Abstract
As a result of many advantages, such as absence of radiation exposure, non-invasiveness, low cost, safety, and ready availability, transthoracic ultrasonography (TUS) represents an emerging and useful technique in the management of pleural and pulmonary diseases. In this first part of a comprehensive review dealing with the role of TUS in pleuropulmonary pathology, the examination technique, limits, normal findings, and sonographic artefacts and morphology of the most important and frequent pleural diseases are described. In particular, this first part deals with the capability of TUS in detecting pleural effusion and differentiating pleural fluid from pleural thickening; its usefulness in detecting pneumothorax on the basis of the changes in the artefacts detectable in the normally aerated lung and the appearance of pathologic artefacts; and its role in detecting pleural-based lesions and classifying them into extrapleural, pleural, and parenchymal lesions. Finally, the limits of TUS when compared with computed tomography of the chest are described, highlighting the inability of TUS to depict lesions that are not in contact with the pleura or are located under bony structures, poor visualization of the mediastinum, and the need for very experienced examiners to obtain reliable results.
Keywords: Ultrasonography, Pleural diseases, Lung diseases
INTRODUCTION
Thoracic diseases are commonly investigated using chest radiography and chest computed tomography (CT). In recent years, new techniques have emerged in thoracic imaging, such as spiral CT scanning, high-resolution CT scanning, and magnetic resonance imaging (MRI). At the same time, transthoracic ultrasonography (TUS) of the chest wall, lung and pleura has gained in popularity in the diagnostic work-up of pleuropulmonary diseases, particularly in critically ill patients. Several studies have recently proved its accuracy in detecting and characterizing pleural-based nodules, and identifying and quantifying pleural effusion. TUS is a feasible technique that can be performed in the emergency room, or directly at the patient’s bedside. Moreover, it provides many other advantages including the absence of radiation exposure, non-invasiveness, low cost, safety, and the possibility to guide real-time aspiration of pleural effusion, and percutaneous biopsy of peripheral pulmonary lesions abutting the pleura.
The aim of this paper is to describe the sonographic appearance of the most important pleural and pulmonary diseases in order to define the actual role of TUS in the diagnostic work-up of lung pathology, and to introduce some preliminary data about the role of contrast-enhanced ultrasonography in the study of pulmonary pleural-based lesions. In the first part of this comprehensive review on the role of TUS in pleuropulmonary pathology, the examination technique, limits, normal findings, and sonographic artefacts and morphology of the most important and frequent pleural diseases (pleural effusion, pneumothorax, focal pleural lesions) are described.
EXAMINATION TECHNIQUE, NORMAL ANATOMY AND ARTEFACTS IN TUS
Ultrasonographic examination of the lung and pleura can be performed using conventional ultrasound equipment with linear and convex probes of 3.5-7.5 MHz, or even 10.0 MHz, using the intercostal spaces as an acoustic window. In some cases, this can be supplemented by the use of color Doppler and ultrasound contrast agents.
Most important, it must always be kept in mind that the diagnostic information provided by TUS is limited by the total reflection of sound waves by tissues that contain air and by the absorption of sound waves by bony structures. It follows that lesions within the aerated lung not in contact with the pleura, and subscapular, paravertebral, retrosternal or rear mediastinal lesions cannot be visualized with TUS. Likewise, TUS cannot provide any useful information in the presence of subcutaneous emphysema. However, a total of about 70% of the pleural surface is accessible to ultrasound examination. To perform posterior scans of the thorax, the patient should stay in a sitting or lateral position, while to perform anterior scans, the patient can be in a supine or sitting position. For optimal examination of the anterior and posterior parts of the chest, the arms of the patient should be elevated and the hands clasped behind the neck. The probe should be moved in longitudinal and transverse directions to visualize the pleura and the lung surface through the intercostal spaces, thereby avoiding the ribs.
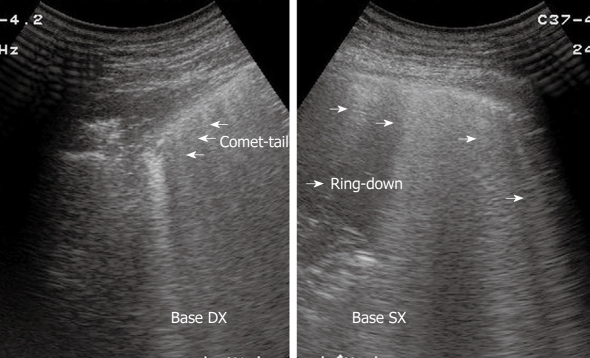
The pleural band of reflections that can be readily delimited from the soft tissues of the thoracic wall consists of several components (the endothoracic fascia, parietal and visceral pleura, and interface reflections to aerated lung), and is created mainly by high-amplitude echoes at the boundary between the pleura and the aerated lung. Distal to this band of reflection, a largely homogeneous area of reverberation echoes is visible, which is elicited by total reflection of the sound waves at the lung surface[1-5]. Based on these concepts, the normal artefacts originating from the lung and pleura should be well known, before approaching the study of pleural and pulmonary diseases. Indeed, respiration-dependent movement of the visceral pleura and lung surface with respect to the parietal pleura and chest wall (“gliding sign” or “lung sliding”) can be easily identified by real-time sonography, particularly in healthy lungs[1-6]. Moreover, intensive band-like reverberation echoes (reverberation and comet-tail artefacts) evoked during breathing movements can be seen at the boundary between the pleura and the ventilated lung tissue[1-4]. As a consequence, it is assumed that reverberation and comet-tail artefacts can be evoked only at the boundary between the visceral pleura and the normally aerated lung. Comet-tail artefacts are generally sporadic in healthy lungs, and become more frequent in diffuse parenchymal diseases[7,8]. These common artefacts can appear as multiple parallel transversal echoes departing from the pleural line (reverberation artefacts, Figure 1), or as some discrete thickening comet-tail-like echoes originating from the pleura (comet-tail artefacts, Figures 2 and 3), depending on the difference in acoustic impedance between the pleura and the tissue nearby[1-4,6]. Moreover, when a fluid component is present in the pulmonary alveoli or interalveolar septa near the pleura, the so called ring-down vertical artefacts (Figures 3 and 4) can be evoked as a series of hyperechoic narrow-based bands or streaks spreading from the pleural line into the lung surface[1-4,6]. It is assumed that the ring-down artefacts are frequent in interstitial lung diseases when a fluid collection is present (e.g. pulmonary edema)[8,9].
Figure 1.
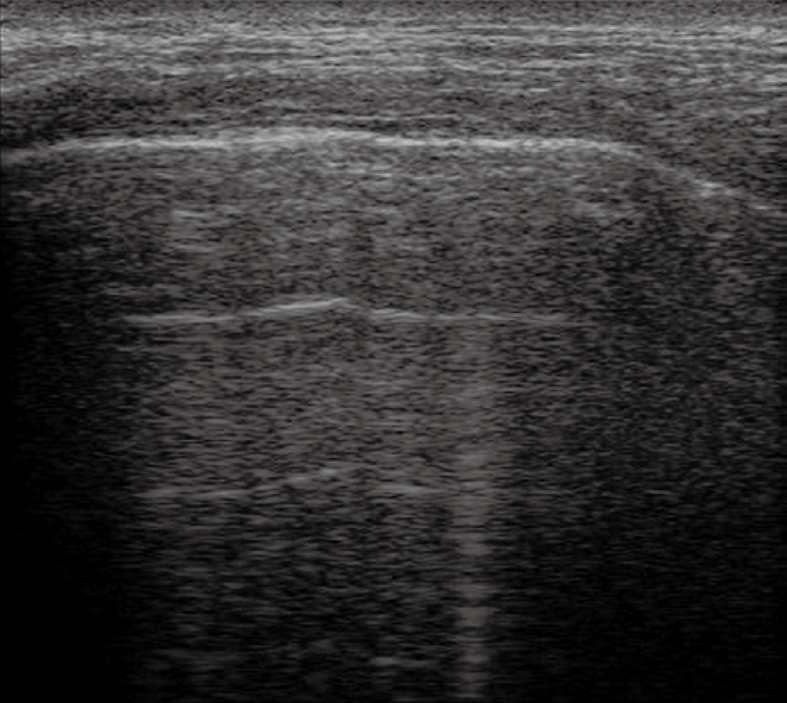
Normal lung. Multiple parallel transversal echoes departing from the pleural line (reverberation artefacts) and a single vertical artefact.
Figure 2.
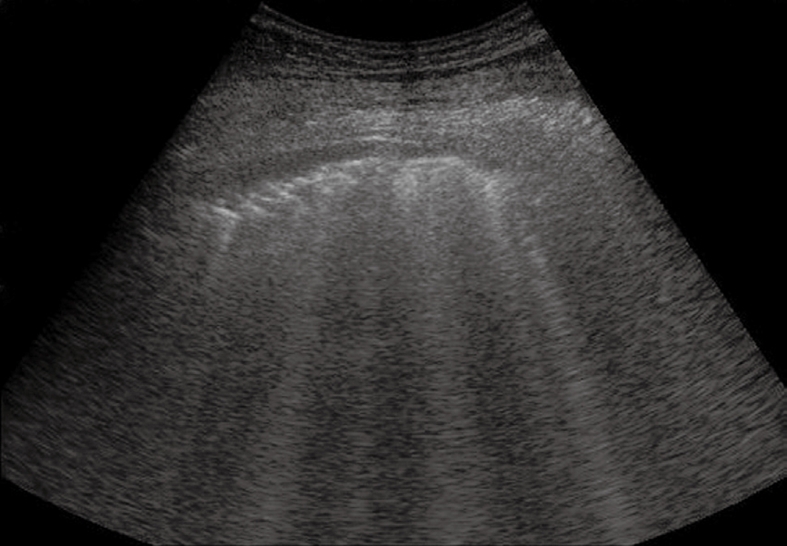
Pulmonary fibrosis. Multiple vertical comet-tail artefacts and sporadic ring-down artefacts. The pleural line appears thick and irregular.
Figure 3.
Right and left lung showing comet-tail and ring-down artefacts, respectively.
Figure 4.
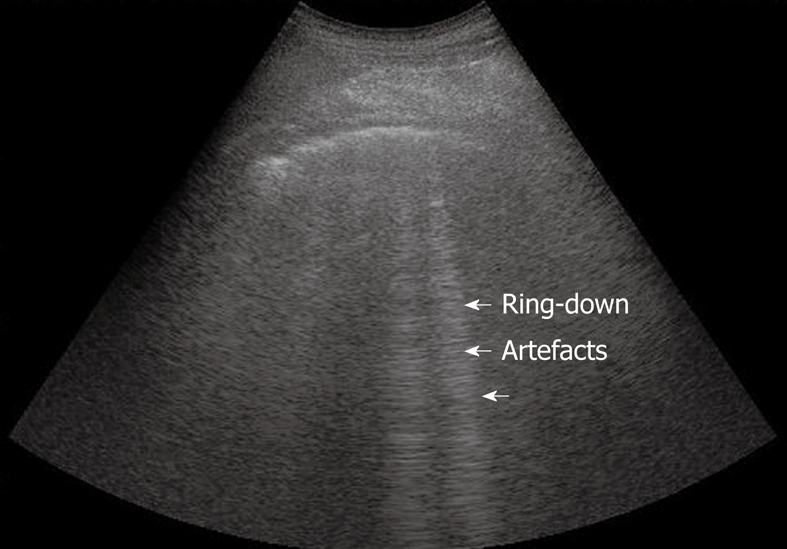
Normal lung. Sporadic ring-down artefacts spreading from the pleural line into the lung surface.
The main artefacts originating from lung and pleura and their description are summarized in the Table 1.
Table 1.
Main findings in TUS study of lung and pleura
| TUS finding | Definition |
| Gliding sign or lung sliding | Respiration-dependent movement of the visceral pleura and lung surface with respect to the parietal pleura and chest wall. Normal finding |
| Reverberation artefacts | Multiple parallel transversal echoes departing from and reproducing the pleural line. Normal finding |
| Comet-tail artefacts | Discrete thickening comet-tail like echoes originating from the pleura. Normal finding1 |
| Ring-down artefacts | Vertical spreading in the depth echoes. Edema; interstitial diseases |
| Lung point | Boundary between pneumothorax and aerated lung |
| Lung pulse | Slow sliding depending on heart beats. It excludes PNX; can suggest atelectasis |
| Bronchogram | Air inside the bronchi2 |
Generally sporadic, becoming more numerous in diffuse parenchymal diseases;
If air moves inside the bronchi (dynamic bronchogram), atelectasis is excluded. If air does not move and appears as multiple parallel and fixed echogenic strains, it suggests atelectasis. TUS: Transthoracic ultrasonography.
PLEURAL DISEASES
For the purposes of this article, the main pleural diseases are divided into pleural effusion, pneumothorax, and focal solid pleural lesions.
Pleural effusion
The capability of TUS in detecting pleural effusion and differentiating pleural fluid and pleural thickening has been well established in recent years[1,3,4,6,10-14]. Moreover, TUS has been proved to be more accurate and preferable to radiographic measurement in the quantification of pleural effusion[11,15]. Consequently, according to its ready availability, TUS has become a major imaging modality in determining the presence and nature of pleural effusion, and in guiding the aspiration of pleural fluid or the placement of chest tubes[3,16].
Pleural effusion can be divided into transudates (protein concentration < 3 g/100 mL) caused by systemic factors, and exudates (protein concentration > 3 g/100 mL) caused by inflammatory or neoplastic diseases[3]. To determine ultrasonographically the nature of pleural effusion, Yang et al[13] have suggested to classify pleural effusion into anechoic, complex non-septated, complex septated, and homogeneously echogenic effusion. According to this classification, the effusion is defined as anechoic if echo-free spaces are present between the visceral and parietal pleura (Figure 5); complex non-septated if heterogeneous echogenic material is inside the anechoic pleural effusion (Figure 6); complex septated if fibrin strands or septa are floating inside the anechoic pleural effusion (Figure 7A and B); and homogeneously echogenic if homogeneously echogenic spaces are present between visceral and parietal pleura (Figure 8A and B). Transudates usually appear anechoic with an echo-free pattern, although partially treated transudates of congestive heart failure may occasionally be echogenic[17]. However, anechoic effusion can be either a transudate or an exudate. Moreover, although hemorrhagic effusion, empyema, and chylothorax usually present as homogeneously echogenic effusion, differential diagnosis among these entities is not possible with TUS, and explorative thoracentesis with macroscopic and physicochemical examination of the fluid may be required to clarify the nature of the effusion.
Figure 5.
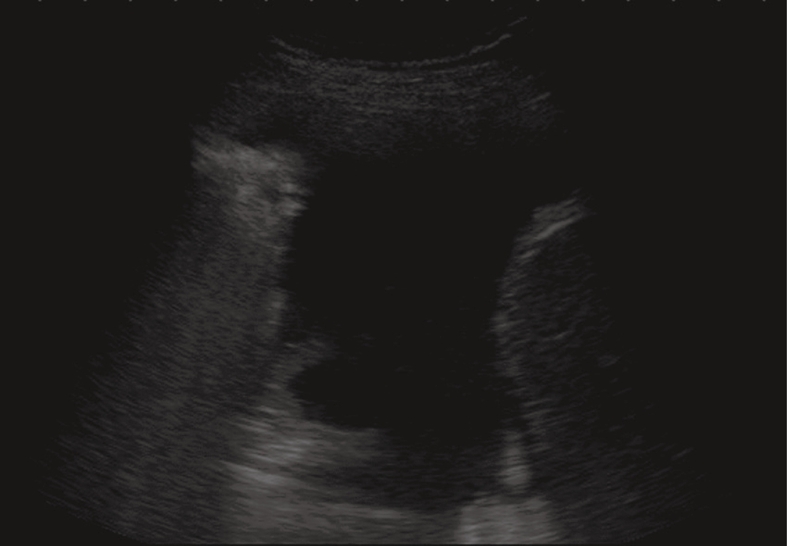
Anechoic echo-free pleural effusion.
Figure 6.
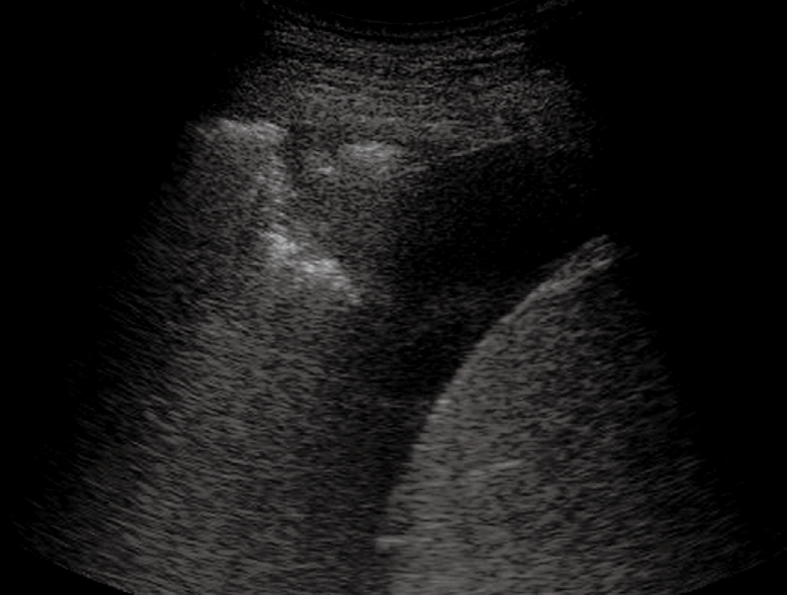
Heterogeneous echogenic material inside the anechoic pleural effusion.
Figure 7.
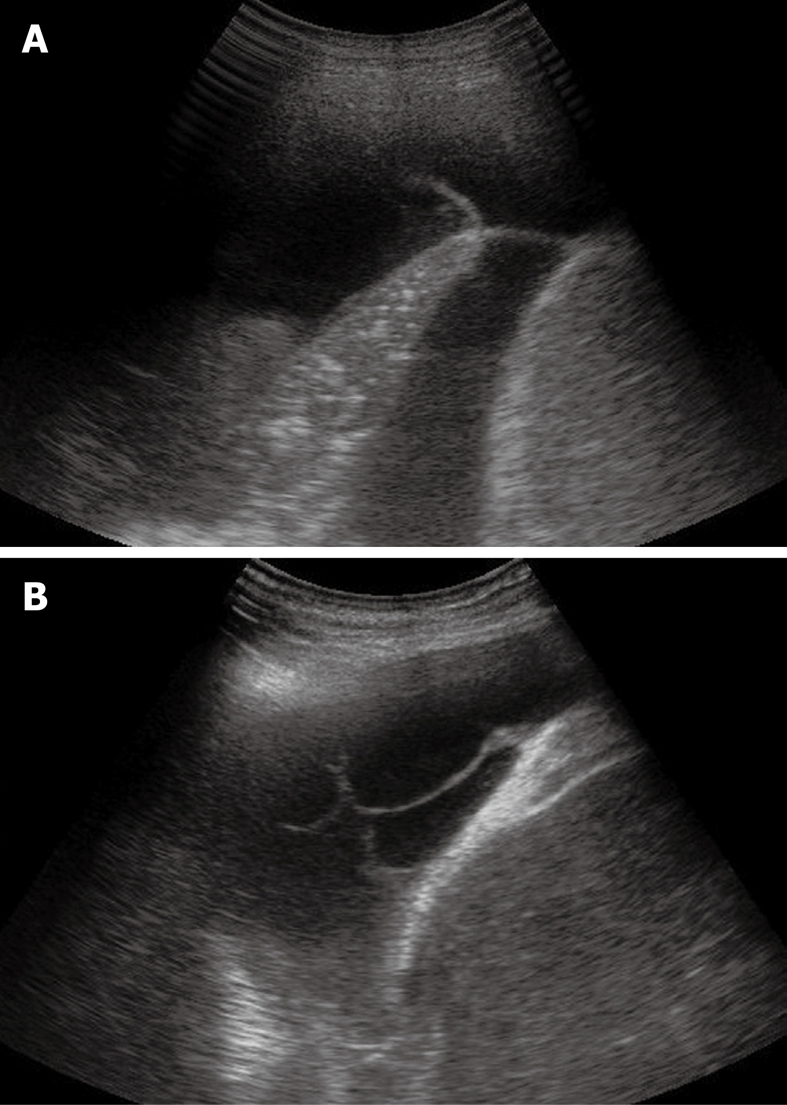
Sporadic (A) and multiple (B) fibrin strands or septa floating inside the anechoic pleural effusion.
Figure 8.
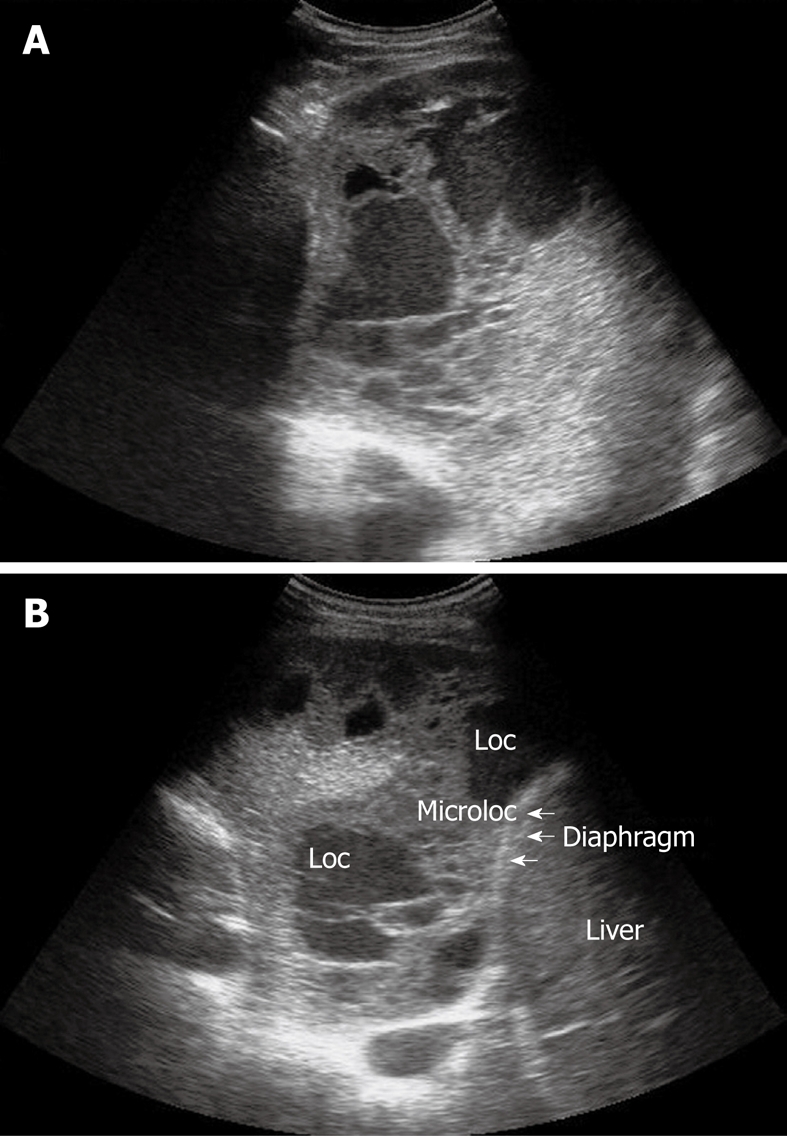
Homogeneous echogenic material inside the pleural space (A and B). Loc: Loculation; Microloc: Microloculation.
Several formulas have been proposed to quantify pleural effusion, but their usefulness in clinical practice is questionable. However, TUS is considered preferable to radiographic measurement to obtain an empirical estimate of the volume. Since the distribution of pleural fluid in the pleural space is dependent on the position of the patient, the use of a standard sonographic method of evaluation with the patient in the sitting position is recommended[3].
Pneumothorax
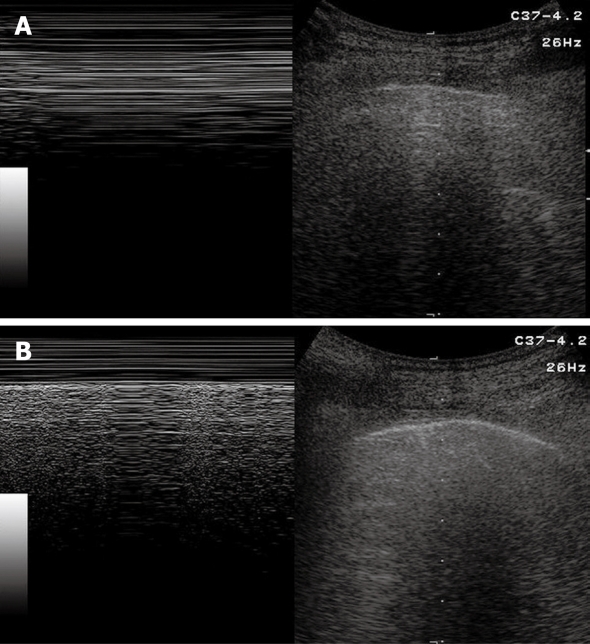
Sonographic images of the lung are composed exclusively of artefacts because air prevents transmission of the ultrasound beam[1-4]. Consequently, it would seem theoretically inconceivable that air in the lungs can be distinguished ultrasonographically from air in the pleural space. However, TUS is able to depict a pneumothorax, as the artefacts in air-containing lungs differ from those induced by air in the pleural space. Indeed, the presence of air in the pleural space prevents sonographic visualization of visceral pleural movements during breathing with disappearance of the gliding sign and comet-tail artefacts, which can be evoked only at the boundary between the visceral pleura and ventilated pulmonary alveoli. Moreover, the presence of air in the pleural space generates reverberation artefacts that form parallel horizontal echoic lines characterized by artefactual immobility during breathing movements. These artefacts (the so-called “frozen echoes”) can be well documented with M-mode imaging (Figure 9A), and can be easily distinguished from the normal “frosted glass-like” artefacts due to the breath-dependent movements (Figure 9B)[18-24].
Figure 9.
Pneumothorax. A: B-mode image (right image) shows horizontal reverberation artefacts corresponding to frozen echoes in M-mode image (left image), due to loss of breathing-dependent motion of pleural line; B: After resolution of pneumothorax, B-mode image (right image) shows pleural line and a single comet-tail artefact; in M-mode image (left image), breathing-dependent movements appear as “frosted glass” artefacts, quite different from frozen echoes seen in A.
Based on these findings, some studies have demonstrated that TUS enables us to detect even very small amounts of air when parasternal anterior scans with supine patients are performed. Such ability plays a very important role particularly in severely ill or traumatized patient. Chest radiography in standing patients is commonly considered the method of choice for the diagnosis of pneumothorax. However, as a rule, the severely ill or traumatized patients can only be subjected to radiography in the recumbent position, which makes diagnosis of a small pneumothorax much more difficult. As a consequence of this limit of chest radiography, in recent years, bedside TUS has gained increasing consideration as a first-line approach in emergency departments and intensive care units to evaluate unstable patients[25-30]. However, TUS cannot replace CT in the diagnostic work-up of critically ill patients, especially if they are traumatized, and CT should always be performed when the patients have become stable.
TUS has been proven to be at least as effective as chest radiography in detecting or excluding pneumothorax after interventional thoracic procedures[24,31,32]. In a recent prospective comparison with chest radiography, Sartori et al[24] have reported a sensitivity and specificity of 100% of TUS for detection of pneumothorax after sonographically guided lung biopsy, versus a sensitivity of 87.5% and specificity of 100% for chest radiography. In another study performed in patients undergoing a miscellany of interventional thoracic procedures, Reißig et al[32] have observed a sensitivity and specificity of 100% for TUS in excluding post-interventional pneumothorax, versus a sensitivity of 98% and specificity of 100% for chest radiography. However, despite its high accuracy in detecting pneumothorax, TUS is not yet considered a reliable tool for estimating the volume of pneumothorax. The size of pneumothorax can be roughly approximated by assessment of the lung point, defined as the boundary between the pleural air sickle and the reappearance of normally moving visceral pleura (Figure 10)[33]. However, the depth of pulmonary collapse cannot be evaluated, and chest radiography is necessary to quantify pneumothorax when the lung point is identified far from the site of biopsy needle entry or drainage placement[34].
Figure 10.
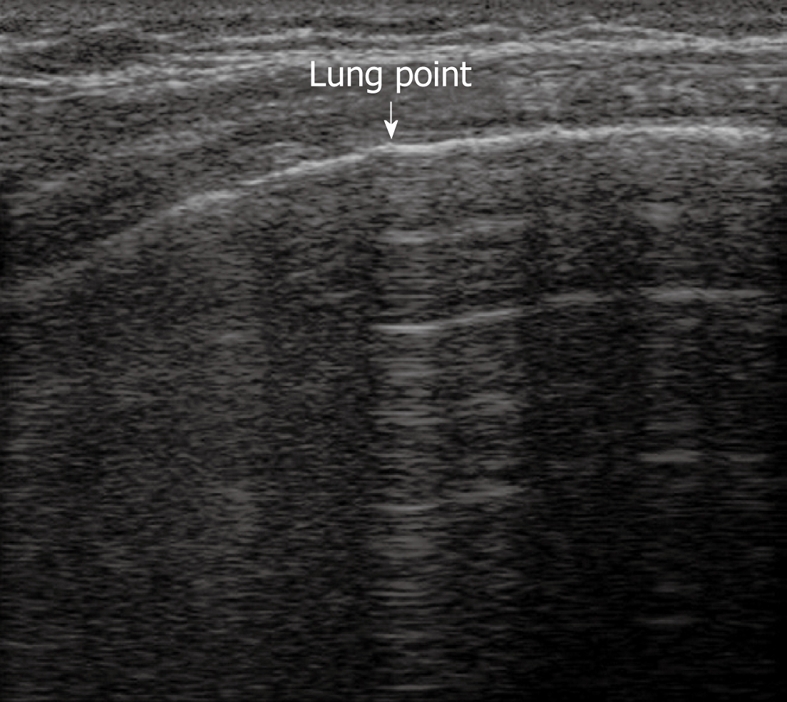
Lung point. Horizontal reverberation artefacts are interrupted by reappearance of irregular, fragmented, thickened pleural line with comet-tail artefacts.
Focal pleural lesions
TUS has been proved to be far superior to chest radiography in classifying pleural-based lesions into extrapleural, pleural, and parenchymal lesions[3]. Extrapleural masses usually displace the overlying parietal and visceral pleura, which results in an obtuse angle between the tumor and the chest wall. If rib destruction or muscle infiltration can also be demonstrated, the extrapleural site of origin of the lesion is further confirmed (Figure 11). Pleural lesions arising from the visceral or parietal pleura are usually confined to the pleural space and show an ovoid or trapezoidal shape with irregular, knotty, or polypoid profiles (Figures 12 and 13), sometimes similar to that of extrapleural lesions. Peripheral pulmonary lesions abutting the pleura typically show an acute angle between the lesion and the chest wall (Figure 14). However, there may be some overlap in the appearance of extrapleural, pleural, and peripheral subpleural lung lesions. Large extrapleural tumors may invade the pleural space and pulmonary parenchyma, pedunculated pleural tumors may prolapse into the adjacent lung parenchyma, which results in acute angles between the lesion and the chest wall, and large pulmonary tumors that broadly infiltrate the pleura may show an obtuse angle with the chest wall. However, the role of TUS in the detection and differential diagnosis of focal pleural lesions is limited. Indeed, unless the tumor is large enough to allow clear depiction and imaging-guided biopsy, the diagnosis of focal pleural lesions is challenging for all imaging techniques. In this regard, some reports have recently suggested a good diagnostic yield of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (PET) and PET/CT[35,36], but at present, thoracoscopy is often needed to achieve a reliable diagnosis of focal pleural lesions.
Figure 11.
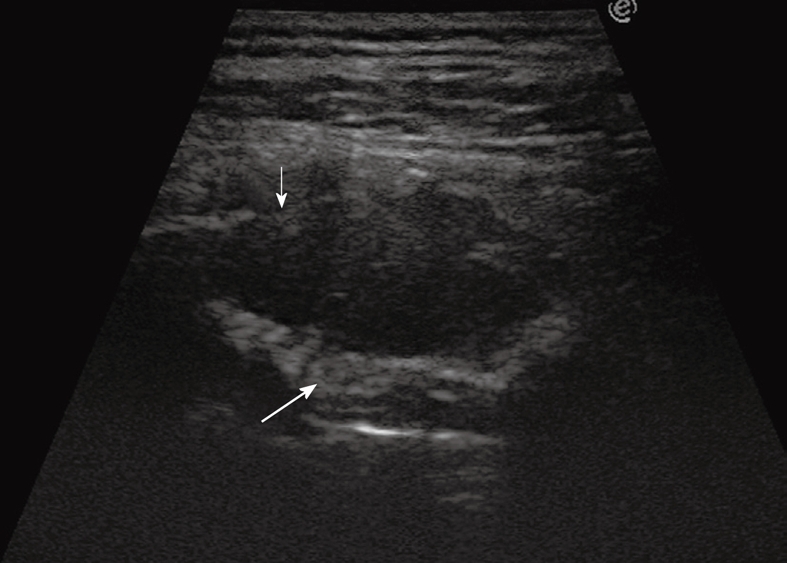
Extrapleural mass disrupting a rib (thin arrow) and displacing and disrupting the pleural line (large arrow).
Figure 12.
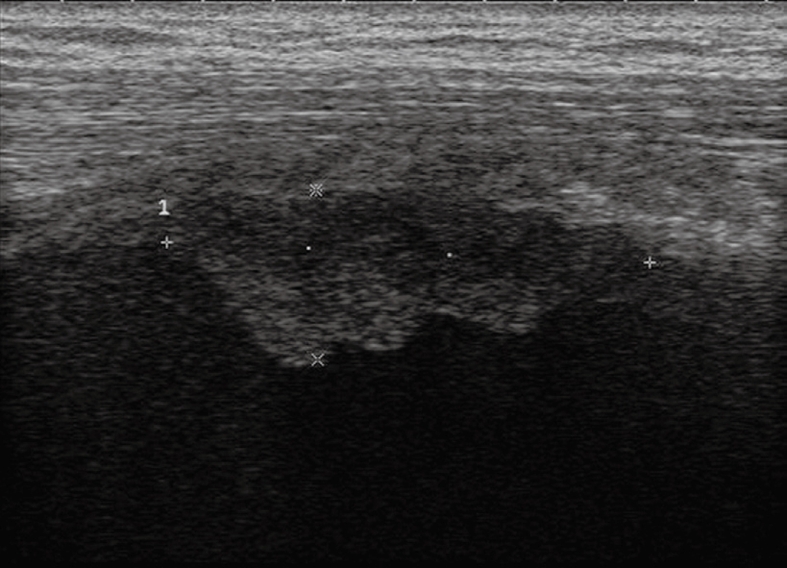
Pleural mesothelioma. Solid pleural mass with irregular lobulated borders.
Figure 13.
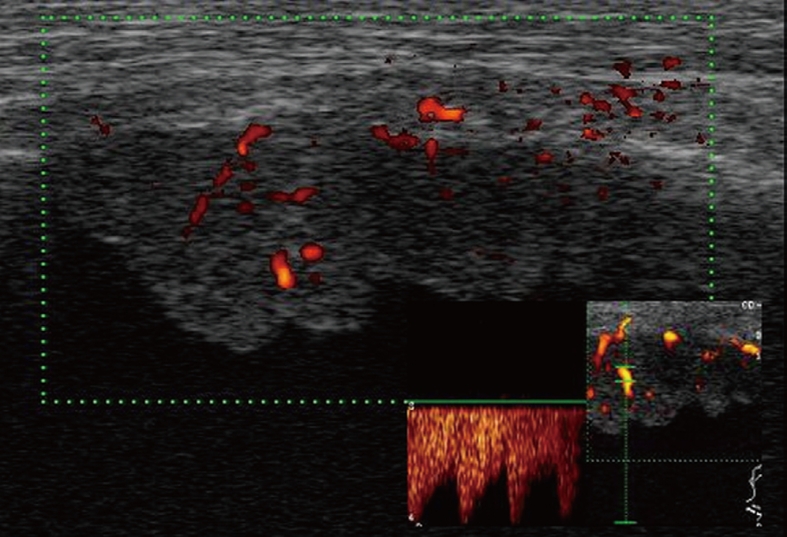
Color Doppler sonography shows flow signals inside the lesion, with mainly arterial vascularization.
Figure 14.
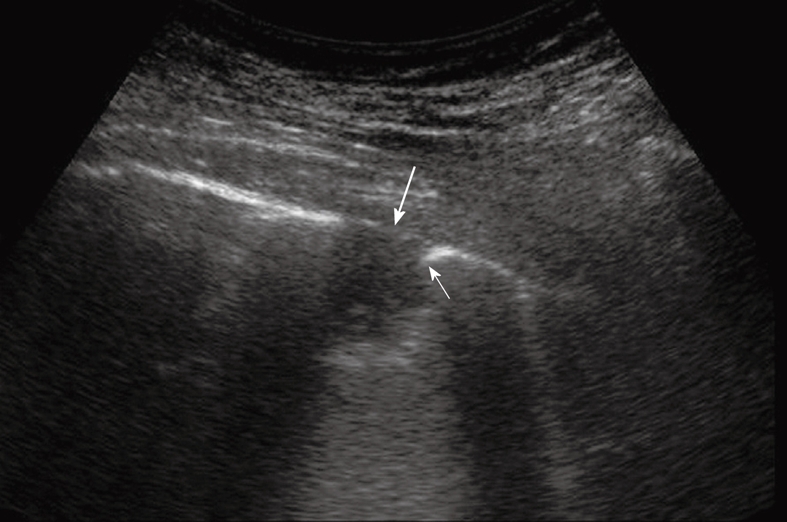
Peripheral pulmonary nodule abutting and infiltrating the pleura. Note the acute angle between the nodule and the pleural line (thin arrow) and the disruption of the pleural line (large arrow).
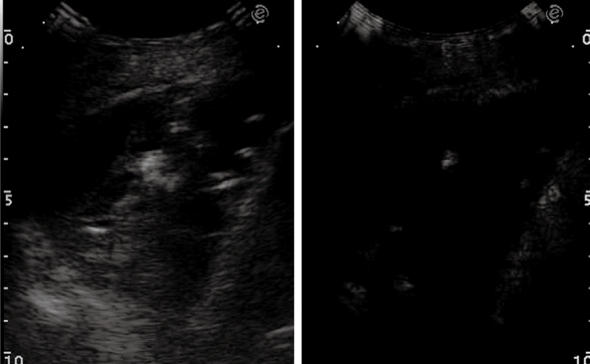
One of the most interesting advantages of TUS is its ability to assess the presence of pleural tumor infiltration by real-time imaging. If the subpleural parenchymal tumor shows breathing-dependent up and down movements, infiltration of the pleura can be excluded. Conversely, if the pulmonary tumor does not move during breathing and the pleural line appears disrupted, fixation to the parietal pleura due to neoplastic infiltration or desmoplastic reaction has occurred. Moreover, in extrapleural lesions infiltration of the visceral pleura can be demonstrated when the comet tail artefacts, which normally move up and down during breathing, become fixed. TUS is also superior to chest radiography in differentiating loculated effusion from solid pleural tumors, and in defining the internal structure of a pleural mass. Color Doppler sonography can help in differentiating homogeneously echogenic loculated effusion from solid lesions, although it cannot depict very slow flow signals. In these cases, low mechanical index, contrast-enhanced sonography with second generation contrast agents could represent a reliable tool to differentiate vascularized tissue lesions (Figure 15A and B) from non-vascularized echogenic effusion (Figure 16). However, at present, there are no definitive data in the literature to support such an assumption.
Figure 15.
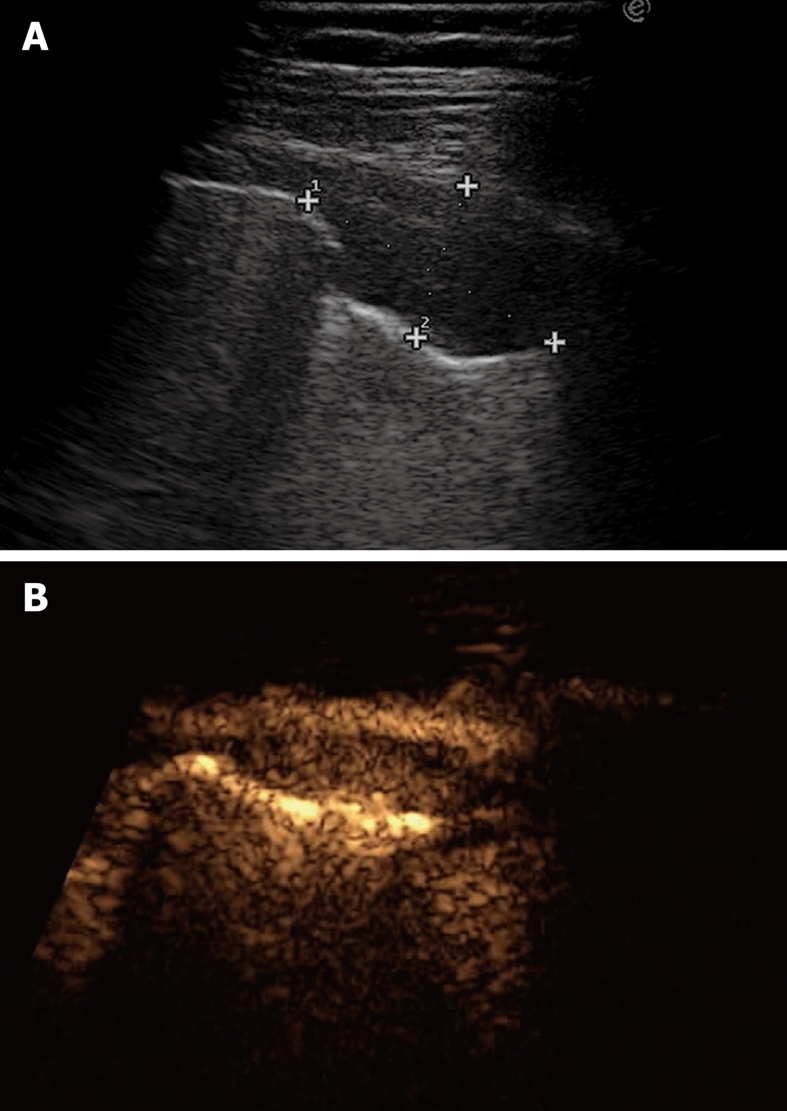
Pleural metastasis. A: B-mode sonogram shows a hypoechoic lesion resembling either a solid mass or a homogeneously echoic saccate effusion; B: Contrast-enhanced sonography shows contrast enhancement of the lesion 20 s after bolus injection of ultrasound contrast agent. Sonographically-guided biopsy confirmed metastasis from breast carcinoma.
Figure 16.
Pleural effusion with broad echoic debris mimicking a solid lesion (left image). Contrast-enhanced sonography shows no enhancement within the effusion (right image).
Benign pleural lesions are quite rare, and include pleural thickening or plaques, fibroma, and lipoma. They usually present as hyperechoic, well-defined pleural lesions that form an obtuse angle with the chest wall. Tumors of the peripheral nerves (neurofibroma, schwannoma) are extrapleural masses of the thoracic wall that may not infrequently mimic a pleural tumor. They are generally benign, but sometimes they can also be malignant, are usually round, sharply marginated masses of high, mixed or low echogenicity. However, in most cases, sonographic features of benign pleural lesions and neurogenic tumors cannot provide a definitive diagnosis, and pathological confirmation is often needed[3,37,38].
Malignant pleural lesions include pleural mesothelioma, pleural metastasis, pleural infiltration of bronchogenic carcinoma, and pleural lymphoma (very infrequent). Pleural mesothelioma usually appears as a diffuse pleural thickening or as a hypoechoic or isoechoic vascularized mass with irregular shape or lobulated borders (Figures 12 and 13). Pleural effusion, frequently hemorrhagic, is often present. Pleural metastases constitute the majority of malignant neoplasms that involve the pleura. Bronchogenic carcinoma is the first cause of pleural metastasis, but primary neoplasms of breast, gastrointestinal tract, kidney and ovaries are also frequent sources. Usually, pleural metastases become manifest as pleural effusion, as they are frequently too small (< 1-2 mm) to be detected by the imaging techniques (TUS or CT). However, TUS plays an important role as the method of choice to guide thoracentesis for cytological confirmation. Pleural metastases that can be detected by TUS usually appear as relatively small hypoechoic lesions with obtuse margins with the chest wall (Figure 15A), or as large masses with complex echogenicity. Conversely, pleural involvement from bronchogenic carcinoma typically appears as a hypoechoic mass with acute angulation between the lesion and the chest wall. As previously described, TUS can easily exclude or document infiltration of the parietal pleura by assessing the mobility of the tumor during breathing movements. However, desmoplastic reaction and inflammation can fix the tumor to the adjacent pleura and disrupt the pleural line, thus simulating an invasive lesion. Although in some cases the sonographic features of the lesions can help to establish the nature of the tumor, cyto-histological confirmation is often needed, and TUS is the method of choice to guide pleural biopsy. TUS-guided biopsy of pleural lesions is a safe and effective technique, and when the lesions can be well identified, it represents a feasible tool to obtain tissue samples with a very low risk of pneumothorax, as the procedure can be monitored in real-time and aerated lung tissue is usually not penetrated by the biopsy needle[16,24,39].
CONCLUSION
TUS is gaining increasing importance in the diagnostic work-up of pleural pathology, and in recent years, several studies have dealt with its usefulness and advantages in this setting. Besides the lack of radiation exposure, it appears to be as effective as chest radiography in detecting or excluding pneumothorax, is superior to chest radiography in detecting and characterizing pleural effusion, and is considered the method of choice to guide pleural fluid aspiration and percutaneous biopsy of pleural-based lesions. Moreover, unlike radiological methods, portable ultrasound devices allow examination at almost any location, and such an advantage can play an important role in emergency departments and intensive care units.
However, despite these advantages, TUS has some limits and cannot be considered as an alternative to thoracic CT for the study of pleural pathology. TUS achieves only poor visualization of the mediastinum (in particular the rear mediastinum), and the sonographic waves are hindered by air and bony structures. As a consequence, TUS does not provide any diagnostic information in the presence of subcutaneous emphysema, and cannot visualize subscapular, paravertebral and retrosternal lesions. Although TUS can provide useful and reliable information about parietal pleural infiltration of lung tumors, CT is superior to TUS for investigating focal and diffuse pleural diseases, as it enables one to evaluate all parts of the pleura, including the mediastinal pleura, and can better delineate the pulmonary and pleural components of a mass. Moreover, TUS is strictly operator dependent, and only skilled examiners with a lot of experience can obtain good and reliable results.
Nonetheless, TUS can provide immediate diagnostic information in particular settings, such as the suspicion of pleural effusion and detection or exclusion of pneumothorax after interventional procedures or in critically ill, unstable patients. Moreover, it can add interesting information in nearly all of the most common pleural diseases, and in our opinion, TUS should be included as a useful, complementary tool in an up-to-date multimodality approach to the diagnostic work-up of pleural pathology.
Footnotes
Peer reviewers: Yasunori Minami, MD, PhD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, 377-2 Ohno-higashi Osaka-sayama Osaka 589-8511, Japan; Tilo Niemann, MD, Department of Radiology, University Hospital Basel, Petersgraben 4, CH-4031 Basel, Switzerland
References
- 1.Soldati G, Copetti R. Ecografia toracica. Torino: CG Edizioni Medico-Scientifiche; 2006. [Google Scholar]
- 2.Soldati G. Lung sonography: artifact, movement or echotexture? J Ultrasound. 2001;4:329–338. [Google Scholar]
- 3.Wernecke K. Ultrasound study of the pleura. Eur Radiol. 2000;10:1515–1523. doi: 10.1007/s003300000526. [DOI] [PubMed] [Google Scholar]
- 4.Reuss J. Sonographic imaging of the pleura: nearly 30 years experience. Eur J Ultrasound. 1996;3:25–39. [Google Scholar]
- 5.Beckh S, Bölcskei PL, Lessnau KD. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759–73. doi: 10.1378/chest.122.5.1759. [DOI] [PubMed] [Google Scholar]
- 6.Mathis G. Thoraxsonography--Part I: Chest wall and pleura. Ultrasound Med Biol. 1997;23:1131–1139. doi: 10.1016/s0301-5629(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 8.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 9.Avruch L, Cooperberg PL. The ring-down artifact. J Ultrasound Med. 1985;4:21–28. doi: 10.7863/jum.1985.4.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Acunas B, Celik L, Acunas A. Chest sonography. Differentiation of pulmonary consolidation from pleural disease. Acta Radiol. 1989;30:273–275. [PubMed] [Google Scholar]
- 11.Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hörmann MF, Grabenwöger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191:681–684. doi: 10.1148/radiology.191.3.8184046. [DOI] [PubMed] [Google Scholar]
- 12.Müller NL. Imaging of the pleura. Radiology. 1993;186:297–309. doi: 10.1148/radiology.186.2.8421723. [DOI] [PubMed] [Google Scholar]
- 13.Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159:29–33. doi: 10.2214/ajr.159.1.1609716. [DOI] [PubMed] [Google Scholar]
- 14.McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156:1145–1153. doi: 10.2214/ajr.156.6.2028857. [DOI] [PubMed] [Google Scholar]
- 15.Gryminski J, Krakówka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70:33–37. doi: 10.1378/chest.70.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Mathis G, Bitschnau R, Gehmacher O, Dirschmid K. [Ultrasound-guided transthoracic puncture] Ultraschall Med. 1999;20:226–235. doi: 10.1055/s-1999-8920. [DOI] [PubMed] [Google Scholar]
- 17.Feldman DJ. Localized interlobar pleural effusion in heart failure. J Am Med Assoc. 1951;146:1408–1409. doi: 10.1001/jama.1951.63670150003012a. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DJ Pneumothorax. In: Fraser RG, Pare JAP, Pare PD, Fraser RS, Generux GP, editors. Diagnosis of diseases of the chest. Philadelphia: W.B. Saunders; 1991. pp. 2741–2750. [Google Scholar]
- 19.Wernecke K, Galanski M, Peters PE, Hansen J. Pneumothorax: evaluation by ultrasound--preliminary results. J Thorac Imaging. 1987;2:76–78. [PubMed] [Google Scholar]
- 20.Targhetta R, Bourgeois JM, Chavagneux R, Coste E, Amy D, Balmes P, Pourcelot L. Ultrasonic signs of pneumothorax: preliminary work. J Clin Ultrasound. 1993;21:245–250. doi: 10.1002/jcu.1870210406. [DOI] [PubMed] [Google Scholar]
- 21.Goodman TR, Traill ZC, Phillips AJ, Berger J, Gleeson FV. Ultrasound detection of pneumothorax. Clin Radiol. 1999;54:736–739. doi: 10.1016/s0009-9260(99)91175-3. [DOI] [PubMed] [Google Scholar]
- 22.Garofalo G, Busso M, Perotto F, De Pascale A, Fava C. Ultrasound diagnosis of pneumothorax. Radiol Med. 2006;111:516–525. doi: 10.1007/s11547-006-0047-y. [DOI] [PubMed] [Google Scholar]
- 23.Chung MJ, Goo JM, Im JG, Cho JM, Cho SB, Kim SJ. Value of high-resolution ultrasound in detecting a pneumothorax. Eur Radiol. 2005;15:930–935. doi: 10.1007/s00330-004-2518-7. [DOI] [PubMed] [Google Scholar]
- 24.Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188:37–41. doi: 10.2214/AJR.05.1716. [DOI] [PubMed] [Google Scholar]
- 25.Soldati G, Rossi M. Traumatic pneumothorax: urgent sonographic diagnosis. J Ultrasound. 2000;3:269–274. [Google Scholar]
- 26.Dulchavsky SA, Schwarz KL, Kirkpatrick AW, Billica RD, Williams DR, Diebel LN, Campbell MR, Sargysan AE, Hamilton DR. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001;50:201–205. doi: 10.1097/00005373-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Targhetta R, Bourgeois JM, Chavagneux R, Marty-Double C, Balmes P. Ultrasonographic approach to diagnosing hydropneumothorax. Chest. 1992;101:931–934. doi: 10.1378/chest.101.4.931. [DOI] [PubMed] [Google Scholar]
- 28.Di Bartolomeo S, Sanson G, Nardi G, Scian F, Michelutto V, Lattuada L. A population-based study on pneumothorax in severely traumatized patients. J Trauma. 2001;51:677–682. doi: 10.1097/00005373-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108:1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 30.Rowan KR, Kirkpatrick AW, Liu D, Forkheim KE, Mayo JR, Nicolaou S. Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT--initial experience. Radiology. 2002;225:210–214. doi: 10.1148/radiol.2251011102. [DOI] [PubMed] [Google Scholar]
- 31.Targhetta R, Bourgeois JM, Chavagneux R, Balmes P. Diagnosis of pneumothorax by ultrasound immediately after ultrasonically guided aspiration biopsy. Chest. 1992;101:855–856. doi: 10.1378/chest.101.3.855. [DOI] [PubMed] [Google Scholar]
- 32.Reißig A, Kroegel C. Accuracy of transthoracic sonography in excluding post-interventional pneumothorax and hydropneumothorax: Comparison to chest radiography. Eur J Radiol. 2005;53:463–470. doi: 10.1016/j.ejrad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenstein D, Mezière G, Biderman P, Gepner A. The "lung point": an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 34.Sistrom CL, Reiheld CT, Gay SB, Wallace KK. Detection and estimation of the volume of pneumothorax using real-time sonography: efficacy determined by receiver operating characteristic analysis. AJR Am J Roentgenol. 1996;166:317–321. doi: 10.2214/ajr.166.2.8553938. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam RM, Wilcox B, Aubry MC, Jett J, Peller PJ. 18F-fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging of malignant pleural mesothelioma. J Med Imaging Radiat Oncol. 2009;53:160–169; quiz 170. doi: 10.1111/j.1754-9485.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- 36.Mavi A, Basu S, Cermik TF, Urhan M, Bathaii M, Thiruvenkatasamy D, Houseni M, Dadparvar S, Alavi A. Potential of dual time point FDG-PET imaging in differentiating malignant from benign pleural disease. Mol Imaging Biol. 2009;11:369–378. doi: 10.1007/s11307-009-0212-5. [DOI] [PubMed] [Google Scholar]
- 37.Müller NL. Imaging of the pleura. Radiology. 1993;186:297–309. doi: 10.1148/radiology.186.2.8421723. [DOI] [PubMed] [Google Scholar]
- 38.Wernecke K. Sonographic features of pleural disease. AJR Am J Roentgenol. 1997;168:1061–1066. doi: 10.2214/ajr.168.4.9124116. [DOI] [PubMed] [Google Scholar]
- 39.Yang PC. Ultrasound-guided transthoracic biopsy of peripheral lung, pleural, and chest-wall lesions. J Thorac Imaging. 1997;12:272–284. doi: 10.1097/00005382-199710000-00005. [DOI] [PubMed] [Google Scholar]