Abstract
Specific motor symptoms of Parkinson’s disease (PD) can be treated effectively using direct electrical stimulation of deep nuclei in the brain. However, this is an invasive procedure, and the fraction of eligible patients is rather low according to currently used criterion. Spinal cord stimulation (SCS), a minimally invasive method, has more recently been proposed as a therapeutic approach to alleviate PD akinesia, in light of its proven ability to rescue locomotion in rodent models of PD. The mechanisms accounting for this effect are unknown but from accumulated experience using SCS in the management of chronic pain, it is known that the pathways most likely activated by SCS are the superficial fibers of the dorsal columns. We suggest that the prokinetic effect of SCS is due to direct activation of ascending pathways reaching thalamic nuclei and the cerebral cortex. The afferent stimulation may, in addition, activate brain stem nuclei contributing to initiation of locomotion. Based on the striking change in the cortico-striatal oscillatory mode of neuronal activity induced by SCS, we propose that through activation of lemniscal and brainstem pathways, the locomotive increase is achieved by disruption of antikinetic low frequency (< 30 Hz) oscillatory synchronization in the cortico-basal ganglia circuits.
Keywords: spinal cord, electrical stimulation, dorsal column, Parkinson’s disease
Introduction
Parkinson’s disease, estimated to afflict more than six million people all over the world, is a neurodegenerative disorder which severely impacts the lives of both patients and their families. The chronic and progressive nature of the disorder hampers the efficacy of available pharmacological symptomatic treatments for long-term use. For example, one of the best pharmacological treatments available, L-DOPA, exhibits decreased effectiveness over years of use, expressed as motor fluctuation and dyskinesias (Olanow & Obeso, 2000; Rascol et al., 2000; Ahlskog & Muenter, 2001). In the last two decades, direct electrical stimulation of subcortical nuclei in the brain, generically known as deep brain stimulation (DBS) have advanced and expanded as a therapeutic approach for a variety of neurological conditions (reviewed by Perlmutter & Mink, 2006). In particular, high-frequency electrical stimulation of sub-thalamic nucleus has proven very effective in alleviating certain motor symptoms of PD (Benabid et al., 2009). In controlled randomized trials, sub-thalamic DBS appears to be the most effective PD symptomatic treatment available; nevertheless, it also appears to have a significant increased risk of serious adverse effects (Weaver et al., 2009). DBS requires chronic implantation of lead electrodes inserted several centimeters deep in the brain and is associated with an increased risk of intracranial hemorrhage (~4% of the patients), although only a fraction of these cases present severe sequelae (Kleiner-Fisman et al., 2006). Other side effects, such as infection, device dysfunction or electrode migration, occur with most of the techniques involving direct electrical microstimulation of intra or extra-cranial locations. Currently, the selection criteria restrict the fraction of DBS eligible patients to only 1.6-4.5% (Morgante et al., 2007). Spinal cord stimulation (SCS), a less invasive and therefore potentially more accessible approach involving electrical stimulation, was recently proposed by our laboratory as a complement for PD treatment. Indeed, epidural electrical stimulation of the dorsal spinal cord was shown to restore the locomotive ability in hypokinetic chronic and acute PD rodent models. If this approach turns out to be equally effective in humans it may well become an important complement to currently used therapeutic strategies for many patients. In this review we will discuss possible neural mechanisms explaining how SCS promotes locomotion in akinetic Parkinsonian animals. Firstly, we will briefly describe the main neural circuits known to be involved in the generation of PD motor symptoms. Secondly, we refer to the electrophysiological hallmarks of PD, especially the oscillatory neural activity patterns related to movement and akinetic states. Finally, we will further develop the concept of the effect of SCS on neural oscillatory patterns and how this could contribute to restoration of locomotive activity in akinetic states related to dopamine depletion.
1.1 Basal ganglia circuits and their involvement in PD motor symptoms
Basal ganglia are a group of subcortical nuclei that contribute to the generation of goal-directed behavior by integrating sensory, cognitive and executive information (reviewed by Haber, 2003). The cerebral cortex, thalamus and brainstem send glutamatergic projections to the basal ganglia’s input nucleus, the striatum. The striatal projection neurons, the medium spiny neurons (MSNs), send inhibitory projection in two rather segregated pathways that express molecular differences. In the direct pathway, MSNs preferentially express D1 dopamine receptors and project directly to the basal ganglia output nuclei, the substantia nigra pars reticulate (SNr), globus pallidus pars interna (GPi) and ventral pallidum. The indirect pathway corresponds to a second group of MSNs expressing predominantly D2 receptors; these neurons project to the globus pallidus pars externa (GPe) which has inhibitory connections to the subthalamic nucleus (STN) that, in turn, sends glutamatergic projections to the pallidal output nuclei. Thus, pallidal output nuclei are subjected to inhibition by activation of the direct pathway, and to activation through the indirect pathway. Since the output nuclei have a tonic inhibitory activity, their inhibition activates their targets, while their activation further inhibits them. The two targets that receive major projections from the pallidal nuclei are the brainstem and thalamic nuclei. Sometimes the same pallidal neurons bifurcate to both structures. Different ways to understand the control of motor function have been proposed based on which of these two structures is emphasized in the brain circuit. One model proposed by Albin in 1989, emphasizes the thalamic nuclei as receptors of pallidal projections. Thus, the activation of the direct pathway would relieve the pallidal inhibition of thalamic nuclei, causing facilitation of thalamocortical activity, and hence cortically directed movement. On the other hand, the activation of the indirect pathway would cause termination or prevention of movement (Albin et al., 1989). This model has received major attention in PD research, where motor symptoms have been considered to arise from the imbalance of activity in the direct/indirect pathways (decrease of the direct, or increase of the indirect pathway) due to a lack of the modulatory influence of dopamine in the striatum. An alternative model emphasizes the brainstem as the receptor of pallidal projections, and using the same concept of activation of direct/indirect pathways, formulates the key motor role of basal ganglia as the selective initiation and termination of stereotyped or rhythmic motor sequences, typically controlled by brainstem and spinal cord circuits, often referred to as motor programs (reviewed by Mink, 1996; Hikosaka et al., 2000; Grillner et al., 2005).
Regardless of emphasizing thalamic or brainstem output, the integrity of the massive dopaminergic projections from the substantia nigra pars compacta (SNc) to the sensorimotor striatum still appears to be crucial for the proper initiation and execution of volitional motor actions. Indeed, postmortem analysis of PD patients shows extensive neurodegeneration of the nigrostriatal pathways, as observed for the first time in 1919 by Tretiakoff (reviewed by Fahn, 2003). Although PD is the result of a complex neurodegenerative process, many of the motor symptoms can be attributed to the lack of dopamine in the striatum, as suggested by the fact that any intervention aimed at directly or indirectly reducing the amount of striatal dopamine [ i.e. chronic chemical lesioning of dopaminergic neurons (Mendez & Finn, 1975; Burns et al., 1983); severe inhibition of dopamine synthesis (Costa et al., 2006); genetically targeted dopaminergic mitochondrial injury (Ekstrand et al., 2007); transgenic or viral vector induced over-expression of alpha-synuclein (Masliah et al., 2000; Kirik et al., 2002) ] will reproduce the cardinal symptoms of hypokinesia and rigidity observed in PD.
In the two models previously described, the lack of dopamine is considered to affect the physiological processing in these circuits mainly by altering the firing rate modulation of the involved neuronal populations. However, in the last two decades, both in human and animal models, extensive evidence of oscillatory unitary and population activity has been collected in all parts of the basal ganglia, raising the possibility of a specific role of these alternating activity patterns (for a review see Hammond et al., 2007).
2.1 Synchronization and oscillatory neuronal activity in PD
A common finding when recording neural activity in animal models of PD is an excessive synchronization of neuronal activation occurring periodically in an oscillatory fashion in practically all areas of the cortico-basal ganglia circuits (Bergman et al., 1994; Nini et al., 1995; Sharott et al., 2005; Costa et al., 2006). Similar oscillatory patterns have also been observed in PD patients when electrophysiological recordings have been obtained in conjunction with deep brain stimulation (DBS) electrode implantations (Levy et al., 2000; Levy et al., 2002). Indeed, synchronization as measured by the power of local field potential (LFP) signals in the 8-35 Hz frequency bands has been shown to directly correlate with the severity of motor symptoms in PD patients (Kuhn et al., 2006). In accordance with these data, both dopamine replacement therapy and DBS reduce the amplitude of low frequency LFP oscillatory activity in the basal ganglia along with clinical improvements (Brown et al., 2001; Brown et al., 2004, however see also Rossi et al., 2008). Consequently, great effort has recently been directed toward identifying the key mechanisms underlying synchronization of neuronal activity in basal ganglia circuits (Hammond et al., 2007).
2.2 The filtering function of the striatum
A prerequisite for signal transmission through the basal ganglia pathways is that the MSNs are switched to an active state, more likely to discharge when receiving input from other brain areas. The MSNs are normally maintained in a hyperpolarized state by inward rectifying K+ channels that remain open under hyperpolarized conditions, but a more depolarized ‘upstate’ can be induced by strong and synchronized input to MSNs via the cortical and the thalamic glutamatergic projections. In the upstate, additional excitatory drive might cause the neurons to reach spiking threshold leading to a period of spiking activity. The high activation threshold of MSNs, requiring convergent and synchronized input from many areas, implies that the majority of signals from different cortical areas are effectively filtered out. Nonetheless, stronger and synchronized cortical signals have the ability to pass through the striatum to the various output nuclei of the basal ganglia and eventually trigger motor actions. Other important components in the striatal filtering function are interconnectivity and network dynamics between groups of local interneurons, and the direct synaptic connections between MSNs. Complex feedforward and feedback signals in the network with either depolarizing or hyperpolarizing effects depending on the state of the target cell (Plenz, 2003), have been observed and are assumed to be part of the selection process leading to activation of specific subgroups of MSNs. In addition to the excitatory drive via thalamic and cortical inputs and local modulation by GABAergic and cholinergic neurons within the striatum, the MSNs activity is also modulated by the dopaminergic projection from the SNc. The dopaminergic input to MSNs is subjected to variations depending on external stimuli; salient external events leading to periods of fast spiking in SNc neurons give rise to a transient but substantial release of dopamine in the striatum, thus enabling activation of low affinity D1 receptors. D1-activation has been shown to alter the response of MSNs to glutamatergic input making the cells more likely to make the state transition from down- to upstate and to reach spiking threshold (Surmeier et al., 2007). On the other hand, in the absence of significant external stimuli, D1-activation is less likely to occur due to the lower dopamine levels, while activation of the higher affinity D2 receptors may still take place. D2-activation reduces the excitability of MSNs expressing this receptor type (Surmeier et al., 2007), which probably contributes to make the two-step transition (from the down- to the upstate and subsequently from the upstate to actual spiking) a rarely occurring event.
2.3 Altered signal transduction after loss of dopaminergic input
In contrast to the normal physiological state encompassing episodes of transient dopamine increase, during a period of dopamine depletion or following chronic lesions to the nigrostriatal pathway, decoupled upstates in individual MSNs are less frequent and instead the spiking of MSNs tends to occur time-locked to depolarizing phases of local field potential oscillations, reflecting an altered response to thalamic and cortical glutamatergic input (Tseng et al., 2001; Costa et al., 2006). The strong entrainment of spiking activity to population activity essentially shapes the basal ganglia output patterns towards the periodic oscillatory input patterns typical of thalamocortical networks. Due to the strong feedback through the basal ganglia-thalamocortical loop, even minor synchronization tendencies can potentially be rapidly amplified (Tseng et al., 2001). Besides intracellular changes in MSN excitability, basal ganglia oscillatory activity in the dopamine depleted state is probably also induced by mechanisms on a network level. For example, in PD patients and animal models of PD, the firing pattern of neurons in the STN is frequently altered so that the firing of individual neurons is changed from an irregular pattern to rhythmic bursts that occur coherently with cortical LFP oscillations below 30 Hz (Bevan et al., 2006). This pattern might be induced by oscillatory activity being passed from the striatum through the indirect pathway, or by alternating states in cortical ensembles influencing the STN through direct connections. Because alternating activity patterns are faithfully transmitted by the STN to target neurons in globus pallidus, SNr and the PPN, the STN is thought to have a particularly important role as a generator of rhythmic output patterns that potentially interfere with normal signal relay (Wichmann et al., 1994).
2.4 Oscillatory activity and akinesia
It can be concluded that network mechanisms and cellular properties of the basal ganglia neurons may cause synchronization of neuronal activity, and the available evidence suggests a pathogenic role of the resulting low-frequency oscillations. Yet, it is important to note that the co-occurrence of synchronous oscillatory activity with motor symptoms does not necessarily implicate a causal link. Actually, low-frequency oscillations in corticostriatal circuits as well as in cortico-spinal motor systems have been suggested to have important physiological functions (Baker et al., 2003; Courtemanche et al., 2003). To test the hypothesis of a pathogenic role of the neuronal synchronization in PD, it is necessary to compare the neural activity patterns during normal and Parkinsonian states, and also during different behaviors. Neural data collected during different types of spontaneous behavior in an inducible PD-model showed that powerful low-frequency oscillations are indeed a normal physiological pattern during inactive or resting state conditions in the motor corticostriatal circuits (Fuentes et al., 2009). However, when comparing control animals with dopamine-depleted animals during both active and resting periods it was shown that the entrainment of striatal spiking to LFP was particularly strong in the dopamine-depleted state. Yet, during the rare instances when these otherwise akinetic animals actually displayed locomotion, the degree of spike-LFP entrainment was reduced to control levels. These experiments also showed that spontaneous initiation of locomotion (not triggered by any evident external input) is accompanied by changes in LFP oscillations, powerful low-frequency (~1-20 Hz) activity decreases, and increases in power at higher frequencies (30-55 Hz). Remarkably, this change in oscillatory pattern preceded the onset of movement by a few seconds, suggesting that the absence of strong synchronization at low-frequencies in the cortico-basal ganglia circuit is a prior necessary condition to initiate movement. Consistent with the concept of a synchronization-induced akinetic state, it was recently shown that slowing of initiation of voluntary movements can be experimentally induced in patients by the use of low frequency STN-DBS (instead of the higher frequencies used in therapeutic DBS) (Timmermann et al., 2004; Chen et al., 2007).
2.5 Locomotive aspects in PD
A better understanding of mechanisms underlying locomotive impairments in PD-patients would be advantageous in linking the electrophysiological data obtained from rodent PD-models to pharmacological data since hypokinetic symptoms affecting this motor behavior are easily detected and scored in these animal models.
Fluid bipedal ambulation is severely affected in PD, especially at the late stages of the disease (Narabayashi, 1993). Freezing of gait, probably the least understood symptom of PD (Okuma, 2006), is defined as “an episodic inability (lasting seconds) to generate effective stepping in the absence of any known cause other than parkinsonism or high-level gait disorders. ” (Giladi & Nieuwboer, 2008). Freezing of gait is significantly associated with festinating gait (Giladi et al., 2001), a peculiar locomotive symptom , described originally by James Parkinson himself in 1817 along with the cardinal PD symptoms of bradykinesia, rigidity and resting tremor. During festinating gait, the patient’s strides become progressively shorter and quicker, bringing the patient off balance and increasing the risk for falls. The pathophysiology of freezing of gait is poorly understood although studies suggest that it transcends the basal ganglia circuit, involving the pedunculopontine nucleus (PPN) (Zweig et al., 1989), parietal-lateral premotor circuits, and orbitofrontal-striatal loops reviewed by (Bartels & Leenders, 2008). The neural correlate of freezing of gait has been elusive to study since this symptom is episodic and unpredictable (Giladi & Nieuwboer, 2008) and because of the limited availability to neurophysiological data. A combination of two currently available experimental tools in non-human primate models could provide a solution. The neural correlate can be obtained using chronic multi-structure electrophysiological recordings in behaving animals (Nicolelis et al., 2003), while detailed quantitative and qualitative aspects of locomotive behavior can be acquired through video image analysis (Fitzsimmons, 2009; Liu et al., 2009).
As pointed out earlier, locomotive activity is severely affected in rodents with parkinsonian lesions. This impairment has recently been directly linked to differential activation of basal ganglia circuits using optogenetic tools. Selective activation of the basal ganglia indirect pathway in normal mice resulted in a decreased frequency and duration of locomotive bouts, while activation of the direct pathway had the opposite effect. Further, in 6-OHDA lesioned mice, selective activation of the direct pathway restored locomotive activity to pre-lesion levels (Kravitz et al., 2010).
2.6 Basal ganglia oscillations and muscular resting tremor in PD
The most obvious tentative link between neuronal oscillatory activity and motor disabilities in the Parkinsonian state is the connection to resting tremor; a cardinal symptom of the disease. In a number of studies, a causal relation between basal ganglia oscillations and resting tremor has been suggested (Bergman et al., 1994; Hutchison et al., 1997). Indeed, basal ganglia oscillations are often found to occur at the same frequency (or at higher oscillation harmonics) as present in muscle tremor. However, a growing body of evidence does not support a causal relation. For example, a substantial fraction of PD-patients do not display resting tremor despite having pronounced oscillatory activity in parts of the basal ganglia and by the same token, tremor is not consistently displayed in all PD animal models (Bergman et al., 1998; Rivlin-Etzion et al., 2006; Rosin et al., 2007). Moreover, even in when central and peripheral oscillations are found to occur at roughly corresponding frequencies a causal link has not been firmly established (Raz et al., 2000; Hurtado et al., 2004).
2.7 Further studies in behaving animals will be needed to clarify PD pathophysiology
Clearly, from a theoretical standpoint it could be argued that the maximum amount of information transmittable in neuronal signals must be considerably reduced in highly synchronized states which could in certain situations compromise the functionality of the circuits involved (Hammond et al., 2007 and as suggested by the findings of Fuentes et al. 2009). But this line of reasoning can hardly explain all the different motor symptoms of PD. Further studies employing chronic recordings from different parts of the basal ganglia circuits in animal models of PD will be needed to help distinguish between normal and pathological neuronal activity patterns in different motor states (e.g. stereotypic movements, locomotion, resting) in cortico-basal ganglia networks.
3.1 Which structure should be stimulated to alleviate akinesia in PD?
Although DBS of the STN or the GPi is very effective in reducing dyskinetic symptoms in L-DOPA medicated patients, this intervention is less effective in reducing the major hypokinetic symptoms that essentially render advanced PD-patients unable to move, that is akinesia (‘freezing’) and postural instability (Plaha & Gill, 2005; Rodriguez-Oroz et al., 2005). It has been proposed that this differential effect may occur because electrical stimulation of these targets primarily alters the activity patterns in the basal ganglia output to thalamocortical motor areas but influences to a much less extent the direct connections to brain stem nuclei controlling locomotion and posture (Stein, 2009).
3.2 The role of the brain stem nuclei in control of locomotion
Almost half a century ago, influential experiments showed that locomotor programs in decerebrate animal preparations could be called into action by application of low intensity microstimulation in an area located in the posterior midbrain encompassing the cuneiform and the PPN (Shik et al., 1966). This area was hence physiologically defined as the mesencephalic locomotor region. In later studies it was argued that in primates, and in particular in humans, the PPN is probably particularly important for activation of locomotor programs (Garcia-Rill, 1986) as it has been shown to activate axial muscle groups that are crucial for control of locomotion and posture. The PPN, which is located dorsal to the substantia nigra, receives a large part of the descending projections from major basal ganglia output nuclei (GPi, and SNr) and also returns connections to these nuclei (Garcia-Rill, 1986; Stein, 2009). In late stages of PD, pronounced cell death - of the same magnitude as that of dopaminergic cells in SNc - has been observed in the cholinergic neurons of the PPN, and the severity of late symptoms (akinesia, postural instability) has been suggested to correlate with the gradual loss of these neurons (Zweig et al., 1989; Pahapill & Lozano, 2000). Additional evidence comes from studies in non-human primates where bilateral PPN lesions caused severe and sustained akinesia (Munro-Davies et al., 1999). Based on these findings, PPN-DBS has been tested in late stage PD-patients suffering from hypokinetic symptoms, and the first studies have shown encouraging results (Stefani et al., 2007; Ferraye et al., 2010; Moro et al., 2010). However, chronic brainstem electrical stimulation, involves risks of serious adverse effects, and in that respect a less invasive stimulation paradigm activating these circuits would be preferable.
3.3 SCS restores locomotion in dopamine depleted animals
SCS in humans has been practiced for at least 4 decades to manage intractable chronic pain. The “gate theory of pain” – published in 1965 by Melzack and Wall – stated that activation of large-diameter fibers of the spinal cord would close a “gate” at the dorsal horns, preventing transmission of the neural activity signaling pain to supraspinal structures (Melzack & Wall, 1965). Based on this concept, in 1967 SCS was used for the first time in patients for chronic pain management (Shealy et al., 1967; Wall & Sweet, 1967). Although other mechanisms are now thought to be responsible for the analgesic effects, SCS has continued to be an important means to treat a variety of chronic pain syndromes.
Using a similar stimulator design as in pain treatment, SCS was shown to restore locomotion in rats with 6-hydroxydopamine striatal lesions (Fuentes et al., 2009). Briefly, the rats received bilateral striatal 6-OHDA injections, which locally reduced the dopaminergic projections by 80%. SCS was applied as biphasic square pulses at 300 Hz causing the 6-OHDA rats to increase their locomotive behavior from a nearly akinetic state to more normal wandering in a circular open field. A similar experiment in acute dopamine depleted mice, where additionally, neural activity from sensorimotor striatum and primary motor cortex (M1) was recorded, showed similar results. Severe inhibition of dopamine synthesis in these mice reduced their striatal dopamine levels by 70% and caused akinesia and rigidity. In this state, neural activity in the striatum and M1, as analyzed from local field potentials, showed increased oscillatory power in the 1.5-4 and 10-15 Hz bands, and decreased power in the 25-55 Hz band. These frequency bands and their relative changes roughly correspond to those observed in PD patients and other animal models. The increase in the 1.5-4 Hz power partially matches the frequency range described in STN and GPi units that have also been associated with limb tremor [4-8 Hz (Bergman et al., 1994), 4-5 Hz (Hurtado et al., 1999), 3-6 Hz (Levy et al., 2002)]. Further, the increase in the 10-15 Hz band is in a similar frequency range to that of the beta band synchronic activity described in PD patients [15-30 Hz (Levy et al., 2002)], non-human primates [10 Hz (Nini et al., 1995)] and rodent models of PD [11-30 Hz (Costa et al., 2006)].
3.4 Spinal cord pathways activated by SCS
To understand the mechanisms by which SCS increases motor activity in the hypodopaminergic state, it is important to know which neural pathways are primarily influenced by this stimulation approach. Two key aspects have to be taken into account to estimate which pathways are activated by SCS: electrode position and current intensity used. In PD-SCS, the cathode and anode plates are located over the meninges covering the dorsal surface of the spinal cord, one on each side of the midline, separated by ~1 mm or less. The electrode plates are in contact with the epidural membrane and the closest spinal cord structures to the electrode surface are the dorsal columns. Stimulation intensity used in PD-SCS was 1.3 (mice) or 1.1 (rats) times the sensory threshold. A previous study - using computer modeling of SCS based on the conductivity, size and location of spinal structures (Struijk et al., 1992) - have concluded that the threshold current intensity to activate a spinal fiber increases exponentially with the fiber’s depth below the pia mater (Holsheimer, 2002), suggesting that low or moderate stimulation intensity is unlikely to activate fibers other than those close to the dorsal surface of the spinal cord. This, together with empirical data from SCS in human patients supports the notion that the spinal structures primarily activated by SCS using intensity within the therapeutic range (above sensory and below discomfort threshold) are the outer fibers of the dorsal columns and the dorsal roots (reviewed by Holsheimer, 2002).
3.5 Inducing cortical desynchronization to allow for initiation of movements
In very early experiments involving EEG-recordings from the human cortical surface it was noted that voluntary movements tended to block beta-range oscillations in cortical areas including and surrounding the precentral gyrus, much in the same way that the visual stimulation blocks alpha-range oscillations in the occipital cortex (Jaspers & Penfield, 1949). This finding was later corroborated in a series non-human primate studies using intracortical recordings of local field potentials and single unit activity (see e.g. Sanes & Donoghue, 1993). In many of these primate experiments it was also observed, however, that both during pre-movement periods when the animals were attentively awaiting a go-signal (Donoghue et al., 1998), and in situations where the animals were required to keep a steady precise grip holding a small object (Murthy & Fetz, 1992; Baker et al., 1999), oscillatory bursts in the high beta or gamma range (> 20 Hz) were often recorded in sensorimotor related cortical areas, perhaps indicating a physiological role of these oscillatory bursts in motor behaviors requiring extra attention (Murthy & Fetz, 1996). In contrast, oscillations in the lower part of the beta-band have more often been associated with resting or inattentive states (MacKay & Mendonca, 1995). In view of that, it has been hypothesized that the strong coherent beta oscillation seen in motor cortex in PD could perpetuate the inactive state, preventing the state transition necessary for initiation of voluntary movements (Brown, 2003; see also Marceglia et al., 2006 for a discussion on high vs. low beta-range oscillations in PD-patients). Along these lines of reasoning the idea has been put forward that an important mechanism of STN DBS may be the direct antidromic activation of cortical pyramidal cells, which by interfering with low-frequency cortical oscillations, facilitate the change into an active desynchronized state (Li et al., 2007). This concept has gained additional support from studies in non-human primates and patients using direct stimulation of motor cortex to alleviate Parkinsonian symptoms (Canavero et al., 2002; Drouot et al., 2004), and also from a recent study utilizing selective expression of light activated ion-channels (thereby eliminating the concern for uncontrolled current spread in the tissue) proving that stimulation of the cortical afferents to the STN, rather than the cells within the nucleus, is required for motor improvements in rodent PD models (Gradinaru et al., 2009). The classical and considerably less invasive method to induce cortical desynchronization is however through stimulation of afferent pathways. Signals ascending through the thalamocortical projections and to some extent through the reticular formation can rapidly induce a switch to a desynchronized state (Adrian & Matthews, 1934; Chatrian et al., 1960). This approach, aimed at therapeutic interference with cortical synchronization, has in fact proven to reduce massive aberrant synchronous activity during epileptic seizures in both animal models (Fanselow et al., 2000) and in pilot clinical studies (DeGiorgio et al., 2003; DeGiorgio et al., 2006). These findings evidently provided a rationale for evaluating the effect of afferent stimulation in PD models. As it turned out, electrical stimulation of peripheral nerves (trigeminal) or other modes of stimulation aimed at inducing a strong arousal response (air-puffs) were not sufficient to promote locomotion in the Parkinsonian animals. The alleviation of akinesia was only seen when directly stimulating the afferent pathways within the dorsal columns of the spinal cord. Thus, synchronous activation of a number of tactile afferent fibers terminating in the dorsal column nuclei and ascending through the lemniscal pathway to cortical areas through the thalamus appears to be required in this model in order to unlock the basal ganglia-cortical circuits and make the transition into a state permitting initiation of locomotion. In this context it is also worth noting that the thalamic nuclei most directly activated by SCS differ from those primarily affected by STN-/GPi-DBS (ventral posterior as opposed to ventral anterior).
However, bearing in mind that anatomical tracer studies indicate that some afferents from the cervical and thoracic spinal cord dorsal horns project directly to PPN and that descending fibers from the PPN project directly to the spinal cord (Jenkinson et al., 2009), a further mechanism contributing to the recovery of locomotive capability in hypodopaminergic conditions could be the activation of this and related brainstem nuclei through ascending and descending connections to the spinal cord. Activating the PPN via ascending pathways would in turn have a dual effect; firstly to directly facilitate the initiation of movement by descending drive to locomotor circuits and secondly, to do this indirectly through ascending thalamocortical pathways by activation and desynchronization of cortical motor areas and structures within the basal ganglia (Hikosaka, 1991; Jenkinson et al., 2009).
3.6 Combination of dopamine replacement therapy and SCS
In the PD-SCS study it was reported that combined treatment with SCS and L-DOPA was superior to L-DOPA alone. On the other hand, SCS in severely dopamine depleted animals (where dopamine levels were decreased to < 1% of normal levels) could not restore locomotive capability without a small L-DOPA dose (20% of the amount required with L-DOPA alone). These data indicate that a minimum amount of dopamine is indeed required for alleviation of akinesia by SCS. This is perhaps not surprising given that dopaminergic projections are known to exist to practically all motor structures thought to regulate locomotor behavior; i.e. spinal cord, brain stem nuclei, thalamus, cortex and basal ganglia (Qu et al., 2006; Bjorklund & Dunnett, 2007; Smith & Villalba, 2008). When evaluating the mechanisms of therapeutic electrical stimulation, the focus is usually on the electrophysiological changes; however considering the crucial role of dopamine in the basal ganglia circuits it is clearly of interest to further investigate the relationship between dopamine release and changes in neuronal activity patterns. For example, it is feasible that electrical stimulation of the targets used to treat PD acts partially by boosting release of intracellular pools of dopamine in relevant brain circuits. Support for a mechanistic connection to dopamine release of this kind was presented in a recent study showing striatal release of dopamine in response to high frequency stimulation of the STN in pigs (Shon et al., 2010) and related findings have been reported for electrical stimulation of peripheral nerves in cats (Inoue et al., 2004). A better understanding of how to optimally combine dopamine replacement therapy and electrical stimulation will be a very important future goal in order to develop better strategies to alleviate motor symptoms in PD. Another interesting possibility is that a neuroprotective effect may be achieved by electrical stimulation alone or in combination with pharmacological treatments that potentially could significantly delay the progression of the disease (Gubellini et al., 2009).
3.7 Translating animal studies to novel therapies- the devil is in the details
Shortly after the publication of the prokinetic effect of SCS in PD rodent models (Fuentes et al., 2009), a study reported that SCS was ineffective when tested in PD patients (Thevathasan et al., 2010). In this study, longitudinal lead electrodes were implanted at a high cervical position (Insola et al., 2008) in two PD patients and stimulation was performed at similar frequencies to those used in the rodent study. No improvement of symptoms was observed as assessed with the motor subsection of the Unified Parkinson’s Disease Rating Scale. Possible reasons for the lack of therapeutic effect in this study, which sharply contrasts the findings in the rodent study, can be linked to the stimulation method; specifically to i) the geometry of the stimulation electrode (Correspondence to Neurology, In Press) and ii) the spinal level of the implant.
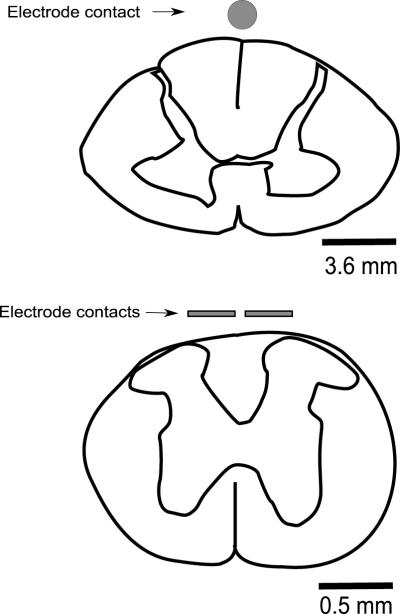
Geometry of electrodes. The electrode used by Thevathasan et al. (2010) makes contact in a longitudinal (rostral-caudal axis) display over the spinal cord, thus when a bipolar configuration is used, the effect is restricted to a narrow elongated area between the contacts. The electrode contacts used in the rodent study had a transversal configuration with a flat contact surface which allowed for coverage of most of the dorsal surface of the rodent spinal cord. Considering the configurati1on of the poles (transversal vs. longitudinal), the shape (flat vs. cylindrical) and the relative size, the electrodes used in the rodent study are estimated to have a contact surface 4 to 7 times larger than the longitudinal lead electrodes used in the human study when compared in a transversal section (Fig. 1), suggesting a significant difference in the number of dorsal column fibers recruited during the stimulation with the two methods. An even more detailed comparison of the respective electrode designs, taking into consideration parameters such as distance between poles and thickness of the cerebrospinal fluid layer at stimulation site, are beyond the scope of this review, but can be modeled with precision with computational tools (Struijk et al., 1991; Holsheimer, 1998).
Figure 1.
Transversal section comparing the contact area of the longitudinal lead electrodes used in humans at cervical level (top) and the electrodes used for SCS in rodent PD models at high thoracic level (bottom). Both drawings are in different scales but are sized equally for comparative purposes.
Spinal level of stimulation. While the human study stimulated the high cervical level, the rodent study stimulated the high thoracic level. It can be argued that the more rostral cervical location could potentially recruit more fibers than a lower location, since the total number of fibers in the ascending tract is higher. However, data gathered from patients with spinal stimulators used for chronic pain conditions (Barolat et al., 1993), show that stimulation at a high cervical level recruits preferentially dorsal column sensory fibers from upper limbs and chest, and very seldom recruits fibers from the lower half of the body (Holsheimer & Barolat, 1998), as indicated by the high probability of paresthesias induced in these areas (Fig. 2). Stimulation at a high thoracic level, where the SCS has been shown to successfully restore locomotion in the rodent study, has a nearly equitable chance to recruit fibers from the upper limbs, torso and lower limbs, respectively (Fig. 2), which suggests that stimulation at this level could in fact activate a greater number of dorsal column fibers.
Figure 2.
Probability of paresthesia in different body areas as a function of the vertebral level location of the stimulation electrode (from Holsheimer, J. & Barolat, G. : Spinal Geometry and Paresthesia Coverage in Spinal Cord Stimulation. Neuromodulation, 1: 129-136, 1998).
Overall, the differences in geometry, the relative size of the electrodes and perhaps also the spinal level at which stimulation is delivered, all suggest that the method used in the rodent study likely recruits more dorsal column fibers than the method used in the human study and could, as a consequence, have a better chance of inducing the required changes in oscillatory brain activity to allow for volitional movement initiation according to the mechanisms discussed in this review.
The concept of SCS having a positive effect on the symptoms of neurological motor impairment has been proved in upper motor neuron disorder (Dimitrijevic et al., 1980) and orthostatic tremor (Krauss et al., 2006). Further, the positive results obtained in rodent models of PD (Fuentes et al., 2009), and more recently the replication of this effect in a non-human primate model (unpublished results Fuentes et al.) are encouraging evidence for the potential benefits of this technique in PD. Yet, the early negative results of SCS in PD patients (Thevathasan et al., 2010) stress the need for further studies in animal models before the translation of this method to clinical practice.
Conclusions
Currently available empirical and modeling data suggest that the effect of SCS is mediated chiefly by dorsal column activation; nonetheless the data do not allow us to disambiguate the paths followed by this signal in supraspinal structures. Two candidate structures for explaining the locomotive increase are the PPN and/or the ventroposterior lateral thalamic nucleus (VPL), both of which affect cortical and basal ganglia activity. Whether the effect is achieved by changes in the firing rates or involves the modulation of oscillatory patterns, as suggested by the observed synchrony changes, must be determined by further studies. Nonetheless, considering the cumulative evidence relating akinesia with specific neural oscillatory regimes, it is attractive to suggest that SCS, via either route, is unlocking the cortico-basal ganglia circuit from the strong low-frequency (1-30 Hz) synchronous activity considered ‘antikinetic’ by many researchers within the field (Brown, 2003; Hutchison et al., 2004). This unlocking allows the circuits to enter a state which is more receptive to cortical input and thus permissive of initiation of volitional movement sequences.
Acknowledgements
We thank S. Halkiotis for outstanding assistance proofreading the manuscript. Research in our laboratory was supported by the National Institute of Neurological Disorders and Stroke (NINDS) R33NS049534 and the International Neuroscience Network Foundation to M.A.L.N., Ruth K. Broad Postdoctoral Award to R.F., and NRC, VR and Olle Engkvist Foundation to P.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
Abbreviations
- DBS
deep brain stimulation
- GPi
globus pallidus pars interna
- MSN
medium spiny neuron
- PD
Parkinson’s disease
- PPN
pedunculopontine nucleus
- SCS
spinal cord stimulation
- STN
subthalamic nucleus
- VPL
ventroposterior lateral thalamic nucleus
References
- Adrian ED, Matthews BH. The interpretation of potential waves in the cortex. J Physiol. 1934;81:440–471. doi: 10.1113/jphysiol.1934.sp003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. effect of oscillatory activity on corticospinal output. J Neurophysiol. 2003;89:1941–1953. doi: 10.1152/jn.00832.2002. [DOI] [PubMed] [Google Scholar]
- Barolat G, Massaro F, He J, Zeme S, Ketcik B. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78:233–239. doi: 10.3171/jns.1993.78.2.0233. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord. 2008;23(Suppl 2):S461–467. doi: 10.1002/mds.21912. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord. 1998;13(Suppl 3):29–34. doi: 10.1002/mds.870131305. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr Opin Neurobiol. 2006;16:621–628. doi: 10.1016/j.conb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol. 2004;188:480–490. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S, Paolotti R, Bonicalzi V, Castellano G, Greco-Crasto S, Rizzo L, Davini O, Zenga F, Ragazzi P. Extradural motor cortex stimulation for advanced Parkinson disease. Report of two cases. J Neurosurg. 2002;97:1208–1211. doi: 10.3171/jns.2002.97.5.1208. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Petersen MC, Uihlein A. Electrical stimulation of the human brain through implanted electrodes: preliminary observations. Dis Nerv Syst. 1960;21:321–326. [PubMed] [Google Scholar]
- Chen CC, Litvak V, Gilbertson T, Kuhn A, Lu CS, Lee ST, Tsai CH, Tisch S, Limousin P, Hariz M, Brown P. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp Neurol. 2007;205:214–221. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia. 2006;47:1213–1215. doi: 10.1111/j.1528-1167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Shewmon DA, Whitehurst T. Trigeminal nerve stimulation for epilepsy. Neurology. 2003;61:421–422. doi: 10.1212/01.wnl.0000073982.42650.57. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Dimitrijevic MM, Sherwood AM, Faganel J. Neurophysiological evaluation of chronic spinal cord stimulation in patients with upper motor neuron disorders. International rehabilitation medicine. 1980;2:82–85. doi: 10.3109/09638288009163962. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, Dauguet J, Lefaucheur JP, Dolle F, Conde F, Bottlaender M, Peschanski M, Keravel Y, Hantraye P, Palfi S. Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron. 2004;44:769–778. doi: 10.1016/j.neuron.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Reid AP, Nicolelis MA. Reduction of pentylenetetrazole-induced seizure activity in awake rats by seizure-triggered trigeminal nerve stimulation. J Neurosci. 2000;20:8160–8168. doi: 10.1523/JNEUROSCI.20-21-08160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardes S, Pollak P. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133:205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons L, Peikon Nicolelis. Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity Front. Integr. Neurosci. 2009;3 doi: 10.3389/neuro.07.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science. 2009;323:1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. 1986;396:47–63. [PubMed] [Google Scholar]
- Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Rozenberg E, Shabtai E. Gait festination in Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:135–138. doi: 10.1016/s1353-8020(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Salin P, Kerkerian-Le Goff L, Baunez C. Deep brain stimulation in neurological diseases and experimental models: from molecule to complex behavior. Prog Neurobiol. 2009;89:79–123. doi: 10.1016/j.pneurobio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia--possible role in motor coordination and learning. Curr Opin Neurobiol. 1991;1:638–643. doi: 10.1016/s0959-4388(05)80042-x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological reviews. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531–540. doi: 10.1038/sj.sc.3100717. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Which Neuronal Elements are Activated Directly by Spinal Cord Stimulation. Neuromodulation. 2002;5:6. doi: 10.1046/j.1525-1403.2002._2005.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Barolat G. Spinal Geometry and Paresthesia Coverage in Spinal Cord Stimulation. Neuromodulation. 1998;1:129–136. doi: 10.1111/j.1525-1403.1998.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Hurtado JM, Gray CM, Tamas LB, Sigvardt KA. Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc Natl Acad Sci U S A. 1999;96:1674–1679. doi: 10.1073/pnas.96.4.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado JM, Rubchinsky LL, Sigvardt KA. Statistical method for detection of phase-locking episodes in neural oscillations. J Neurophysiol. 2004;91:1883–1898. doi: 10.1152/jn.00853.2003. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Lozano AM, Tasker RR, Lang AE, Dostrovsky JO. Identification and characterization of neurons with tremor-frequency activity in human globus pallidus. Exp Brain Res. 1997;113:557–563. doi: 10.1007/pl00005606. [DOI] [PubMed] [Google Scholar]
- Inoue M, Katsumi Y, Hayashi T, Mukai T, Ishizu K, Hashikawa K, Saji H, Fukuyama H. Sensory stimulation accelerates dopamine release in the basal ganglia. Brain Res. 2004;1026:179–184. doi: 10.1016/j.brainres.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Insola A, Padua L, Mazzone P, Valeriani M. Unmasking of presynaptic and postsynaptic high-frequency oscillations in epidural cervical somatosensory evoked potentials during voluntary movement. Clin Neurophysiol. 2008;119:237–245. doi: 10.1016/j.clinph.2007.09.132. [DOI] [PubMed] [Google Scholar]
- Jaspers H, Penfield W. Electrocorticograms in man: effect of the voluntary movement upon the electrical activity of the precentral gyrus. Arch. Psychiatr. Z Neural. 1949;183:163–174. [Google Scholar]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24:319–328. doi: 10.1002/mds.22189. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, Lang AE, Deuschl G. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Weigel R, Blahak C, Bazner H, Capelle HH, Grips E, Rittmann M, Wohrle JC. Chronic spinal cord stimulation in medically intractable orthostatic tremor. J Neurol Neurosurg Psychiatry. 2006;77:1013–1016. doi: 10.1136/jnnp.2005.086132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010 doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Liu N, Yue F, Tang WP, Chan P. An objective measurement of locomotion behavior for hemiparkinsonian cynomolgus monkeys. J Neurosci Methods. 2009;183:188–194. doi: 10.1016/j.jneumeth.2009.06.037. [DOI] [PubMed] [Google Scholar]
- MacKay WA, Mendonca AJ. Field potential oscillatory bursts in parietal cortex before and during reach. Brain Res. 1995;704:167–174. doi: 10.1016/0006-8993(95)01109-9. [DOI] [PubMed] [Google Scholar]
- Marceglia S, Foffani G, Bianchi AM, Baselli G, Tamma F, Egidi M, Priori A. Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease. J Physiol. 2006;571:579–591. doi: 10.1113/jphysiol.2005.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mendez JS, Finn BW. Use of 6-hydroxydopamine to create lesions in catecholamine neurons in rats. J Neurosurg. 1975;42:166–173. doi: 10.3171/jns.1975.42.2.0166. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Morgante L, Morgante F, Moro E, Epifanio A, Girlanda P, Ragonese P, Antonini A, Barone P, Bonuccelli U, Contarino MF, Capus L, Ceravolo MG, Marconi R, Ceravolo R, D’Amelio M, Savettieri G. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat Disord. 2007;13:528–531. doi: 10.1016/j.parkreldis.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Munro-Davies LE, Winter J, Aziz TZ, Stein JF. The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Exp Brain Res. 1999;129:511–517. doi: 10.1007/s002210050921. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Narabayashi H. Three types of akinesia in the progressive course of Parkinson’s disease. Adv Neurol. 1993;60:18–24. [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Okuma Y. Freezing of gait in Parkinson’s disease. J Neurol. 2006;253(Suppl 7):VII27–32. doi: 10.1007/s00415-006-7007-2. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Obeso JA. Preventing levodopa-induced dyskinesias. Ann Neurol. 2000;47:S167–176. discussion S176-168. [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep Brain Stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–156. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16:629–637. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Rosin B, Nevet A, Elias S, Rivlin-Etzion M, Israel Z, Bergman H. Physiology and pathophysiology of the basal ganglia-thalamo-cortical networks. Parkinsonism Relat Disord. 2007;13(Suppl 3):S437–439. doi: 10.1016/S1353-8020(08)70045-2. [DOI] [PubMed] [Google Scholar]
- Rossi L, Marceglia S, Foffani G, Cogiamanian F, Tamma F, Rampini P, Barbieri S, Bracchi F, Priori A. Subthalamic local field potential oscillations during ongoing deep brain stimulation in Parkinson’s disease. Brain Res Bull. 2008;76:512–521. doi: 10.1016/j.brainresbull.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci U S A. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesthesia and analgesia. 1967;46:489–491. [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Shon YM, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, Bennet K, Blaha CD, Chang SY. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci Lett. 2010;475:136–140. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl 3):S534–547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Stein JF. Akinesia, motor oscillations and the pedunculopontine nucleus in rats and men. Exp Neurol. 2009;215:1–4. doi: 10.1016/j.expneurol.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Struijk JJ, Holsheimer J, van der Heide GG, Boom HB. Recruitment of dorsal column fibers in spinal cord stimulation: influence of collateral branching. IEEE Trans Biomed Eng. 1992;39:903–912. doi: 10.1109/10.256423. [DOI] [PubMed] [Google Scholar]
- Struijk JJ, Holsheimer J, van Veen BK, Boom HB. Epidural spinal cord stimulation: calculation of field potentials with special reference to dorsal column nerve fibers. IEEE Trans Biomed Eng. 1991;38:104–110. doi: 10.1109/10.68217. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Mazzone P, Jha A, Djamshidian A, Dileone M, Di Lazzaro V, Brown P. Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology. 2010;74:1325–1327. doi: 10.1212/WNL.0b013e3181d9ed58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson’s disease. Mov Disord. 2004;19:1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–109. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr., Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol. 1989;26:41–46. doi: 10.1002/ana.410260106. [DOI] [PubMed] [Google Scholar]