
A study employing the Sternberg verbal working memory task reported similar levels of performance with respect to reaction time and accuracy in alcoholic and nonalcoholic subjects. In these same subjects, however, activations were greater in alcoholics than in controls in the left prefrontal cortex and the right superior cerebellum (Desmond et al., 2003). Another study revealed that the processing of redundant targets relative to a single target was associated with a significant BOLD response in bilateral extrastriate cortices in controls. By contrast, although alcoholics activated only the left extrastriate cortex, they also showed significant BOLD responses in the thalamus, pallidum, and left cerebellum (Schulte et al., 2010). These fMRI studies provide evidence for the role of the cerebellum in augmenting performance and compensating for the functional deficits attributable to frontal cortical disruption in alcoholics.
Brain Structure/Function Relationships in Alcohol Use Disorders
Quantitative studies of brain structure and motor function have revealed the traditionally accepted relationship between postural instability and small volume of the anterior superior cerebellar vermis in alcoholics (Sullivan et al., 2000b), infratentorial tissue volumes (Sullivan et al., 2010a), and postural sway (Sullivan et al., 2006a). Components of sway may be the consequence of damage to other nodes in the frontocerebellar circuit. For example, using posturography and balance platform testing, truncal tremor was observed at two frequencies in alcoholic men (Sullivan et al., 2006a): the tremor at 5-7 Hz could indicate direct damage to the thalamus (Guehl et al., 2003).
In addition to these motor-based relations with the condition of the cerebellum and pons, other analyses have reported correlations between frontally-based cognitive impairment that can be related to compromised cerebellar or pontine structures and even of the white matter connecting the various nodes of the circuitry. Indeed, the volumes of selective regions of the cerebellum have been shown to be better predictors than frontal lobe volumes of executive and visuospatial deficits in alcoholics (Sullivan, 2003). Also in alcoholics, the volume of the pons and a white matter region in the midbrain common to both afferent corticocerebellar and efferent cerebellocortical fibers correlated with performance on neuropsychological tests including fluency, letter-number sequencing, trail-making B, Stroop interference, and the Wisconsin Card Sorting test (Chanraud et al., 2007). The number of fibers per volume coursing between the midbrain and pons correlated with performance on Part B of the Trail Making Test, which assesses visual search, working memory, and cognitive flexibility (Chanraud et al., 2009). Alcoholics clinically asymptomatic for pontine signs of CPM reveal significant correlations between poorer verbal and nonverbal fluency production (tests long considered sensitive to lesions of lateral frontal cortex, Lezak, 1995; Kolb and Whishaw, 1996) and longer pontine relaxation times (Sullivan and Pfefferbaum, 2001). Regarding anatomical connectivity, it is possible that the relationships between fluency output and pontine relaxivity arise from compromise of frontal connections to central pontine sites (Schmahmann and Pandya, 1997).
Conclusion
This overview proposes the guiding hypothesis that disruption of frontocerebellar circuitry is one of the principal neural mechanisms underlying behavioral deficits in both uncomplicated alcoholism and alcoholics with neurological complications such as WKS (cf., Wijnia and Goossensen, 2010). Compromise of the gray matter nodes of this circuit or disruption of the white matter tracts connecting the nodes may adversely influence remote regions within that circuit, resulting in characteristic alcoholism-related cognitive and motor deficits. This network, even when compromised, may be invoked by alcoholics for compensation in the performance of challenging cognitive procedures. The cerebellum, therefore, exerts substantial primary and modulatory influence on behavior with its long-reaching loops to frontal sites. Even modest alterations within this frontocerebellar circuitry in alcoholics have the potential to contribute to vulnerability for relapse by virtue of executive function impairment. Involvement of frontocerebellar circuitry in compensatory activity may also be a source of maintenance of addictive behavior, given the roles of the cerebellum (Grafman et al., 1992; Doyon et al., 1997; Hubert et al., 2009) and basal ganglia (Heindel et al., 1989; Pascual-Leone et al., 1993; Witt et al., 2002) in implicit and procedural learning (see this issue Bostan and Strick, 2010). By its nature, implicit learning is accomplished with little to no conscious awareness and therefore can skirt the purview of therapy and contribute to denial (cf., Le Berre et al., 2010), the first “step” alcoholics must overcome to reduce harmful drinking levels (Wilson, 2001).
Acknowledgments
We would like to thank our colleague, Adolf Pfefferbaum, M.D. for thoughtful discussion on brain and behavioral sequelae of alcoholism and for production of the imaging figures.
Funding support: This work was funded by grants from the U.S. National Institute on Alcohol Abuse and Alcoholism (AA010723, AA017168, AA017923).
Footnotes
Disclosure: The authors have no conflict of interest to disclose.
References
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. Archives of Neurology and Psychiatry. 1959;81:154–172. [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Fewer pigmented neurons in the locus coeruleus of uncomplicated alcoholics. Brain Res. 1994;650:1–8. doi: 10.1016/0006-8993(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Badsberg-Jensen G, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Baker K, Harding A, Halliday G, Kril J, Harper C. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 1999;91:429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Belzunegui T, Insausti R, Ibanez J, Gonzalo LM. Effect of chronic alcoholism on neuronal nuclear size and neuronal population in the mammillary body and the anterior thalamic complex of man. Histol Histopathol. 1995;10:633–638. [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. Interactions between the cerebellum and basal ganglia. Neuropsychology Review. 2010;20 doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Sullivan EV. Structural imaging of alcohol abuse. In: Shenton ME, Turetsky BI, editors. Understanding Neuropsychiatric Disorders. Cambridge University Press; 2010a. [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: Relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010b doi: 10.1038/npp.2010.56. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, Cointepas Y, Martelli C, Martinot JL. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of Alcohol on the Nervous System of Man. Los Angeles: San Lucas Press; 1955. [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- De la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, De Rosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased fronto-cerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Laforce R, Jr, Castonguay M, Bedard PJ, Bedard F, Bouchard JP. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain and cognition. 1997;34:218–245. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004;32:63–67. doi: 10.1016/j.alcohol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P. Sensation seeking in long-term abstinent alcoholics, treatment-naive active alcoholics, and nonalcoholic controls. Alcoholism, clinical and experimental research. 2010;34:1045–1051. doi: 10.1111/j.1530-0277.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LE, Jackson M, Crowe SF. The relationship between alcoholic cerebellar degeneration and cognitive and emotional functioning. Neuroscience and biobehavioral reviews. 2008;32:466–485. doi: 10.1016/j.neubiorev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Fuster J. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand. 1999;99:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. 13th Proceedings of theInternational Society for Magnetic Resonance in Medicine; Miami, FL. 2005. Abstract no 1337. [Google Scholar]
- Grafman J, Litvan I, Massaquoi S, Stewart M, Sirigu A, Hallett M. Cognitive planning deficit in patients with cerebellar atrophy. Neurology. 1992;42:1493–1496. doi: 10.1212/wnl.42.8.1493. [DOI] [PubMed] [Google Scholar]
- Guehl D, Pessiglione M, Francois C, Yelnik J, Hirsch EC, Feger J, Tremblay L. Tremor-related activity of neurons in the ‘motor’ thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur J Neurosci. 2003;17:2388–2400. doi: 10.1046/j.1460-9568.2003.02685.x. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Halliday G, Ellis J, Heard R, Caine D, Harper C. Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff's psychosis. J Neuropathol Exp Neurol. 1993;52:567–579. doi: 10.1097/00005072-199311000-00003. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? Neuropathology and Experimental Neurology. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril JJ. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. Journal of Neurology, Neurosurgery, and Psychiatry. 1985;48:211–217. doi: 10.1136/jnnp.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J. If you drink your brain will shrink: Neuropathological considerations. Alcohol Alcohol Supplement. 1991;1:375–380. [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I, Pfefferbaum A, Adalsteinsson E, Sullivan EV, Lewoh IJ, Dodd PR, Taylor MJ, Fein G, Landman B. The pathophysiology of ‘brain shrinkage’ in alcoholics structural and molecular changes and clinical implications. Alcoholism: Clinical and Experimental Research. 2005;29:1106–1115. [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: A pathological study. Br Med J. 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Brain shrinkage in alcoholics is not caused by changes in hydration: a pathological study. Journal of Neurology, Neurosurgery, and Psychiatry. 1988;51:124–127. doi: 10.1136/jnnp.51.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Kumagai H. MR imaging of chronic alcoholism. Acta Radiol. 1992;33:201–206. [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. The Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert V, Beaunieux H, Chetelat G, Platel H, Landeau B, Viader F, Desgranges B, Eustache F. Age-related changes in the cerebral substrates of cognitive procedural learning. Human brain mapping. 2009;30:1374–1386. doi: 10.1002/hbm.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imaging. 1996;14:553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Jones DK. Diffusion MRI: Theory, Methods, and Applications. First. Oxford: Oxford University Press; 2010. [Google Scholar]
- Karhunen PJ, Erkinjuntti T, Laippala P. Moderate alcohol consumption and loss of cerebellar purkinje cells. Br Med J. 1994;308:1663–1667. doi: 10.1136/bmj.308.6945.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003a;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003b;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Anderson CA, Rubinstein D. Asymptomatic pontine lesions found by magnetic resonance imaging: are they central pontine myelinolysis? Journal of Neurological Science. 1997;149:27–35. doi: 10.1016/s0022-510x(96)05333-6. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. Fourth. New York: W. H. Freeman and Company; 1996. [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of the alcoholic brain. Acta Neuropathol (Berl) 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Butterworth RF. Diencephalic and cerebellar pathology in alcoholic and nonalcoholic patients with end-stage liver disease. Hepatology. 1997;26:837–841. doi: 10.1002/hep.510260405. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, Pitel AL, Alliain P, Eustache F, Beaunieux H. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcoholism: Clinical & Experimental Research. 2010 doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Chiricozzi FR, Clausi S, Tedesco AM, Molinari M. The neuropsychological profile of cerebellar damage: The sequencing hypothesis. Cortex. 2009 doi: 10.1016/j.cortex.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wixey J, Harper CG, Dodd PR. Expression of MBP, PLP, MAG, CNP, and GFAP in the Human Alcoholic Brain. Alcohol Clin Exp Res. 2005;29:1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Third. New York: Oxford University Press; 1995. [Google Scholar]
- Mann K, Agartz I, Harper C, Shoaf S, Rawlings R, Momenan R, Hommer D, Pfefferbaum A, Sullivan EV, Anton R, Drobes D, George M, Bares R, Machulla HJ, Mundle G, Reimold M, Heinz A. Neuroimaging in alcoholism: Ethanol and brain damage. Alcoholism: Clinical and Experimental Research (supplement) 2001;25:104–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional Topography of the Cerebellum in Verbal Working Memory. Neuropsychology Review. 2010 doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Fujito T, Kimura H, Omori M, Itoh H, Wada Y. Serial MRI and (1)H-MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry research. 2001;108:49–55. doi: 10.1016/s0925-4927(01)00304-3. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Olsson NU, Harding AJ, Harper C, Salem N., Jr High-performance liquid chromatography method with light-scattering detection for measurements of lipid class composition: analysis of brains from alcoholics. J Chromatogr B Biomed Appl. 1996;681:213–218. doi: 10.1016/0378-4347(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No 34. Bethesda, MD: National Institutes of Health; 2000. pp. 437–472. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21:65–75. [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Greenberg DS, Nickel MK, Dietrich MS, Rogers BP, Martin PR. Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcoholism, clinical and experimental research. 2010;34:1098–1109. doi: 10.1111/j.1530-0277.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- Parks MH, Morgan VL, Pickens DR, Price RR, Dietrich MS, Nickel MK, Martin PR. Brain MRI activation associated with self-paced finger-tapping in chronic alcohol dependent patients. Alcoholism: Clinical and Experimental Research. 2003;27:704–711. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism: Clinical and Experimental Research. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in Parkinson's disease and cerebellar degeneration. Annals of neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pentney RJ. Alterations in the structure of the cerebellum after long-term ethanol consumption. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage: NIAAA Research Monograph No 22. Rockville, MD: National Institute of Health; 1993. pp. 249–276. [Google Scholar]
- Petroff OA, Pleban LA, Spencer DD. Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magnetic resonance imaging. 1995;13:1197–1211. doi: 10.1016/0730-725x(95)02033-p. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging. 2003;18:427–433. doi: 10.1002/jmri.10377. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: Selective relations to dissociable functions. Alcohol Clin Exp Res. 2010;34:1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–314. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix L, Virta A, Basser PJ. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multi-channel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J. The Cerebellum and Cognition. San Diego: Academic Press; 1997. [Google Scholar]
- Schmahmann J. The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. The Journal of comparative neurology. 1989;289:53–73. doi: 10.1002/cne.902890105. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The Cerebellum and Cognition. San Diego: Academic Press; 1997. pp. 31–60. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44:1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring E, Rohlfing T, Pfefferbaum A, Sullivan EV. White Matter Fiber Degradation Attenuates Hemispheric Asymmetry When Integrating Visuomotor Information. Journal for Neuroscience. 2010 doi: 10.1523/JNEUROSCI.2160-10.2010. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcoholism: Clinical and Experimental Research. 2001;25:924–934. [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Shear PK, Butters N, Jernigan TL, DiTraglia GM, Irwin M, Schuckit MA, Cermak LS. Olfactory loss in alcoholics: correlations with cortical and subcortical MRI indices. Alcohol (Fayetteville, NY. 1992;9:247–255. doi: 10.1016/0741-8329(92)90061-e. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Renshaw PF, Demicheli E, Wurtman R, Pillay SS, Cohen BM. Choline ingestion increases the resonance of choline-containing compounds in human brain: an in vivo proton magnetic resonance study. Biol Psychiatry. 1995;37:170–174. doi: 10.1016/0006-3223(94)00120-R. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No 34. Bethesda, MD: National Institutes of Health; 2000. pp. 473–508. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism, clinical and experimental research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Magnetic resonance relaxometry reveals central pontine abnormalities in clinically asymptomatic alcoholic men. Alcoholism, clinical and experimental research. 2001;25:1206–1212. [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, clinical and experimental research. 2000a;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch, and stance on cerebellar vermian-related sway and tremor: A quantitative MRI and physiological study. Cereb Cortex. 2006a;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006b;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology. 2010a;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A. Alcohol's effects on brain and behavior. Alcohol Research & Health. 2010b;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Lim KO, Marsh L, Pfefferbaum A. Patterns of regional cortical dysmorphology distinguishing schizophrenia and chronic alcoholism. Biol Psychiatry. 1998;43:118–131. doi: 10.1016/S0006-3223(97)00264-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology. 2000b;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A. Effects of alcohol dependence comorbidity and anti-psychotic medication on volumes of the thalamus and pons in schizophrenia. Am J Psychiatry. 2003;160:1110–1116. doi: 10.1176/appi.ajp.160.6.1110. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006a;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006b;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung E, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Bertolino A, Righini A, Campbell G, Raman R, Duyn JH, Moonen CTW, Alger JR, Di Chiro G. Brain regional distribution pattern of metabolite signal intensities in young adults by proton magnetic resonance spectroscopic imaging. Neurology. 1995;45:1384–1391. doi: 10.1212/wnl.45.7.1384. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J. 2010;39:527–540. doi: 10.1007/s00249-009-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M. The irrelevance of mammillary body lesions in the causation of the Korsakoff amnesic state. Int J Neurol. 1987;21-22:51–57. [PubMed] [Google Scholar]
- Victor M, Adam RD, Mancall EL. A restricted form of cerebellar degeneration occurring in alcoholic patients. Arch Neurol. 1959;1:577–688. [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- Wijnia JW, Goossensen A. Cerebellar neurocognition and Korsakoff's syndrome: an hypothesis. Medical hypotheses. 2010;75:266–268. doi: 10.1016/j.mehy.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Wilson W. The Big Book. Fourth 2001. [Google Scholar]
- Witt K, Nuhsman A, Deuschl G. Dissociation of habit-learning in Parkinson's and cerebellar disease. Journal of cognitive neuroscience. 2002;14:493–499. doi: 10.1162/089892902317362001. [DOI] [PubMed] [Google Scholar]
- Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. Neuroimage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


