Abstract
Sonazoid (Daiichi Sankyo, Tokyo, Japan), a second-generation of a lipid-stabilized suspension of a perfluorobutane gas microbubble contrast agent, has been used clinically in patients with liver tumors and for harmonic gray-scale ultrasonography (US) in Japan since January 2007. Sonazoid-enhanced US has two phases of contrast enhancement: vascular and late. In the late phase of Sonazoid-enhanced US, we scanned the whole liver using this modality at a low mechanical index (MI) without destroying the microbubbles, and this method allows detection of small viable hepatocellular carcinoma (HCC) lesions which cannot be detected by conventional US as perfusion defects in the late phase. Re-injection of Sonazoid into an HCC lesion which previously showed a perfusion defect in the late phase is useful for confirming blood flow into the defects. High MI intermittent imaging at 2 frames per second in the late phase is also helpful in differentiation between necrosis and viable hypervascular HCC lesions. Sonazoid-enhanced US by the coded harmonic angio mode at a high MI not only allows clear observation of tumor vessels and tumor enhancement, but also permits automatic scanning with Sonazoid-enhanced three dimensional (3D) US. Fusion images combining US with contrast-enhanced CT or contrast-enhanced MRI have made it easy to detect typical or atypical HCC lesions. By these methods, Sonazoid-enhanced US can characterize liver tumors, grade HCC lesions histologically, recognize HCC dedifferentiation, evaluate the efficacy of ablation therapy or transcatheter arterial embolization, and guide ablation therapy for unresectable HCC. This article reviews the current developments and applications of Sonazoid-enhanced US and Sonazoid-enhanced 3D US for diagnosing and treating hepatic lesions, especially HCC.
Keywords: Sonazoid, Contrast-enhanced ultrasonography, Contrast-enhanced three-dimensional ultrasonography, Hepatic tumor, Hepatocellular carcinoma, Fusion image
INTRODUCTION
Since the late 1990s the first-generation agent Levovist (SH U 508A; Schering AG, Berlin, Germany), which consists of air-microbubbles, has been introduced as an ultrasound (US) contrast agent to enhance liver lesions mainly in Japan but also in other countries. In addition to its vascular phases (arterial and portal), this contrast agent has a late phase (parenchyma-specific phase, delayed phase) and can accumulate for up to 20 min within the liver[1-3]. Using both phases, Levovist-enhanced US is useful for characterization of liver tumors[4-13], evaluation of the efficacy of ablation therapy or transcatheter arterial embolization[14-16], and for guiding ablation therapy[17-20] in unresectable hepatocellular carcinoma (HCC). However, the parenchyma-specific contrast of Levovist was effective only when imaging was performed at high acoustic power using a high mechanical index (MI), and the effect was transient. Therefore, it cannot be used in real-time examinations in the late phase, and visualization of the whole liver is limited to a single scan[21,22].
SonoVue (Bracco, Milan, Italy) is widely available in Europe and China, replacing Levovist for radiology applications. Unlike Levovist, SonoVue contrast visualization can be achieved with low MI imaging, a far less destructive technique[23-29]. Thus, low MI techniques with SonoVue enable continuous real-time imaging. SonoVue is thought to be a truly intravascular, blood-pool contrast agent, with no interstitial equilibrium phase. SonoVue is minimally phagocytosed by reticuloendothelial cells (Kupffer cells)[30,31]. Parenchyma-specific contrast images with SonoVue can be seen only 3 to 5 min after the injection. Therefore, SonoVue requires repeated injections during intraoperative contrast-enhanced US to perform a whole liver examination[26,32]. SonoVue is thus not approved in Japan.
A newly developed second-generation ultrasound contrast agent, Sonazoid (Daiichi Sankyo, Tokyo, Japan), a lipid-stabilized suspension of perfluorobutane gas microbubbles was exclusively approved for clinical use in patients with liver lesions and for phase inversion harmonic gray-scale US in Japan in January 2007. Sonazoid is associated with a low occurrence of side effects, i.e. diarrhea 1.6%, albuminuria 1.6% and neutropenia 1.0%[33]. There is no contraindication for patients with renal dysfunction or iodine-allergy while one contraindication of Sonazoid was reported for patients allergic to eggs[33].
Like Levovist, contrast-enhanced US with Sonazoid has two phases of contrast enhancement: vascular and late (parenchyma-specific phase, delayed phase). Both Levovist and Sonazoid microbubbles enhance the liver parenchyma during the late phase, i.e. more than 5 min after injection of the contrast agent, and most of the malignant tumor tissues are not enhanced. Based on the results obtained in rabbit liver tumor models, Sonazoid microbubbles are retained within hepatic reticuloendothelial cells for 10-30 min after injection and enhance the liver parenchyma in late phase contrast-enhanced US at a low (0.4)[34] or moderate MI setting (0.6)[35]. In clinical use for human beings, enhancement of liver parenchyma and non-enhancement of malignant tumors in the late phase of Sonazoid-enhanced US is not completely understood but is thought to be due to phagocytosis by reticuloendothelial (Kupffer) cells or to adherence of microbubbles to the hepatic sinusoids and tumor vascular spaces[29,36,37], or recirculation of Sonazoid microbubbles[37]. Because malignant tumors contain few or no reticuloendothelial cells[38], they appear as perfusion defects in late phase Sonazoid-enhanced US[37,39,40].
It has been recently noted that more Sonazoid microbubbles than Levovist are taken up by reticuloendothelial cells in the rat liver[30]. The high stability of Sonazoid has overcome the deficiency of Levovist and microbubbles of Sonazoid have the advantage of long-lasting imaging and strong contrast effects that allows detailed observations[37,41]. Moreover, in the late phase of Sonazoid-enhanced US, we scanned the whole liver using this modality at a low MI without destroying the Sonazoid microbubbles, and this method allowed detection of small malignant lesions as perfusion defects. This method also allows the detection of small hypervascular HCC lesions that cannot be detected by conventional US[37]. Moreover, we can use Sonazoid in combination with low MI and high MI to obtain the advantages of both conditions. Because of these features, Sonazoid is widely used in Japan.
This article mainly reviews the current developments and applications of Sonazoid-enhanced US and Sonazoid-enhanced three dimensional (3D) US for diagnosing and treating hepatic lesions, especially HCC.
HEPATIC LESIONS
Injection dose of Sonazoid
The recommended dose of Sonazoid for liver tumor enhancement is 0.015 mL/kg. This dose was decided on the basis of clinical research conducted approximately 7 years ago[33]. With the development of US equipment, the image quality of Sonazoid-enhanced US is sufficiently good at doses lower than this recommended dose. Injection of the recommended dose of Sonazoid enhances the liver parenchyma excessively, which in turn sometimes induces the attenuation of the deep portion of the liver. This phenomenon prolongs the duration of washout of the malignant tumor in the late phase, resulting in a prolonged examination time. Therefore, most authors injected a decreased dose of Sonazoid to evaluate the vascularity of liver lesions, especially HCC[37,39,42-44] (Table 1).
Table 1.
Sonazoid-enhanced US at a low mechanical index (MI)
| Authors | Injection volume of Sonazoid | MI in late phase | Scan starting time in late phase |
| Moriyasu et al[33] | 0.015 mL/kg | 0.3-0.5 | 10 min afterinjection |
| Korenaga et al[42] | 0.01 mL/kg | 0.2-0.3 | 30 min after injection |
| Hatanaka et al[39] | 0.01 mL/kg | 0.2 | At least 10 min after injection |
| Maruyama et al[44] | 0.0075 mL/kg | 0.24-0.3 | 5-10 min after injection |
| Numata et al[37] | 0.2 mL/body | 0.2 | At least 5 min after injection |
All examinations were conducted using contrast-enhanced phase inversion harmonic US.
Sonazoid-enhanced US at a low MI
Sonazoid-enhanced US at a low MI is easy to use, and allows visualization, even using non-high-end equipment, and therefore, dependence on the operator’s skills/equipment is decreased, which may facilitate the widespread use of contrast-enhanced US[45].
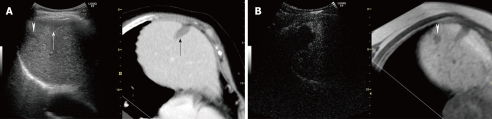
Sonazoid-enhanced US at a low MI made it easy to prevent destruction of the microbubbles around the tumor and adjacent to the liver parenchyma, which in turn made it easy to obtain perfusion images of hepatic lesions in the vascular phase (Figure 1A). Sonazoid-enhanced US at a low MI also made it possible to scan the whole liver in the late phase, facilitating detection of perfusion defect images of hepatic malignant lesions[37,39,46]. Employing both vascular and late phases, Sonazoid-enhanced US is useful for characterization of liver tumors[39], histological grading of HCC lesion[42], early recognition of HCC dedifferentiation[47], and guiding ablation therapy for unresectable HCC[44,48]. Xia et al[49] reported that Sonazoid-enhanced US was significantly more sensitive than dynamic CT in depicting the residual tumor blood supply to HCCs 1 wk after (transcatheter arterial chemoembolization (TACE) (P < 0.01, Chi-squared test). Sonazoid-enhanced US appeared to be a highly sensitive and accurate modality for evaluating responses of HCCs shortly after TACE.
Figure 1.
A 67-year-old man with recurrent HCC (maximum diameter 25 mm) in segment IV. A: Fusion image combining arterial phase contrast-enhanced CT (right side) and early phase Sonazoid-enhanced US employing the coded phase inversion (CPI) mode at a low MI (left side). Arterial phase contrast-enhanced CT shows a high attenuation area (arrowheads) adjacent to the non-enhanced area treated by radiofrequency ablation (RFA) (arrows) in segment IV. Early phase Sonazoid-enhanced US by the CPI mode at a low MI shows homogeneous enhancement in the recurrent viable area (arrowheads) and no enhancement in the necrotic area (arrows). These enhanced and non-enhanced areas correspond well to the arterial phase contrast-enhanced CT; B: Late phase Sonazoid-enhanced US by CPI mode at a low MI shows a perfusion defect (wash out) in both the recurrent viable area and the necrotic area (arrowheads). It is difficult to differentiate between the necrotic and viable areas because both appear as a perfusion defect; C: Defect on re-perfusion image with Sonazoid-enhanced US by the CPI mode at a low MI shows tumor enhancement in the recurrent viable area which previously showed a perfusion defect in the late phase (see B) (arrowheads). The necrotic area appears as a perfusion defect (arrows).
Sonazoid-enhanced US at a low MI allows the detection of small viable HCC lesions that cannot be detected by conventional US. However, it was difficult to differentiate between necrotic and viable areas because both appeared as perfusion defects in the late phase[37] (Figure 1B). To solve this problem, Kudo et al[45] re-injected Sonazoid into HCCs which had previously shown a perfusion defect in the late phase. They devised a method called “defect re-perfusion imaging” which confirms blood flow into the defects (Figure 1C). Using this method, Hatanaka et al[43] reported that Sonazoid-enhanced US has a higher sensitivity and accuracy for the diagnosis of hepatic malignancies than contrast-enhanced CT. There were significant differencesin sensitivity and accuracy between Sonazoid-enhanced US and contrast-enhanced CT (P < 0.05, respectively).
Korenaga et al[42] reported that Sonazoid-enhanced US was useful for estimating the histological grades of HCCs. Results of late phase images of Sonazoid-enhanced US and MRI with superparamagnetic iron oxide (SPIO) [Ferucarbotran (Resovist), Bayer, Osaka, Japan] matched perfectly (100%) in all of the moderately and poorly differentiated HCCs. They conclude that Sonazoid-enhanced US is a modality that can potentially replace SPIO-MRI. This result is similar to a report by Inoue et al[50]. In the late phase of Sonazoid-enhanced US, all of the moderately and poorly differentiated HCCs appeared hypoechoic and were detected as perfusion defects, whereas the majority (9 of 13 cases, 69.2%) of the well-differentiated HCCs were of an isoechoic pattern[42]. We must remain aware that we cannot detect the majority of well-differentiated HCCs as perfusion defects in the late phase of Sonazoid-enhanced US.
Advantages and disadvantages of Sonazoid-enhanced US at a low MI and a high MI
Vascular phase Sonazoid-enhanced US: Table 2 shows the setting conditions of Sonazoid-enhanced US at a low MI and a high MI. Table 3 compares the images of Sonazoid-enhanced US at a low MI and a high MI for evaluating the vascularity of liver lesions.
Table 2.
Setting conditions of Sonazoid-enhanced US at a low and a high MI
| Parameters | Low MI | High MI |
| Transducer | 3.5 or 4.0-MHz convex transducer | |
| Imaging mode | Coded phase inversion (A), Contrast pulse sequence (B), Pulse inversion harmonic (C), Amplitude modulation (D) | Coded harmonic angio (A) |
| MI | 0.12-0.3 (average 0.2) | 0.7-1.0 (average 0.8) |
| Frame rate | 7-13 frames | 2 -13 frames |
| Focus position | Bottom of the liver (A, D), Bottom of the lesion (B, C) | Bottom of the lesion (A) |
A: LOGIQ 7 ultrasound system (GE Healthcare, Milwaukee, WI). Both imaging software programs consist of phase inversion harmonic and coded technology. These programs restrict signal components from tissues and emphasize signals from blood vessels; B: ACUSON Sequoia ultrasound system (Mochida Siemens Medical System, Tokyo, Japan); C: Aplio-XV ultrasound system (Toshiba, Tokyo, Japan); D: LOGIQ E9 ultrasound system (GE Healthcare, Milwaukee, WI).
Table 3.
Comparison between Sonazoid-enhanced US images at low and high MI for evaluation of liver lesion vascularity
| Parameters | Low MI | High MI | |
| Vascular phase images | |||
| Tumor vessel image | Good | < | Very good |
| Tumor enhancement image | Good | < | Very good |
| Tumor enhancement image of the lesion located in deep portion or high echoic lesions | Poor | << | Good |
| Real time image (frame rate) | Good (high frame rate) | > | Good to poor (high to low frame rate) |
| Late phase images | |||
| Scan starting time | At least 10 min | < | At least 5 min |
| Perfusion defect image of the lesion | Good | < | Very good |
| Perfusion defect images of the lesion located in deep portion or high echoic lesion | Poor | << | Good |
| Real-time image (frame rate) | Very good (high frame rate) | >> | Poor (low frame rate) |
| Examination time | More than 10 min | < | More than 5 min |
| Injection volume of Sonazoid | Half or the volume recommended | < | Quarter or third of the volume recommended |
Grades are classified as poor, good and very good. >> or >: Low MI is superior to high MI; << or <: High MI is superior to low MI.
Sonazoid-enhanced US at a low MI permits visualization of tumor vessels and tumor enhancement of liver lesions. However, not only relatively large vessels, such as tumor vessels and portal veins, but also microvessels within the liver parenchyma are rapidly fulfilled with Sonazoid microbubbles under low MI conditions, which in turn permit rapid enhancement of the tumor and liver parenchyma simultaneously. This phenomenon induces a short duration of detailed observation for tumor vessels and tumor enhancement in the vascular phase of Sonazoid-enhanced US. Microflow imaging (MFI) is one of the methods of solving this problem[27,51,52]. MFI is an imaging method combining flash scanning with a high MI US exposure in a fixed plane which includes a distinctive cross section of the focal hepatic lesion and accumulates maximum intensity by holding the images obtained with contrast-enhanced low MI pulse subtraction imaging in the same fixed plane[27,51,52]. After all microbubbles disappeared from the fixed plane, circulated microbubbles flowed into the hepatic lesion with accumulation of maximum intensity holding images. However, this method does not evaluate the first pass of Sonazoid microbubbles into liver lesions. These accumulated maximum intensity holding images consist of combined arterial flow, portal venous flow, and hepatic venous flow.
Sonazoid-enhanced US at a high MI with coded harmonic angio (CHA) mode allows observation of vessels in the early phase by eliminating microbubbles in microvessels but not those in relatively large vessels, such as tumor vessels and portal veins[37] (Figure 2A), which permits prolongation of the observation time of tumor vessels, clear tumor enhancement, and also obtaining automatically scanned Sonazoid-enhanced three dimensional (3D) US. However, to obtain clear tumor enhancement by Sonazoid-enhanced US, intermittent scanning is sometime needed. This condition cannot provide real-time images of tumor vascularity. However, we can accumulate maximum intensity holding images during the first pass of Sonazoid microbubbles (Figure 2B).
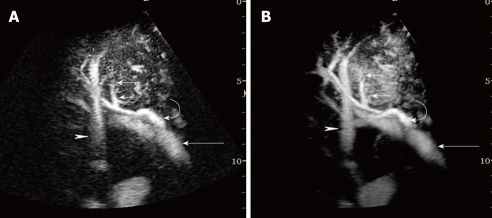
Figure 2.
A 76-year-old man with HCC (maximum diameter 40 mm) in segment VIII. A: Early phase Sonazoid-enhanced US by CHA mode at a high MI shows intratumoral vessels (small arrows), right portal vein (arrow), hepatic artery (curved arrow), and hepatic vein (arrowhead); B: Accumulation maximum intensity holding image in the early phase. Sonazoid-enhanced US by CHA mode at a high MI more clearly shows the serial images of intratumoral vessels (small arrows), right portal vein (arrow), hepatic artery (curved arrow), and hepatic vein (arrowhead) than the images in A.
Late phase Sonazoid-enhanced US: A low MI setting enables repeated observation of the whole liver in real time. However, because of US attenuation, we cannot evaluate whether the hepatic lesion located in the deep portion of the liver exhibits a perfusion defect or not. Moreover, due to enhancement of background B-mode, we cannot adequately evaluate whether or not the high echoic lesions exhibit perfusion defects in the late phase of Sonazoid-enhanced US at a low MI (Figure 3A and B).
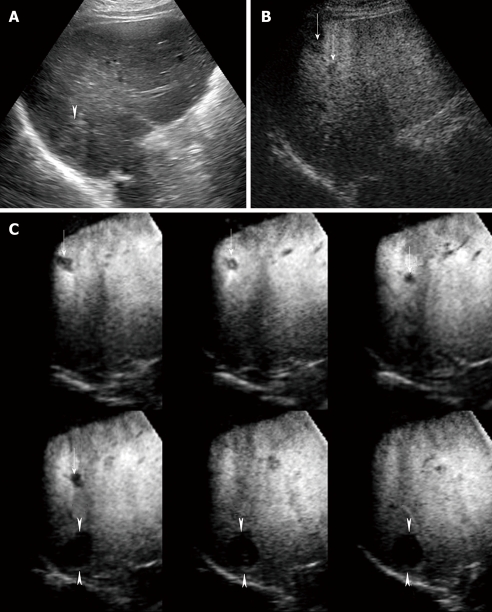
Figure 3.
A 75-year-old man with multiple HCC lesions (maximum diameter 22 mm, 12 mm and 10 mm, respectively) in segment VI. A: Conventional US shows one hyper-echoic HCC lesion alone; B: Late phase Sonazoid-enhanced US by CPI mode at a low MI shows two perfusion defects not detected by conventional US (arrows). However, one hyper-echoic lesion located in the deep portion far from the skin surface cannot be visualized by Sonazoid-enhanced US by CPI mode at a low MI; C: Late phase Sonazoid-enhanced 3D US by CHA mode at a high MI shows three HCC lesions as clear perfusion defects, as depicted on tomographic ultrasound images in plane A, which can be translated from front to back (arrows and arrowheads). The hyper-echoic HCC lesion which was not detected by Sonazoid-enhanced US at a low MI is clearly seen (arrowheads).
In the late phase of Sonazoid-enhanced US, we scanned the whole liver at a high MI with CHA mode, which enables us to observe a perfusion defect image clearly even when a hyper-echoic nodule is located in a deep portion of the liver (Figure 3C). However, the opportunity to scan with a high MI is limited because of microbubble destruction.
Sonazoid-enhanced US at combined high MI and low MI
Procedure: To obtain the advantages of both the low and the high MI setting of Sonazoid-enhanced US, we combined the high with the low MI setting for detection and diagnosis of hypervascular HCC lesions.
We injected only 0.2 mL/body in order to easily destroy the Sonazoid microbubbles within and around the tumor (Table 1). However, no significant differences in the imaging quality of Sonazoid-enhanced US were observed when the same amount of contrast agent was injected to the patients with different body weight. We consider this amount to be sufficient to evaluate the vascularity of hepatic tumors using the CHA mode at a high MI in the early (20-60 s after injection) (Figure 4) and middle (80-120 s after injection) phases (Figure 4E), using the coded phase inversion (CPI) mode at low MI in the late phase (more than 5 min after injection) (Figure 4F), and finally, using the CHA mode with high MI intermittent imaging in the late phase[37] (Figure 4G-J).
Figure 4.
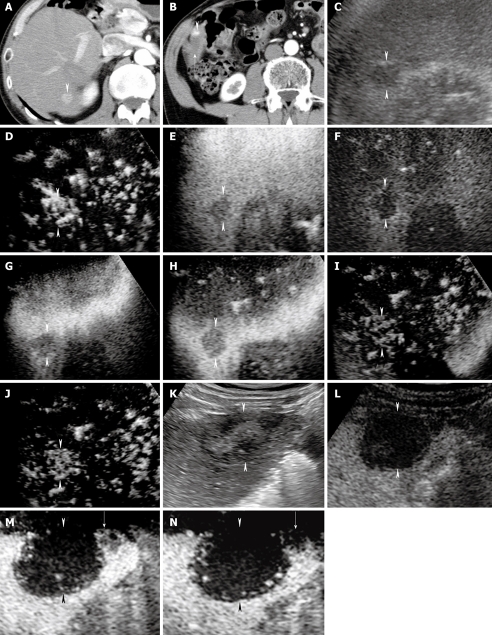
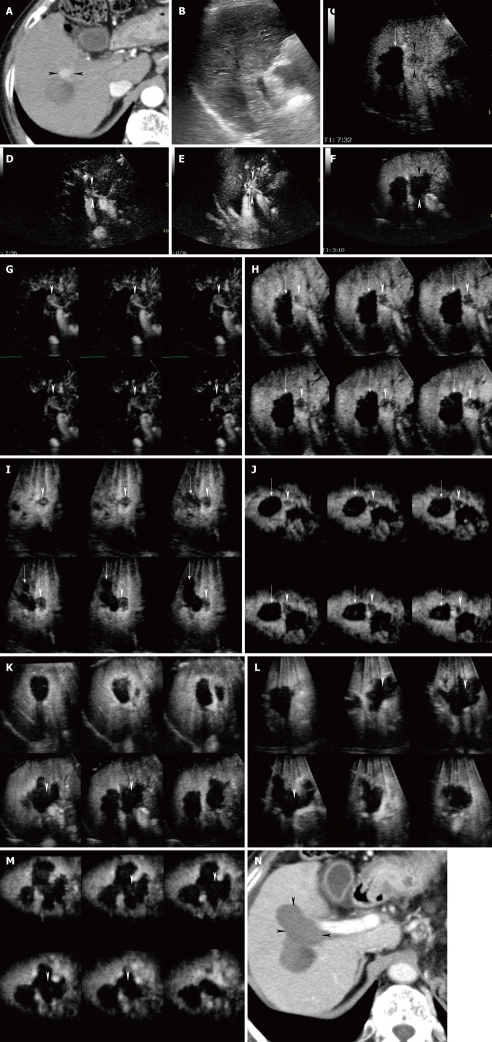
A 70-year-old man with newly developed HCC (maximum diameter 15 mm) in segment VI and residual viable lesion in segment V (maximum diameter 8 mm). A-B: Arterial phase contrast-enhanced CT shows a high attenuation area in segment VI (arrowhead) (A) and a high attenuation area (arrowhead) adjacent to the non-enhanced area (arrow) in segment V (B); C: Conventional US shows a hypo-echoic tumor in segment VI (arrowheads); D: Early phase Sonazoid-enhanced US by CHA mode at a high MI shows intratumoral vessels and homogeneous tumor enhancement (arrowheads); E: Middle phase Sonazoid-enhanced US by CHA mode at a high MI shows slightly hypo-echoic (wash out) but homogeneous tumor enhancement (arrowheads); F: Late phase Sonazoid-enhanced US by CPI mode at a low MI shows a perfusion defect (wash out) (arrowheads); G-J: Late phase Sonazoid-enhanced US by CHA mode on a high MI intermittent image shows a perfusion defect (G, H) (arrowheads). Intratumoral vessels (I) and homogeneous enhancement (J) are then seen later (arrowheads); K: Conventional US shows a hypo-echoic tumor in segment V (arrowheads); L: Late phase Sonazoid-enhanced US by CPI mode at a low MI shows a perfusion defect (arrowheads). It is difficult to differentiate between the necrotic and viable areas because both appear as a perfusion defect. The normal liver parenchyma is enhanced; M-N: Late phase Sonazoid-enhanced US by CHA mode at a high MI shows intratumoral vessels in the right side of the lesion (viable area) (arrow) and no enhancement in the left side of the lesion (necrotic area). Arrowheads indicate the margin of the lesion. This enhanced area corresponds closely to the area of high attenuation seen in B.
High MI intermittent imaging: Recently, we used intermittent imaging at 2 frames per second with CHA mode at a high MI (0.7-1.2) to depict the features of tumor vascularity in the late phase. We called this method “high MI intermittent imaging”. As a result of using high acoustic power, high MI intermittent imaging destroyed the microbubbles in and around the lesions, and allowed replenishment of microbubbles in the capillary bed between image acquisitions, which resulted in visualization of both tumor enhancement and the distribution of tumor vessels[37]. When we scanned the tumor lesion with high MI intermittent imaging, the Sonazoid microbubbles within and around the tumor may have been destroyed immediately and the tumor vessels and tumor enhancement may have been seen because of back flow into the tumor vessels and vascular spaces. In contrast, if the tumor became necrotic as a result of transcatheter arterial embolization, ablation therapy, or spontaneous necrosis, the lesions would exhibit neither tumor vessels nor enhancement when scanned using high MI intermittent imaging (Figure 4M and N). We then scanned the lesions again by this method to differentiate necrotic areas from newly developed or residual viable tumor areas detected as perfusion defects by Sonazoid-enhanced US at a low MI (Figures 4, 5 and 6). There were no vascular spaces in the necrotic area, whereas the viable areas contained vascular spaces. We considered these enhanced lesions to be viable HCC lesions and treated them by percutaneous radiofrequency ablation (RFA) therapy guided by late phase Sonazoid-enhanced US (Figure 5) or early phase Sonazoid-enhanced US (Figure 6).
Figure 5.
A 67-year-old woman with newly developed HCC (maximum diameter 12 mm) in segment V. A: Conventional US shows no evidence of tumor; B: Late phase Sonazoid-enhanced US by CPI mode at a low MI shows a perfusion defect (arrowheads); C-D:Late phase Sonazoid-enhanced US by CHA mode on a high MI intermittent image shows a perfusion defect (C) (arrowheads). Intratumoral vessels (D) are seen later (arrowheads). This lesion was treated with percutaneous RFA guided by late phase Sonazoid-enhanced US by CPI mode at a low MI; E-F: Arterial phase contrast-enhanced CT shows a high attenuation area in segment V before RFA (arrowheads) (E). Arterial phase contrast-enhanced CT obtained 4 weeks after treatment shows a low attenuation area (arrowhead) (F). This low attenuation area is larger than that of high attenuation seen in E.
Figure 6.
A 75-year-old woman with newly developed HCC (maximum diameter 15 mm) in segment VIII. A: Arterial phase contrast-enhanced CT shows a high attenuation area in segment VIII (arrowheads); B: Conventional sonogram cannot pinpoint the location of a tumor; C: Late phase Sonazoid-enhanced US by CHA mode on a high MI intermittent image shows a perfusion defect (arrowheads). Intratumoral vessels are faintly visible. Note the non-enhanced area caused by previous percutaneous RFA therapy (arrow); D: Late phase Sonazoid-enhanced US by CHA mode at a high MI intermittent image shows intratumoral vessels (arrowheads); E: Early phase Sonazoid-enhanced US CHA mode on a high MI shows homogeneous tumor enhancement (arrowheads) and the tip of the RFA electrode (arrow) in the tumor; F: Middle phase Sonazoid-enhanced US obtained 1 d after RFA guided by early phase Sonazoid-enhanced US shows the tumor as a perfusion defect with an oval shape and distinct margins (arrowheads). This non-enhanced area is larger than the area of tumor enhancement seen in C or D. Normal liver parenchyma is enhanced; G-J: Sonazoid-enhanced 3D US images show that before RFA the HCC lesion located adjacent to the right anterior portal vein is distinctly enhanced in the early phase (arrowheads), as shown on tomographic ultrasound images in plane A, which can be translated from front to back (G). Late phase Sonazoid-enhanced 3D US images show that the HCC lesion and areas previously treated by RFA appear as perfusion defects (arrowheads) and completely non-enhanced areas (arrows), respectively, as shown on tomographic ultrasound images in plane A, which can be translated from front to back (H), plane B, which can be translated from right to left (I), and plane C, which can be translated from down to up (J); K-M: One day after RFA treatment, adequate ablation in the absence of enhancement was detected as shown in the middle phase on the tomographic ultrasound images in plane A, which can be translated from front to back (K), plane B, which can be translated from right to left (L), and plane C, which can be translated from down to up (M). Arrowheads indicate the HCC lesion margin, as seen in G or H-J. This non-enhanced area is larger than the areas seen in G or H-J. Normal liver parenchyma is enhanced; N: Arterial phase contrast-enhanced CT obtained 4 wk after treatment shows a low attenuation area (arrowhead). This low attenuation is larger than the area of high attenuation seen in A.
Advantages of this method include that sufficient information can be obtained in patients with multiple lesions. If the multiple lesions are located close to each other, the enhancement patterns can be observed simultaneously during a single scanning process, and if they are located in different lobes or segments, repeated scanning is possible, because of the long duration of late phase Sonazoid-enhanced US imaging (Figure 4). This method is regarded as an alternative to additional injection of contrast agents when tumor vascularity requires further observation. This method can be used when there is a lack of medical personnel or to save time. We finally concluded that high MI intermittent imaging in the late phase is potentially useful for evaluating the enhancement patterns of focal liver tumors[53] and differentiation between necrosis and viable hypervascular HCC lesions after treatment[37].
Sonazoid-enhanced 3D US
Acquisition and reconstruction of Sonazoid-enhanced 3D US: Sonazoid-enhanced 3D US is being used as a clinical diagnostic method in Japan, especially for liver lesions[54]. With the assistance of CHA mode and high MI contrast conditions, which reduce microbubbles in microvessels but not in relatively large vessels, such as tumor vessels and portal veins, Sonazoid-enhanced three-dimensional (3D) US facilitated detailed observation of tumor vessels. Compared with Sonazoid-enhanced (two dimensional) US, Sonazoid-enhanced 3D US allows viewing the volume of interest (VOI)in three orthogonal planes, supplying more spatial information and facilitating easier anatomic assessment.
The process of Sonazoid-enhanced 3D US includes two steps: data acquisition and image reconstruction. With equipment development, a volume probe which scans automatically with internal sectorial mechanical tilt movement to obtain the data has become available. This type of probe supplies us a convenient and fast means for data acquisition in the contrast-enhanced phase. The scanning parameters, including volume angle and number of scanning frames, need to be adjusted before the examination.
After data acquisition, the raw data are stored in the hard disk and image reconstruction can be performed at any time. Two important functions in reconstruction of imaging are to obtain tomographic images and sonographic angiograms. In tomographic images, enhancement of the VOI can be demonstrated in three orthogonal dimensions, and in each dimension, the enhancement of VOI can be presented in multiple parallel planes with an adjustable inter-plane distance (Figure 6). Sonographic angiograms are reconstructed using a rendering mode, such as “gray surface”, “texture”, “maximum intensity”, or “average intensity” in the GE LOGIQ 7 ultrasound system. Different rendering modes can be employed to depict different enhancement characteristics[55] (Figure 7).
Figure 7.
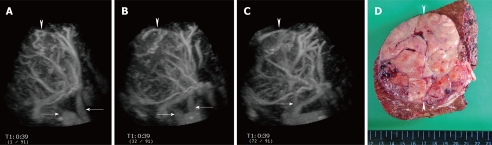
A 72-year-old man with large HCC (maximum diameter 90 mm) in the right lobe of the liver. A-C: Sonographic angiograms acquired by Sonazoid-enhanced 3D US and rendered by maximum intensity with gray surface mode, presented in different directions, and the tumor had tortuous intratumoral vessels. Middle and right hepatic veins are also seen (arrows). An arrowhead points to the margins of the tumor; D: Surgical specimen shows a large HCC lesion located in the right lobe of the liver (arrowheads). Histological specimen shows moderately to poorly differentiated HCC.
Characterization of liver lesions by Sonazoid-enhanced 3D US: Sonazoid-enhanced US has been proved useful for characterizing liver lesions, such as HCC, metastases, hemangioma and focal nodular hyperplasia[39]. In addition to the information from Sonazoid-enhanced US, Sonazoid-enhanced 3D US also supplies important spatial views of any part of the VOI. From the early to the late phase, Sonazoid-enhanced 3D US shows tumor vascularity and changes in tumor enhancement patterns in a multiplanar image[55]. Tortuous vessels within the lesion can be clearly depicted (Figure 7).
In a retrospective study on 139 liver lesions, the accuracy of Sonazoid-enhanced 3D US and that of contrast-enhanced 3D CT for differentiating liver lesions were assessed. The sensitivity was 83% or higher with both modalities, the specificity was 87% or higher with Sonazoid-enhanced 3D US and 92% or higher with contrast-enhanced 3D CT, the positive predictive value was at least 71% with both modalities, and the area under the receiver operating characteristics curve (Az) was at least 0.89 with US and at least 0.92 with CT. Inter-reader agreement was good to excellent (κ ≥ 0.76) with both modalities[56].
Evaluation of the effect of ablation therapy by Sonazoid-enhanced 3D US: To evaluate the effect of ablation therapy and to detect the residual tumor exactly in the early phase are very important for further treatment. Immediately after the RFA procedure and during the early follow-up period on contrast-enhanced CT images, the presence of hyper-attenuation due to dehydration produced by coagulative necrosis, and periablational enhancement due to reactive hyperemia, arteriovenous shunts, or fibrosis/giant cell reaction, hinder accurate use of this modality for evaluating RFA[57-59]. Compared with CE-CT images, contrast-enhanced US images acquired soon after RFA have shown very few artifacts and have thus been proven to be useful for early evaluation of the therapeutic efficacy of RFA[60-62].
Sonazoid-enhanced 3D US images allowed observers to compare HCC tumors before treatment with ablated areas after treatment employing a 3D visualization. When the lesion sites were evaluated on Sonazoid-enhanced 3D US images after ablation, the ablation was evaluated as adequate if a non-enhancing area in the early, middle, and late phases covered the hypervascular enhancement seen in the early and middle phases before ablation. Residual tumor on Sonazoid-enhanced 3D US images was diagnosed when an area within the tumor was detected with hypervascular enhancement in the early and middle phases, and was hypoechoic or isoechoic in the late phase of Sonazoid-enhanced 3D US after ablation (Figure 6).
In a previous study, 63 cases of HCC were treated by RFA. Sonazoid-enhanced 3D US was performed 5-7 d before and 1 d after RFA. Contrast-enhanced 3D CT was performed 5-7 d before and 1 mo after the ablation, and during the follow-up period. When 1-mo contrast-enhanced 3D CT scans were used as the reference standard, the sensitivity, specificity and accuracy of 1 d Sonazoid-enhanced 3D US for detecting residual tumor were 100%, 97% and 97%, respectively, and the Kappa value for agreement between the findings of the two modalities was 0.65. By demonstrating the ablated areas and residual tumors in three dimensions, Sonazoid-enhanced 3D US was shown to be helpful in evaluating the therapeutic effect of RFA for HCC lesions at an earlier time than contrast-enhanced 3D CT[63]. Sonazoid-enhanced 3D US can be used also to evaluate the immediate therapeutic effect of high-intensity focused ultrasound (HIFU) on small HCC lesions. This modality has the potential to enable us to detect residual portions of HCCs and perform additional HIFU ablation of the untreated portions[64].
Fusion images combining US with dynamic CT or US with Gd-EOB-DTPA
For detection of HCC lesions, a combination of modalities using different imaging techniques is recommended to increase the sensitivity and specificity of diagnosis. To achieve this combination of different imaging modalities, we used the fusion feature which can fuse and synchronize US images with multiplanar reconstruction CT or MRI images on the same screen in real time using LOGIQ E9 (GE Healthcare, Milwaukee, WI). A new ultrasound transducer together with a navigation system and dynamic positioning system (volume navigation system: “VNav”) make it possible to combine current ultrasonic images with uploaded CT or MRI data[65].
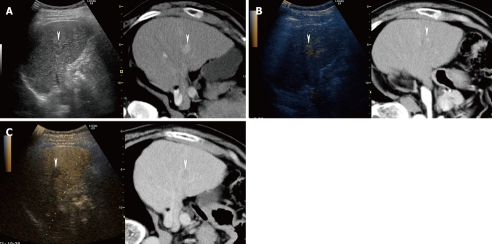
Fusion images combining conventional US and arterial phase contrast-enhanced CT can detect hypervascular HCC lesions easily (Figures 1A and 8). On the contrary, fusion images combining conventional US and hepatobiliary phase contrast-enhanced MRI with gadolinium-ethoxybenzyl-diethylenetriamine (Gd-EOB-DTPA; Primovist®, Bayer Schering Pharma AG, Berlin, Germany) obtained at 20 min after injection can exhibit small hypervascular (typical) HCC lesions or non-hypervascular (atypical) HCC lesions as hypo-intense areas (Figure 9). Recently, Kim et al[66] reported that hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA obtained at 20 min after injection can detect smaller HCC lesions better than contrast-enhanced CT.
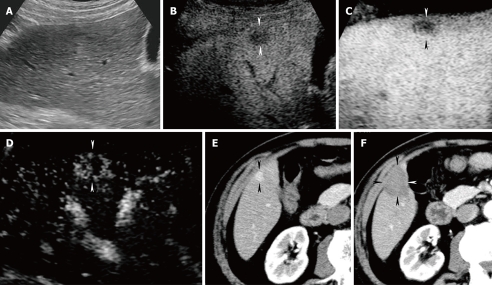
Figure 8.
A 66-year-old man with newly developed HCC (maximum diameter 15 mm) in segment V. A: Fusion image combining arterial phase contrast-enhanced CT (right side) and conventional US (left side). Due to advanced liver cirrhosis, the echogenicity of the liver parenchyma is heterogeneous. Arterial phase contrast-enhanced CT shows a high attenuation area in segment V (arrowheads). Arterial phase contrast-enhanced CT as the reference standard, allows conventional US to detect the target HCC lesion easily; B: Fusion image combining arterial phase contrast-enhanced CT (right side) and early phase Sonazoid-enhanced US at a low MI (left side). Early phase Sonazoid-enhanced US at a low MI shows a homogeneous enhancement in segment V (arrowhead). This enhanced area corresponds well to a high attenuation area, as shown on the arterial phase contrast-enhanced CT image (arrowhead); C: Fusion image combining late phase contrast-enhanced CT (right side) and late phase Sonazoid-enhanced US at a low MI (left side). Late phase Sonazoid-enhanced US at a low MI shows a perfusion defect in a viable HCC lesion (arrowhead). This perfusion defect corresponds well to a low attenuation area, as shown on the late phase contrast-enhanced CT.
Figure 9.
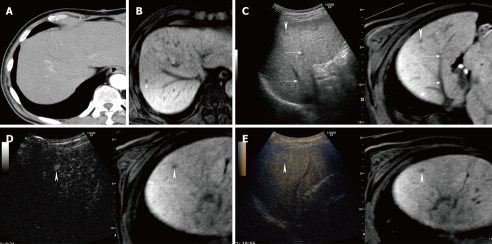
A 58-year-old man with newly developed HCC (maximum diameter 10 mm) in segment VIII. A: Arterial phase contrast-enhanced CT can not detect a tumor; B:Hepatobiliary phase contrast-enhanced MRI with gadolinium-ethoxybenzyl-diethylenetriamine (Gd-EOB-DTPA) obtained at 20 min after injection shows a hypo-intense area in segment VIII (arrow); C: Fusion image combining hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA (right side) and conventional US (left side). Hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA shows a hypo-intense area in segment VIII. Hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA as the reference standard, allows conventional US to detect the target HCC lesion easily (arrowhead). Arrows indicate the hepatic vein; D: Fusion image combining hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA (right side) and early phase Sonazoid-enhanced US at a low MI (left side). Early phase Sonazoid-enhanced US at a low MI shows a small homogeneous enhancement in segment VIII (arrowhead). This enhanced area corresponds to a hypo-intense area, as shown on hepatobiliary phase contrast-enhanced MRI with a Gd-EOB-DTPA image (arrowhead); E: Fusion image combining hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA (right side) and late phase Sonazoid-enhanced US at a low MI (left side). Late phase Sonazoid-enhanced US at a low MI shows a small perfusion defect in segment VIII (arrowhead). This area corresponds to a hypo-intense area, as shown on hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA image (arrowhead).
In our experience, hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA can detect small non-hypervascular (atypical) HCC lesions not detectable by contrast-enhanced CT (Figure 10). Hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA is sensitive for detecting atypical HCC lesions. These lesions were seen as hypo-intense areas in this phase of contrast-enhanced MRI with Gd-EOB-DTPA. In cases of advanced cirrhosis with repeated treatment using various forms of ablation therapy, conventional US detects many hypo- or hyper-echoic areas in the liver parenchyma[17,67], resulting in difficulties recognizing targeted small HCC lesions. However, fusion images combining hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA and conventional US can be used to recognize such atypical HCC lesions not clearly detected by contrast-enhanced CT. Hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA as the reference standard can detect atypical HCC lesions which appear as hypo- or hyper-echoic lesions on conventional US ( unpublished data).
Figure 10.
A 77-year-old woman with newly developed HCC (maximum diameter 12 mm) in segment VIII. A: Fusion image combining arterial phase contrast-enhanced CT (right side) and conventional US (left side). Arterial phase contrast-enhanced CT shows hypo-attenuation area previously treated by RFA alone (arrow). Conventional US shows two hypo-echoic lesions. One is an HCC lesion which was previously treated by RFA (arrow) and the other is a new HCC lesion which was not detectable by dynamic CT (arrowhead); B: Fusion image combining hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA (right side) and middle phase Sonazoid-enhanced US at a low MI (left side). Hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA shows a small hypo-intense area in segment VIII (arrowhead). Middle phase Sonazoid-enhanced US obtained 1 d after RFA shows the tumor as a perfusion defect. This non-enhanced area is larger than the hypo-echoic lesion seen on the hepatobiliary phase contrast-enhanced MRI with Gd-EOB-DTPA. Normal liver parenchyma is enhanced.
CONCLUSION
Sonazoid, a lipid-stabilized suspension of perfluorobutane gas microbubbles, has been approved for clinical use in Japan only since January 2007. Like Levovist, contrast-enhanced US with Sonazoid has two phases of contrast enhancement: vascular and late (parenchyma-specific phase, delayed phase). The high stability of Sonazoid has overcome the deficiency of Levovist, and microbubbles of Sonazoid have the advantage of long-lasting imaging and strong contrast effects allowing detailed observations. Contrast-enhanced US and contrast-enhanced 3D US using Sonazoid are useful in making the diagnosis and assisting with treatment of hepatic, especially HCC, lesions. Moreover, fusion images combining US with CT or MRI allows correct selection of small HCC lesions which appear as hypo- or hyper-echoic lesions on the US.
Acknowledgments
We thank Naoto Sato, Mitsuyoshi Sase, Hiroshi Hashimoto and Takao Higashiizumi of GE Healthcare for providing technical advice, and Kazuya Sugimori, Reiko Kunisaki, and Masahiko Terada of the Gastroenterological Center, Yokohama City University Medical Center for providing additional assistance.
Footnotes
Peer reviewers: Sergio Casciaro, PhD, Institute of Clinical Physiology - National Research Council,Campus Universitario Ecotekne, Via Monteroni, 73100 Lecce, Italy; Roberto Miraglia, MD, Department of Diagnostic and Interventional Radiology, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IsMeTT), Via Tricomi 1, 90100 Palermo, Italy
S- Editor Cheng JX L- Editor Ma JY E- Editor Zheng XM
References
- 1.Albrecht T, Hoffmann CW, Schmitz SA, Schettler S, Overberg A, Germer CT, Wolf KJ. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol. 2001;176:1191–1198. doi: 10.2214/ajr.176.5.1761191. [DOI] [PubMed] [Google Scholar]
- 2.Hauff P, Fritzsch T, Reinhardt M, Weitschies W, Lüders F, Uhlendorf V, Heldmann D. Delineation of experimental liver tumors in rabbits by a new ultrasound contrast agent and stimulated acoustic emission. Invest Radiol. 1997;32:94–99. doi: 10.1097/00004424-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Marelli C. Preliminary experience with NC100100, a new ultrasound contrast agent for intravenous injection. Eur Radiol. 1999;9 Suppl 3:S343–S346. doi: 10.1007/pl00014070. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153–161. doi: 10.1148/radiology.215.1.r00ap08153. [DOI] [PubMed] [Google Scholar]
- 5.Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology. 2000;216:411–417. doi: 10.1148/radiology.216.2.r00jl21411. [DOI] [PubMed] [Google Scholar]
- 6.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000;35:58–71. doi: 10.1097/00004424-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Numata K, Tanaka K, Kiba T, Saito S, Ikeda M, Hara K, Tanaka N, Morimoto M, Iwase S, Sekihara H. Contrast-enhanced, wide-band harmonic gray scale imaging of hepatocellular carcinoma: correlation with helical computed tomographic findings. J Ultrasound Med. 2001;20:89–98. doi: 10.7863/jum.2001.20.2.89. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S, Ioka T, Oshikawa O, Hamada Y, Yoshioka F. Dynamic sonography of hepatic tumors. AJR Am J Roentgenol. 2001;177:799–805. doi: 10.2214/ajr.177.4.1770799. [DOI] [PubMed] [Google Scholar]
- 9.Dill-Macky MJ, Burns PN, Khalili K, Wilson SR. Focal hepatic masses: enhancement patterns with SH U 508A and pulse-inversion US. Radiology. 2002;222:95–102. doi: 10.1148/radiol.2221010092. [DOI] [PubMed] [Google Scholar]
- 10.von Herbay A, Vogt C, Häussinger D. Late-phase pulse-inversion sonography using the contrast agent levovist: differentiation between benign and malignant focal lesions of the liver. AJR Am J Roentgenol. 2002;179:1273–1279. doi: 10.2214/ajr.179.5.1791273. [DOI] [PubMed] [Google Scholar]
- 11.Isozaki T, Numata K, Kiba T, Hara K, Morimoto M, Sakaguchi T, Sekihara H, Kubota T, Shimada H, Morizane T, et al. Differential diagnosis of hepatic tumors by using contrast enhancement patterns at US. Radiology. 2003;229:798–805. doi: 10.1148/radiol.2293020858. [DOI] [PubMed] [Google Scholar]
- 12.Numata K, Isozaki T, Morimoto M, Sugimori K, Kunisaki R, Morizane T, Tanaka K. Prospective study of differential diagnosis of hepatic tumors by pattern-based classification of contrast-enhanced sonography. World J Gastroenterol. 2006;12:6290–6298. doi: 10.3748/wjg.v12.i39.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Numata K, Morimoto M, Isozaki T, Sugimori K, Oka H, Matsuo K, Shimada H, Imada T. Use of accumulation images obtained by arterial phase contrast-enhanced harmonic grayscale sonography to evaluate tumor vessels in focal nodular hyperplasia and other hepatic tumors. J Med Ultrasonics. 2007;34:3–10. doi: 10.1007/s10396-006-0132-9. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K. Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721–730. doi: 10.1148/radiol.2213010358. [DOI] [PubMed] [Google Scholar]
- 15.Numata K, Tanaka K, Kiba T, Saito S, Isozaki T, Hara K, Morimoto M, Sekihara H, Yonezawa H, Kubota T. Using contrast-enhanced sonography to assess the effectiveness of transcatheter arterial embolization for hepatocellular carcinoma. AJR Am J Roentgenol. 2001;176:1199–1205. doi: 10.2214/ajr.176.5.1761199. [DOI] [PubMed] [Google Scholar]
- 16.Wen YL, Kudo M, Zheng RQ, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T, Maekawa K. Radiofrequency ablation of hepatocellular carcinoma: therapeutic response using contrast-enhanced coded phase-inversion harmonic sonography. AJR Am J Roentgenol. 2003;181:57–63. doi: 10.2214/ajr.181.1.1810057. [DOI] [PubMed] [Google Scholar]
- 17.Numata K, Isozaki T, Ozawa Y, Sakaguchi T, Kiba T, Kubota T, Ito A, Sugimori K, Shirato K, Morimoto M, et al. Percutaneous ablation therapy guided by contrast-enhanced sonography for patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2003;180:143–149. doi: 10.2214/ajr.180.1.1800143. [DOI] [PubMed] [Google Scholar]
- 18.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. AJR Am J Roentgenol. 2004;183:153–156. doi: 10.2214/ajr.183.1.1830153. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama H, Kobayashi S, Yoshizumi H, Okugawa H, Akiike T, Yukisawa S, Fukuda H, Matsutani S, Ebara M, Saisho H. Application of percutaneous ultrasound-guided treatment for ultrasonically invisible hypervascular hepatocellular carcinoma using microbubble contrast agent. Clin Radiol. 2007;62:668–675. doi: 10.1016/j.crad.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Minami Y, Kudo M, Chung H, Kawasaki T, Yagyu Y, Shimono T, Shiozaki H. Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: prospective randomized controlled trial. AJR Am J Roentgenol. 2007;188:489–494. doi: 10.2214/AJR.05.1286. [DOI] [PubMed] [Google Scholar]
- 21.Blomley MJ, Albrecht T, Cosgrove DO, Patel N, Jayaram V, Butler-Barnes J, Eckersley RJ, Bauer A, Schlief R. Improved imaging of liver metastases with stimulated acoustic emission in the late phase of enhancement with the US contrast agent SH U 508A: early experience. Radiology. 1999;210:409–416. doi: 10.1148/radiology.210.2.r99fe47409. [DOI] [PubMed] [Google Scholar]
- 22.Blomley MJ, Sidhu PS, Cosgrove DO, Albrecht T, Harvey CJ, Heckemann RA, Butler-Barnes J, Eckersley RJ, Basilico R. Do different types of liver lesions differ in their uptake of the microbubble contrast agent SH U 508A in the late liver phase? Early experience. Radiology. 2001;220:661–667. doi: 10.1148/radiol.2203992044. [DOI] [PubMed] [Google Scholar]
- 23.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–430. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 24.Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551–1559. doi: 10.2214/AJR.05.0138. [DOI] [PubMed] [Google Scholar]
- 25.Nicolau C, Vilana R, Catalá V, Bianchi L, Gilabert R, García A, Brú C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 26.Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, Albrecht T, Hohmann J, Oldenburg A, Ritz JP, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243:236–240. doi: 10.1097/01.sla.0000197708.77063.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Liu GJ, Lu MD, Xu HX, Xie XY. Evaluation of the vascular architecture of hepatocellular carcinoma by micro flow imaging: pathologic correlation. J Ultrasound Med. 2007;26:461–467. doi: 10.7863/jum.2007.26.4.461. [DOI] [PubMed] [Google Scholar]
- 28.Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748–3756. doi: 10.3748/wjg.15.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu GJ, Xu HX, Xie XY, Xu ZF, Zheng YL, Liang JY, Lu MD, Moriyasu F. Does the echogenicity of focal liver lesions on baseline gray-scale ultrasound interfere with the diagnostic performance of contrast-enhanced ultrasound? Eur Radiol. 2009;19:1214–1222. doi: 10.1007/s00330-008-1251-z. [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318–325. doi: 10.1016/j.ultrasmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Lim AK, Patel N, Eckersley RJ, Taylor-Robinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 32.Torzilli G, Del Fabbro D, Palmisano A, Donadon M, Bianchi P, Roncalli M, Balzarini L, Montorsi M. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg. 2005;9:1148–1153; discussion 1153-1154. doi: 10.1016/j.gassur.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009;193:86–95. doi: 10.2214/AJR.08.1618. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe R, Matsumura M, Chen CJ, Kaneda Y, Ishihara M, Fujimaki M. Gray-scale liver enhancement with Sonazoid (NC100100), a novel ultrasound contrast agent; detection of hepatic tumors in a rabbit model. Biol Pharm Bull. 2003;26:1272–1277. doi: 10.1248/bpb.26.1272. [DOI] [PubMed] [Google Scholar]
- 35.Forsberg F, Piccoli CW, Liu JB, Rawool NM, Merton DA, Mitchell DG, Goldberg BB. Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system-specific phase. Radiology. 2002;222:824–829. doi: 10.1148/radiol.2223001786. [DOI] [PubMed] [Google Scholar]
- 36.Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver leasions. Eur Radiol. 2001;11 Suppl 3:E15–E26. doi: 10.1007/pl00014125. [DOI] [PubMed] [Google Scholar]
- 37.Numata K, Morimoto M, Ogura T, Sugimori K, Takebayashi S, Okada M, Tanaka K. Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J Ultrasound Med. 2008;27:395–406. doi: 10.7863/jum.2008.27.3.395. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996;24:807–812. doi: 10.1053/jhep.1996.v24.pm0008855180. [DOI] [PubMed] [Google Scholar]
- 39.Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, Takahashi S, Inoue T, Hagiwara S, Chung H, et al. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51 Suppl 1:61–69. doi: 10.1159/000122600. [DOI] [PubMed] [Google Scholar]
- 40.Kanemoto H, Ohno K, Nakashima K, Takahashi M, Fujino Y, Nishimura R, Tsujimoto H. Characterization of canine focal liver lesions with contrast-enhanced ultrasound using a novel contrast agent-sonazoid. Vet Radiol Ultrasound. 2009;50:188–194. doi: 10.1111/j.1740-8261.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe R, Matsumura M, Chen CJ, Kaneda Y, Fujimaki M. Characterization of tumor imaging with microbubble-based ultrasound contrast agent, sonazoid, in rabbit liver. Biol Pharm Bull. 2005;28:972–977. doi: 10.1248/bpb.28.972. [DOI] [PubMed] [Google Scholar]
- 42.Korenaga K, Korenaga M, Furukawa M, Yamasaki T, Sakaida I. Usefulness of Sonazoid contrast-enhanced ultrasonography for hepatocellular carcinoma: comparison with pathological diagnosis and superparamagnetic iron oxide magnetic resonance images. J Gastroenterol. 2009;44:733–741. doi: 10.1007/s00535-009-0053-7. [DOI] [PubMed] [Google Scholar]
- 43.Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology. 2008;75 Suppl 1:42–47. doi: 10.1159/000173423. [DOI] [PubMed] [Google Scholar]
- 44.Maruyama H, Takahashi M, Ishibashi H, Okugawa H, Okabe S, Yoshikawa M, Yokosuka O. Ultrasound-guided treatments under low acoustic power contrast harmonic imaging for hepatocellular carcinomas undetected by B-mode ultrasonography. Liver Int. 2009;29:708–714. doi: 10.1111/j.1478-3231.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 45.Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl 1:1–12. doi: 10.1159/000181865. [DOI] [PubMed] [Google Scholar]
- 46.Nakano H, Ishida Y, Hatakeyama T, Sakuraba K, Hayashi M, Sakurai O, Hataya K. Contrast-enhanced intraoperative ultrasonography equipped with late Kupffer-phase image obtained by sonazoid in patients with colorectal liver metastases. World J Gastroenterol. 2008;14:3207–3211. doi: 10.3748/wjg.14.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maruyama H, Takahashi M, Ishibashi H, Okabe S, Yoshikawa M, Yokosuka O. Changes in tumor vascularity precede microbubble contrast accumulation deficit in the process of dedifferentiation of hepatocellular carcinoma. Eur J Radiol. 2009:Epub ahead of print. doi: 10.1016/j.ejrad.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y, Terae S, Shirato H. Sonazoid-enhanced sonography for guiding radiofrequency ablation for hepatocellular carcinoma: better tumor visualization by Kupffer-phase imaging and vascular-phase imaging after reinjection. Jpn J Radiol. 2009;27:185–193. doi: 10.1007/s11604-009-0317-4. [DOI] [PubMed] [Google Scholar]
- 49.Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, Hagiwara S, Inoue T, Ishikawa E, Kitai S, et al. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of sonazoid-enhanced harmonic sonography. Oncology. 2008;75 Suppl 1:99–105. doi: 10.1159/000173430. [DOI] [PubMed] [Google Scholar]
- 50.Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, Ishikawa E, Hagiwara S, Minami Y, Chung H, et al. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology. 2008;75 Suppl 1:48–54. doi: 10.1159/000173424. [DOI] [PubMed] [Google Scholar]
- 51.Shiraishi J, Sugimoto K, Moriyasu F, Kamiyama N, Doi K. Computer-aided diagnosis for the classification of focal liver lesions by use of contrast-enhanced ultrasonography. Med Phys. 2008;35:1734–1746. doi: 10.1118/1.2900109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugimoto K, Moriyasu F, Kamiyama N, Metoki R, Yamada M, Imai Y, Iijima H. Analysis of morphological vascular changes of hepatocellular carcinoma by microflow imaging using contrast-enhanced sonography. Hepatol Res. 2008;38:790–799. doi: 10.1111/j.1872-034X.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 53.Luo W, Numata K, Kondo M, Morimoto M, Sugimori K, Hirasawa K, Nozaki A, Zhou X, Tanaka K. Sonazoid-enhanced ultrasonography for evaluation of the enhancement patterns of focal liver tumors in the late phase by intermittent imaging with a high mechanical index. J Ultrasound Med. 2009;28:439–448. doi: 10.7863/jum.2009.28.4.439. [DOI] [PubMed] [Google Scholar]
- 54.Luo W, Numata K, Morimoto M, Nozaki A, Nagano Y, Sugimori K, Zhou X, Tanaka K. Clinical utility of contrast-enhanced three-dimensional ultrasound imaging with Sonazoid: findings on hepatocellular carcinoma lesions. Eur J Radiol. 2009;72:425–431. doi: 10.1016/j.ejrad.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Luo W, Numata K, Morimoto M, Nozaki A, Nagano Y, Sugimori K, Tanaka K. Three-dimensional contrast-enhanced sonography of vascular patterns of focal liver tumors: pilot study of visualization methods. AJR Am J Roentgenol. 2009;192:165–173. doi: 10.2214/AJR.08.1107. [DOI] [PubMed] [Google Scholar]
- 56.Luo W, Numata K, Morimoto M, Kondo M, Takebayashi S, Okada M, Morita S, Tanaka K. Focal liver tumors: characterization with 3D perflubutane microbubble contrast agent-enhanced US versus 3D contrast-enhanced multidetector CT. Radiology. 2009;251:287–295. doi: 10.1148/radiol.2511081324. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 58.Dill-Macky MJ, Asch M, Burns P, Wilson S. Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:S287–S295. doi: 10.2214/AJR.04.1916. [DOI] [PubMed] [Google Scholar]
- 59.Filippone A, Iezzi R, Di Fabio F, Cianci R, Grassedonio E, Storto ML. Multidetector-row computed tomography of focal liver lesions treated by radiofrequency ablation: spectrum of findings at long-term follow-up. J Comput Assist Tomogr. 2007;31:42–52. doi: 10.1097/01.rct.0000232264.36178.1e. [DOI] [PubMed] [Google Scholar]
- 60.Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, Kim SK, Jang KM, Lee JY, Lim JH. Radiofrequency ablation of liver cancer: early evaluation of therapeutic response with contrast-enhanced ultrasonography. Korean J Radiol. 2004;5:185–198. doi: 10.3348/kjr.2004.5.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim CK, Choi D, Lim HK, Kim SH, Lee WJ, Kim MJ, Lee JY, Jeon YH, Lee J, Lee SJ, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol. 2005;56:66–73. doi: 10.1016/j.ejrad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Vilana R, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J, et al. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol. 2006;16:2454–2462. doi: 10.1007/s00330-006-0264-8. [DOI] [PubMed] [Google Scholar]
- 63.Luo W, Numata K, Morimoto M, Oshima T, Ueda M, Okada M, Takebayashi S, Zhou X, Tanaka K. Role of Sonazoid-enhanced three-dimensional ultrasonography in the evaluation of percutaneous radiofrequency ablation of hepatocellular carcinoma. Eur J Radiol. 2009:Epub ahead of print. doi: 10.1016/j.ejrad.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Numata K, Fukuda H, Ohto M, Itou R, Nozaki A, Kondou M, Morimoto M, Karasawa E, Tanaka K. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol. 2009:Epub ahead of print. doi: 10.1016/j.ejrad.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Jung EM, Schreyer AG, Schacherer D, Menzel C, Farkas S, Loss M, Feuerbach S, Zorger N, Fellner C. New real-time image fusion technique for characterization of tumor vascularisation and tumor perfusion of liver tumors with contrast-enhanced ultrasound, spiral CT or MRI: first results. Clin Hemorheol Microcirc. 2009;43:57–69. doi: 10.3233/CH-2009-1221. [DOI] [PubMed] [Google Scholar]
- 66.Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675–1681. doi: 10.2214/AJR.08.1262. [DOI] [PubMed] [Google Scholar]
- 67.Numata K, Tanaka K, Kiba T, Matsumoto S, Iwase S, Hara K, Kirikoshi H, Morita K, Saito S, Sekihara H. Nonresectable hepatocellular carcinoma: improved percutaneous ethanol injection therapy guided by CO(2)-enhanced sonography. AJR Am J Roentgenol. 2001;177:789–798. doi: 10.2214/ajr.177.4.1770789. [DOI] [PubMed] [Google Scholar]