Abstract
There is no standard treatment for peritoneal carcinomatosis (PC) from gastric cancer. A novel multidisciplinary treatment combining bidirectional chemotherapy [neoadjuvant intraperitoneal-systemic chemotherapy protocol (NIPS)], peritonectomy, hyperthermic intraperitoneal chemoperfusion (HIPEC) and early postoperative intraperitoneal chemotherapy has been developed. In this article, we assess the indications, safety and efficacy of this treatment, review the relevant studies and introduce our experiences. The aims of NIPS are stage reduction, the eradication of peritoneal free cancer cells, and an increased incidence of complete cytoreduction (CC-0) for PC. A complete response after NIPS was obtained in 15 (50%) out of 30 patients with PC. Thus, a significantly high incidence of CC-0 can be obtained in patients with a peritoneal cancer index (PCI) ≤ 6. Using a multivariate analysis to examine the survival benefit, CC-0 and NIPS are identified as significant indicators of a good outcome. However, the high morbidity and mortality rates associated with peritonectomy and perioperative chemotherapy make stringent patient selection important. The best indications for multidisciplinary therapy are localized PC (PCI ≤ 6) from resectable gastric cancer that can be completely removed during a peritonectomy. NIPS and complete cytoreduction are essential treatment modalities for improving the survival of patients with PC from gastric cancer.
Keywords: Gastric cancer, Peritoneal carcinomatosis, Chemotherapy
INTRODUCTION
Peritoneal carcinomatosis (PC) is a stage IV factor of gastric cancer and has been generally associated with a grim prognosis[1,2]. No standard treatment for PC has been proposed and surgery or chemotherapy alone has no beneficial effect on survival.
Sugarbaker and Yonemura propose a new multimodal treatment called cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC)[3], which utilizes surgery to reduce the visible tumor burden and HIPEC to eradicate peritoneal micrometastasis and peritoneal free cancer cells (PFCCs). Survival analyses after CRS plus HIPEC have shown that complete cytoreduction is associated with an improvement in survival[3].
Neoadjuvant chemotherapy has been proposed as a method of reducing tumor burden before surgery, resulting in a higher incidence of complete cytoreduction[4]. A new bidirectional chemotherapy regimen [neoadjuvant intraperitoneal-systemic chemotherapy protocol (NIPS)] has been developed to reduce the volume and peritoneal cancer index of PC[4]. NIPS attacks PC from both sides of the peritoneum: from the peritoneal cavity and from the subperitoneal blood vessels. Accordingly, NIPS is known as bidirectional chemotherapy.
Early postoperative intraperitoneal chemotherapy (EPIC) can eradicate residual intraperitoneal cancer cells before fibrin can accumulate around residual cancer cells on the peritoneal surface.
In this review, the latest results of multidisciplinary treatment consisting of bidirectional chemotherapy (NIPS), CRS (peritonectomy), HIPEC and EPIC for patients with established PC from gastric cancer are considered.
MULTIDISCIPLINARY THERAPY FOR THE TREATMENT OF PC FROM GASTRIC CANCER
The median survival period of patients with PC from gastric cancer is reportedly 7 mo after diagnosis and best-supportive care[2]. There are many reports examining the use of systemic chemotherapy but few have focused on PC from gastric cancer. Intravenous 5FU infusion, alone or in combination with other anticancer drugs (FAM[5], FAMTX[6]), has been used in patients with advanced gastric cancer. Recently, Ajani[7] reported that DCF therapy (a combination chemotherapy using docetaxel, CDDP and 5FU) exhibited a significantly better survival outcome than CF (CDDP + 5FU) therapy. However, these regimens have little effect on the survival of patients with PC. To overcome the limitations of systemic chemotherapy, a novel combination chemotherapy regimen comprised of CRS and bidirectional chemotherapy (NIPS and EPIC) has been proposed[8-10].
Recently, a new drug named S-1 has been used for the treatment of PC from gastric cancer in Japan. Positive results for response and for the improvement of survival have been reported after the oral administration of S-1. Accordingly, S-1 or S-1 in combination with other drugs, such as cisplatinum, paclitaxel, docetaxel or irinotecan, has become the standard regimen for the treatment of PC from gastric cancer.
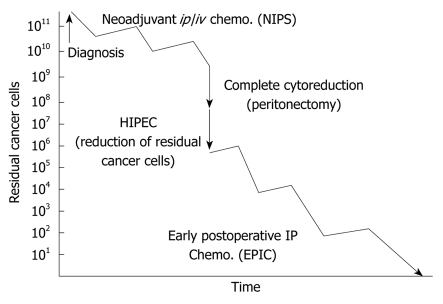
Recently, new multidisciplinary therapies combining CRS and perioperative chemotherapy have been reported[4,10]. These therapies consist of NIPS, CRS, HIPEC and EPIC (Figure 1).
Figure 1.
Treatment strategy for PC from gastric cancer. PC: Peritoneal carcinomatosis; NIPS: Neoadjuvant intraperitoneal-systemic chemotherapy protocol; HIPEC: Hyperthermic intraperitoneal chemoperfusion; EPIC: Early postoperative intraperitoneal chemotherapy.
Patients who have been diagnosed with PC based on the results of an exploratory laparotomy, diagnostic laparoscopy or computed tomography are treated with NIPS followed by CRS to enable complete cytoreduction. Immediately after CRS, HIPEC is performed for one hour. After surgery, EPIC is performed on postoperative days 1 to 5[11], and systemic chemotherapy is performed on postoperative days 30-40.
NEOADJUVANT BIDIRECTIONAL CHEMOTHERAPY (NIPS)
The aims of neoadjuvant chemotherapy (NAC) are stage reduction, eradication of micrometastasis outside the surgical field, and the improvement of resectability. Usually, systemic chemotherapy is used for NAC. In the late 1990s, TS-1, irinotecan, taxanes and docetaxel were introduced and the response rate after monotherapy with these drugs was around 20%. Combination chemotherapy with S-1 and CDDP produced outstanding results, with a response rate of 74%[12]. Yabusaki et al[13] reported the results of NAC with S-1 and CDDP in 37 advanced gastric cancer patients scheduled to undergo non-curative resection. After 2 courses of treatment, the overall response rate was 68%, but the response rate for patients with peritoneal dissemination was only 14% (2/14). S-1 plus CPT-11 and CPT-11 plus CDDP produced a high response rate of 42% and a long period of progression-free survival, but treatment failure as a result of toxicity was also observed[14].
Ajani[7] reported an excellent response rate (55.7%) to systemic DCF therapy combined with docetaxel (75 mg/m2 on day 1, q 3 wk), CDDP (75 mg/m2 on day 1, q 3 wk), and 5FU (750 mg/m2 on days 1 to 5, q 3 wk) However, the effects on PC were not described.
In addition, the one-month mortality rate and the incidence of grade 3 or 4 toxicity after DCF therapy were 8% and 60% respectively. Accordingly, a high incidence of postoperative complications can be expected during the postoperative period after DCF therapy.
These results indicate that systemic chemotherapies have minimal effects on PC[15]. In other words, the peritoneal cavity acts as a sanctuary against systemic chemotherapy probably because of the existence of a blood-peritoneal barrier consisting of stromal tissue between mesothelial cells and submesothelial blood capillaries[8]. This barrier accounts for a total thickness of 90 μm[16]. Accordingly, only a small amount of systemic drugs are capable of penetrating this barrier and passing into the peritoneal cavity so a higher percentage of the administered drugs instead moves to the bone marrow and vital organs other than the peritoneum, resulting in the development of adverse effects.
In contrast, IP chemotherapy offers potential therapeutic advantages over systemic chemotherapy by generating high local concentrations of chemotherapeutic drugs in the peritoneal cavity[9,17]. This concentration difference enables the exposure of small nodules of PC before CRS and lowers the systemic toxicity. This advantage of IP chemotherapy can be expressed by the area under the curve (AUC) ratios of intraperitoneal versus plasma exposure.
Table 1 shows the AUC IP/systemic for various drugs[15]. Relatively high AUC/systemic ratios were obtained after the IP administration of paclitaxel, docetaxel, gemcitabine, 5-fluorouracil and doxorubicin. These drugs may be good candidates for IP chemotherapy. Other important factors in the selection of drugs for IP chemotherapy are a high penetration activity into the PC nodules and chemosensitivity. Each drug has its own penetration depth into the peritoneal surface and the effective diffusion distance into tissues reportedly ranges from 100 to 1000 μm[17,18]. Adriamycin can penetrate only 4-6 cell layers of experimental tumors[18], but cisplatin and carboplatin were confirmed to penetrate 1 to 2 mm from the surface of PC nodules[17]. The penetration distance depends on the specific drug and type of tumor. Thus, any superiority of intraperitoneal chemotherapy over intravenous delivery is limited to those PC patients with very small tumor volumes of less than 2 mm.
Table 1.
Area under the curve ratios of intraperitoneal exposure to systemic agents
| Drugs | Area under the curve ratio |
| 5-Fluorouracil | 250 |
| Carboplatin | 10 |
| Cisplatin | 7.8 |
| Docetaxel | 552 |
| Doxorubicin | 230 |
| Etoposide | 65 |
| Gemcitabin | 500 |
| Irinotecan | N/A |
| Melphalan | 93 |
| Mitomycin C | 23.5 |
| Mitoxantrone | 115-255 |
| Oxaliplatin | 16 |
| Paclitaxel | 1000 |
| Pemetrexed | 40.8 |
An in vitro chemosensitivity test using the collagen-gel method in human gastric cancer tissues[4] showed that the tissues were highly sensitive to 5-FU, carboplatin, cisplatin and docetaxel. In an experimental PC model using a highly metastatic cell line derived from human gastric cancer in the peritoneal cavity, docetaxel, 5-FU, carboplatin and TS-1 plus cisplatin were highly effective for improving the survival of nude mice[19] and the IP administration of these drugs is expected to become standard therapy for gastric cancer patients[10,17,20,21].
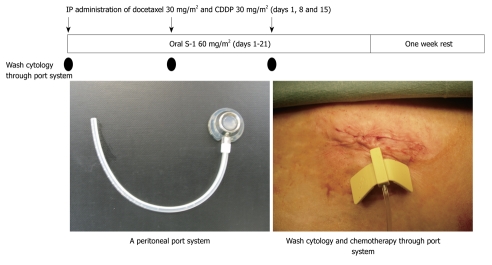
From these experimental results, a new bidirectional chemotherapy combined with the oral administration of S-1 and IP CDDP and docetaxel has been developed. By simultaneously administering intravenous and intraperitoneal chemotherapy, a bidirectional diffusion gradient can create a wider treatment area than single treatment. As shown in Figure 2, a peritoneal port system (Hickman Subcutaneous port; BARD, Salt Lake City, USA) was introduced into the abdominal cavity under local anesthesia, and the tip of the system was placed on the cul-de-sac of Douglas. Then, a peritoneal wash cytology was performed after 500 mL of physiological saline was injected into the peritoneal cavity. To improve the accuracy of the cytology, an immunohistochemical examination using monoclonal antibodies for anti-human carcinoembryonic antigen (TAKARA Bio INC., Tokyo, Japan) and anti-human epithelial antigen (DAKO, Copenhagen, Denmark) was performed. A peritoneal wash cytological examination was performed before and after NIPS.
Figure 2.
Bidirectional chemotherapy for peritoneal carcinomatosis from gastric cancer.
Patients were treated with 60 mg/m2 of oral S-1 (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) for 21 d, followed by a one week rest. On days 1, 8, and 15 after the start of oral S-1 administration, 30 mg/m2 of Taxotere and 30 mg/m2 of cisplatinum with 500 mL of saline were introduced through the port. This regimen was repeated after a one week rest[10].
Bidirectional chemotherapy is used before surgery to reduce the peritoneal surface involved by PC and to eradicate peritoneal free cancer cells (PFCCs). Accordingly, it may facilitate a complete cytoreduction after chemotherapy. This approach was given the acronym Neoadjuvant Intra Peritoneal and Systemic chemotherapy (NIPS)[22]. Yonemura et al[10] reported the outcomes of 79 gastric cancer patients with PC who were treated with NIPS: no chemotherapy-related deaths after NIPS occurred in this series. Furthermore, grade 4 bone marrow toxicity developed in only 1 (1.3%) of the 79 patients. Renal dysfunction occurred in 3 patients (3.8%) but these patients recovered fully. Accordingly, the new bidirectional chemotherapy regimen is considered to be a safe method[10].
A distinctive complication of this treatment is subcutaneous infection around the periportal space, which was observed in 3 patients (3.8%). When infection is detected, the port should be removed under local anesthesia. Peritoneal lavage cytology from a port system detected PFCCs in 65 (82.2%) of 79 patients before NIPS; these positive cytology results became negative in 41 patients (63.0%) after NIPS[10]. Positive cytology results obtained before NIPS became negative in 4 (40.0%) of 10 patients after one treatment cycle. In contrast, 37 (67.2%) of 55 patients with positive cytology results before NIPS obtained negative cytology results after two or more cycles of NIPS. Accordingly, NIPS is a very powerful treatment modality for eradicating PFCCs and two cycles of NIPS is recommended to achieve a negative cytology status.
NIPS reportedly adds to the morbidity and mortality of further surgical treatment[23,24]. Table 2 shows the surgical methods and the rate of complete cytoreduction in our experience with 30 primary gastric cancer patients who had a gastrectomy plus peritonectomy after NIPS[10]. A total gastrectomy and D2 lymphadenectomy were performed in 29 patients and some parts of the peritoneum were removed in combination with the gastrectomy. As a result, a complete cytoreduction was achieved in 24 patients (80%). No postoperative mortalities occurred but morbidities occurred in 5 (16.7%) of the 30 patients, a morbidity rate similar to that after a gastrectomy with an aggressive lymphadenectomy[25].
Table 2.
Operation methods in 30 primary gastric cancer patients with PC after NIPS
| Extent of gastrectomy |
| Total gastrectomy: 29 |
| Distal gastrectomy: 1 |
| LN dissection |
| D2 dissection: 29 |
| D1 dissection: 1 |
| Peritonectomy procedures |
| Diaphragm copula: right side 1, both side 2 |
| Colon resection : 9 |
| Hysterectomy + BSO: 9 |
| Douglasectomy: 7 |
| Small bowel/mesentery resection: 4 |
| Falciform ligament resection: 30 |
| Morrison Pouch resection: 29 |
| Omentectomy: 30 |
| Anterior leaf of transverse colon: 29 |
| Splenectomy: 28 |
| Completeness of cytoreduction |
| Complete cytoreduction: 24 (80.0%) |
PC: Peritoneal carcinomatosis; NIPS: Neoadjuvant intraperitoneal-systemic chemotherapy protocol.
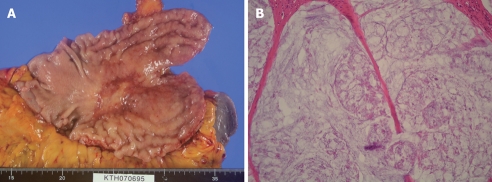
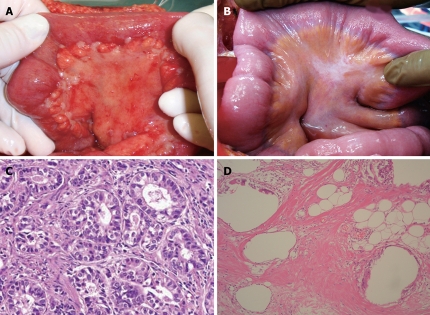
After systemic neoadjuvant chemotherapy, a complete PC response is very rare. Inokuchi et al[26] reported that the response rate of PC after S-1 plus irinotecan was 69% (9/13), but no CR was experienced for PC. Baba also reported the limited effects of systemic S-1+CDDP on PC from gastric cancer[27]. Figure 3A and B shows the macroscopic and histologic changes in the primary tumor after NIPS. Almost all the cancer cells have disappeared and only mucin remains in the primary tumor. A histologic change similar to that seen in Figure 3B corresponds to a histological grade of 3, according to the general rules for gastric cancer treatment in Japan[28]. A histologic grade of 1 means the degeneration of cancer is detected in less than two third of the tumor tissue, while a grade of 3 means the complete disappearance of the cancer cells. Histologic effects on primary tumors were found in 25 of the 30 tumors, and Grade 1, 2 and 3 evaluations were made in 10 cases (33.3%), 14 (46.7%) and 1 (3.3%) respectively. In contrast, the complete histologic disappearance of PC was observed in 15 (50%) of 30 patients (Figure 4A-D). Stage migration from stage 4 to stage 1, 2 or 3 was experienced in 10 patients (33.3%). Accordingly, NIPS is a powerful strategy for eradicating PFCCs and macroscopic PC, resulting in stage migration[29].
Figure 3.
A 34-year old female patient with PC from type 4 gastric cancer treated with NIPS. A: Macroscopic finding of resected stomach of patients treated with NIPS; B: Histologic finding of resected stomach of patient treated with NIPS. Almost all cancer cells disappear and mucin alone was depicted in the primary tumor (histological grade 3).
Figure 4.
A 48-year old male patient with PC from gastric cancer treated with NIPS. A: Macroscopic finding of PC on bowel mesentery; B: After 2 courses of NIPS, PC nodules shows fibrotic changes; C: Histologic findings of PC nodule obtained at the first operation of Figure 4A; D: Complete degeneration of cancer cells in PC nodule obtained at second look operation after NIPS.
CRS USING PERITONECTOMY PROCEDURES
The current state-of-the-art treatment for colorectal peritoneal dissemination is a comprehensive management using CRS and HIPEC. Patients with a low tumor volume, well/moderately differentiated tumors and complete cytoreduction may potentially benefit from combined treatment[30]. In gastric cancer patients with PC, no survival benefit has been reported by cytoreduction alone. In contrast, CRS with peritonectomy plus HIPEC confers a prolonged survival period[10]. Complete cytoreduction is an essential factor for a good outcome and NIPS plus peritonectomy may improve the incidence of complete cytoreduction[29,31]. Glehen et al[32] reported that CRS and HIPEC might have a survival benefit in highly selected patients (good general condition, resectable primary gastric cancer and PC).
However, NIPS might increase the risk of a peritonectomy procedure plus a gastrectomy combined with a lymphadenectomy. Glehen reported a mean operation time of 5.2 h (range 1.5-9.5 h), a 30-d mortality rate of 4% (2/49), and a major complication rate of 27% (13/49)[32]. In our consecutive series of 96 gastric cancer patients with PC, two hospital deaths (2%) occurred in patients who died of MOF from pancreatic fistula and sepsis. Postoperative major complications occurred in 30 (32%) patients (Table 3)[10]. A second operation was necessary in 4 patients who had complications from insufficiency of esophagojejunal anastomosis, bleeding, and ileal and colonic fistula. Glehen reported a higher complication rate of 47% in patients who underwent extensive CRS (gastrectomy combined with the removal of more than 2 peritoneal zones)[32]. The magnitude of surgery, the number of resected organs, the number of anastomoses and the operation time are considered to have contributed to the significantly higher complication rate.
Table 3.
Postoperative complications after 96 peritonectomy for PC from gastric cancer
| Medical complication | |
| Pulmonary complications | 5 (5%) |
| Surgical complication | |
| Anastomotic leakage | 11 (10%) |
| Fistula from small bowel | 4 (4%) |
| Abdominal abscess | 4 (4%) |
| Bleeding | 3 (3%) |
| Pancreatic fistula | 2 (2%) |
| Reoperation | 4 (4%) |
| Bleeding | 1 |
| Drainage of abscess | |
| From leakage | 1 |
| From bowel fistula | 2 |
To avoid futile aggressive treatments, the preoperative stringent selection of patients must be emphasized. Surgeons should have a large amount of surgical experience with gastrointestinal and genitourinary diseases and an extensive knowledge of organ anatomy and physiology. Surgeons must also be able to judge the balance between the postoperative risk associated with the magnitude of the peritonectomy and the survival benefit and quality of life.
Yan et al[33] reported the existence of a learning curve with this procedure and recommended the accumulation of experience to achieve an acceptable morbidity rate. They proposed that at least 70 peritonectomy procedures are needed to obtain a reliable level of surgical proficiency and postoperative care. Surgeons who want to perform this procedure must have a surgical team that includes anesthesiologists, nurses, pathologists, urologists, gynecologists and other experienced surgeons.
QUANTITATIVE ESTIMATION AND PREOPERATIVE DIAGNOSIS OF PC
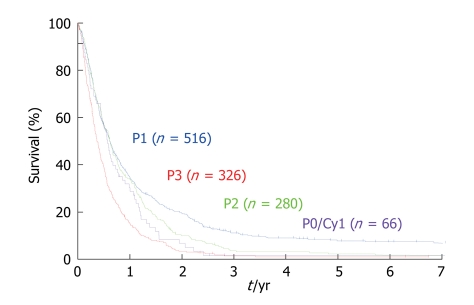
For the objective evaluation of the distribution and volume of PC, a quantitative staging system is needed. In the Japanese Rules of Gastric Cancer, PC is classified into five categories[28]: P0/Cy0, P0/Cy1, P1, P2 and P3. P0/Cy0 means no macroscopic disease and a negative peritoneal wash cytology; P0/Cy1 means no macroscopic PC but a positive peritoneal wash cytology; P1 means PC in the upper abdomen above the transverse colon; P2 means several countable PC in the peritoneal cavity; and P3 means numerous PC in the peritoneal cavity. The size of the PC is not taken into consideration in the Japanese staging system. The Japanese staging system itself has been shown to be an important prognostic factor. Significant survival differences in the survival rates were observed between P1 vs P2, P1 vs P3, and P2 vs P3 group (Figure 5).
Figure 5.
Survival curves of patients with PC, according to the Japanese classification. P1 vs P2: P < 0.025, χ2 = 4.979; P1 vs P3: P < 0.001, χ2 = 61.13; P0/Cy1 vs P2, P3: Not significant.
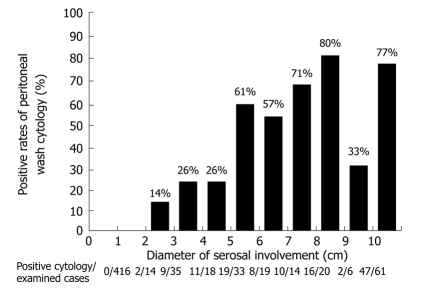
A unique point of the Japanese classification is the performance of a cytological examination using peritoneal wash fluid, even in patients who are scheduled to undergo a potentially curative resection. The peritoneal wash cytology is usually negative when the macroscopic diameter of the serosal invasion of the primary tumor is smaller than 2 cm (Figure 6). Furthermore, for serosal involvement with a diameter larger than 5 cm, the peritoneal wash cytology was reportedly positive in 66% (101/153) of the patients[34].
Figure 6.
Positive rates of peritoneal wash cytology according to the diameter of serosal involvement of primary tumor. Intraoperative cytological examination of the peritoneal wash solution using 200 mL of saline in 637 patients who had no macroscopic PC.
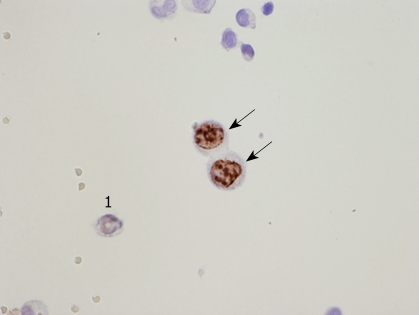
PFCCs have a high proliferative activity (Figure 7) because the Ki-67 labeling rate of PFCCs is high (median value, 60%)[34,35]. The median survival time of patients with a positive cytology result is 6 mo, and the survival curve of patients with P0/Cy1 is not statistically significant, compared with that of patients with P1, 2, or 3 statuses (Figure 5)[35,36]. Accordingly, P0/Cy1 patients are regarded as having stage IV disease. A peritoneal wash cytology is recommended in gastric cancer patients scheduled to undergo curative resection.
Figure 7.
Ki-67 expression in PFCCs (immunocytochemical staining using MIB-1). 1Cancer cell without expression of Ki-67. Arrows indicate Ki-67 positive PFCCs with proliferative activity.
Gilly proposed a new staging system that takes into account the size of the peritoneal nodules and their distribution (localized or diffuse) (Table 4). This staging system has also been confirmed to be an important prognostic indicator[32]. Since the median survival of patients with stage 1 or 2 is significantly better than those with stage 3 or 4 after CRS and HIPEC, patients with stage 1 or 2 could be candidates for CRS and HIPEC.
Table 4.
Gilly staging system
| Stage | Peritoneal carcinomatosis description |
| Stage 0 | No macroscopic disease |
| Stage 1 | PC less than 5 mm in diameter |
| localized in one part of abdomen | |
| Stage 2 | PC less than 5 mm |
| Diffuse in the whole abdomen | |
| Stage 3 | PC 5 mm to 2 cm in diameter |
| Stage 4 | Large PC more than 2 cm |
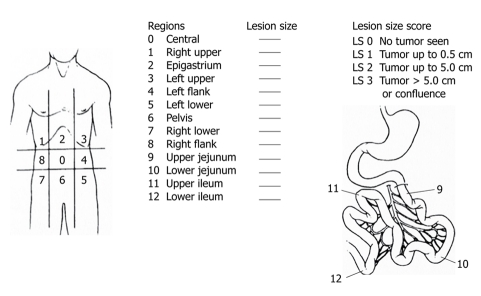
Sugarbaker reported the use of the peritoneal cancer index (PCI)[37]. This staging system accounts for both cancer distribution and size of peritoneal nodules (Figure 8). The abdominal cavity is subdivided into 13 regions. Furthermore, the accurate sizes of the lesions in each region are recorded by direct visual inspection intraoperatively. The macroscopic PC nodules in each zone are meticulously observed during surgery and scored from 0 to 3. Score 0 means that no nodules seen; score 1 has nodules with a maximum visible diameter of up to 0.5 cm; score 2 a diameter of greater than 0.5 cm and up to 5 cm; and score 3 refers to nodules with a diameter of 5 cm or greater. Peritoneal nodules are scored as lesion sizes 0 through 3 (LS 0 to LS 3) (Figure 8). This method quantifies the extent of PC in the peritoneal cavity, which can be summated as a numerical score (from 0 to 39).
Figure 8.
Peritoneal cancer index (PCI). Peritoneal cavity is divided into 13 parts, which ranges from 0 to 12. Accurate measurement of each region is scored as lesion size 0 through 3. LS 0: No implants. LS 1 refers to implants up to 0.5 cm in diameter; LS 2 refers to implants greater than 0.5 cm and up to 5 cm; and SL3 refers to those 5 cm or greater in diameter.
Sugarbaker proposed a means of assessing the completeness of cytoreduction (CC), classified into four categories. CC-0 indicates complete cytoreduction with no residual macroscopic nodule; CC-1 no macroscopic tumor but a positive histological margin or suspicious residual nodules less than 5 mm in diameter; CC-2 apparent macroscopic residual tumors greater than 5 mm but up to 5 cm in diameter; and CC-3 residual PC greater than 5 cm in diameter.
In patients with gastric cancer who have received a CC-0 or CC-1 peritonectomy, involvement was not found on the anterior abdominal wall and liver capsule but was observed in other zones in 10 cases (2.7% to 35%). In contrast, all the zones were involved in the CC-2 or CC-3 group. A higher incidence of diffuse involvement on the serosal surface or mesentery of the small and large bowel was seen in the CC-2 or CC-3 group. In patients with colorectal cancer, Koh et al[38] reported a predilection for the right side, with spreading to the right upper and flank regions being approximately twice as common as to the left regions. The right upper and flank regions correspond to the right diaphragmatic copula and the right para-colic gutter of the ascending colon. Furthermore, they reported that the small bowel segment was the least commonly affected area, with the exception of the distal ileum. During laparoscopic examination and exploratory laparotomy, surgeons should meticulously observe and palpate these 13 zones[36].
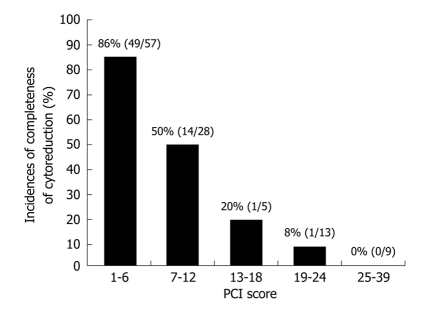
The complete cytoreduction of patients with PCI ≤ 6 and PCI ≥ 7 has been reportedly performed in 86% (49/57) and 39% (14/41) of patients respectively (Figure 9). These percentages were significantly different (P < 0.05). Complete cytoreduction was successfully performed in only 7% (2/27) of patients with a PCI greater than 13.
Figure 9.
PCI scores and completeness of cytoreduction in 92 gastric cancer with PC, who underwent CRS.
The PCI score is believed to be an independent prognostic factor and a PCI score capable of serving as a threshold for favorable versus poor prognosis has been reported. In colorectal cancer, the survival results are significantly better when the PCI was lower than 16[37,39]. In a series of patients with colorectal cancer, Sugarbaker reported a 5-year survival rate of 50% when the PCI was less than 10, a rate of 20% for an index of 11-20, and a rate of 0% for an index > 20[40]. Berthet et al[41] reported that the PCI score predicted a benefit to the visceral organs from the treatment of sarcomatosis. The PCI score system developed by Sugarbaker is now used worldwide for the assessment of PC from various tumor types.
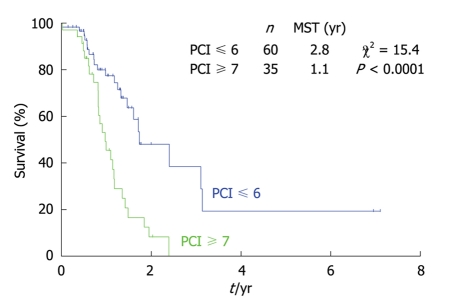
As shown in Figure 10, the survival of gastric cancer patients with a PCI score ≤ 6 was significantly better than those with a PCI score ≥ 7. Gastric cancer is believed to have a more aggressive biological behavior than colorectal cancer. These results suggest that carcinomatosis from gastric cancer with a PCI score greater than 7 should be treated with palliative intent without peritonectomy.
Figure 10.
Survival differences of gastric cancer patients with PC, according to the PCI score.
For the preoperative diagnosis of PC, computed tomography (CT), magnetic resonance imaging (MRI), PET-CT and laparoscopy are performed as part of the stringent patient selection required for peritonectomy[42]. The availability and lower incidence of movement artifacts make multi-sliced CT the most widely used imaging tool for the detection of PC. High-speed spiral CT for the detection of PC from gastric cancer has an accuracy of 78%, a sensitivity of 39%, a specificity of 94%, a positive predictive value of 72% and a non-predictive value of 79%[42].
Koh et al[38] reported the value of preoperative CT in estimating PC in patients with colorectal carcinomatosis. CT portrayed the lesion size accurately in 60% of the cases, underestimated the size in 33% of the cases, and overestimated the size in 7% of the cases. The detection rates depended on the peritoneal zone and lesion size. The detection rates for PC are relatively high in the epigastrium, right upper area, and pelvis with detection rates of greater than 50%. In contrast, the depiction rate of small-bowel involvement had the lowest sensitivity, with a rate of 8%-17%. The sensitivity of CT for detecting PC was influenced by the lesion size and the false-negative rate significantly decreased with the lesion size. Small PC (< 0.5 cm) was visualized using CT with a sensitivity of 11%, in contrast to a sensitivity of 94% for PC with a diameter greater than 5 cm.
The diameter of PC from gastric cancer tends to be smaller than that from colorectal cancer because gastric cancer always has a poorly differentiated histological type. In our study, the PCI estimated from preoperative radiologic studies was compared with the intraoperative PCI score. The mean preoperative radiologic PCI and the operative PCI scores were 5.91 and 5.64 respectively (no statistical significance)[42]. Koh also reported that radiologically determined PCI underestimated the true extent of PC[38].
PET provides a functional image, but this modality has drawbacks for small lesions less than 5 mm in size. Although a PET-CT system seems to be an attractive option, the use of this modality is limited by its high cost as well as its limitations in the assessment of low-volume PC[42]. Since the accuracy of PET-CT for primary gastric cancer and lymph node metastases was 54%[43], PET is not recommended for the diagnosis of lymph node metastases from gastric cancer[43].
Yang et al[42] reported that the accuracy of PET-CT for PC from gastric cancer was 87%, with a sensitivity of 72.7%, a specificity of 93.6%, a positive predictive value of 82.1%, and a negative predictive value of 89.6%, and that PET-CT showed a better sensitivity than high-speed spiral CT (HSSCT). Because peritoneal deposits usually have a low-volume density, all radiological modalities have major limitations in the assessment of PC. Surgeons should keep in mind that the preoperative radiologic PCI scores are always smaller than the intraoperative PCI scores.
Recently, diagnostic laparoscopy has enabled the direct visualization of small PC but technical difficulties can arise in the presence of adhesions caused by prior surgery. Garofalo reported an excellent experience with laparoscopic diagnosis for PC[44]. A good correlation was obtained between the open surgery data and the laparoscopic PCI scores. This method exhibited an excellent diagnostic accuracy for PC on the small bowel mesentery which cannot be correctly diagnosed using CT, MRI or PET-CT.
HYPERTHERMIC INTRAPERITONEAL CHEMO-PERFUSION (HIPEC) AFTER CYTOREDUCTION
An abundance of experimental and clinical evidence has indicated that malignant cells are selectively destroyed by hyperthermia in the range of 41°C to 43°C. Hyperthermia impairs DNA repair, protein denaturation and the inhibition of oxidative metabolism in the microenvironment of malignant cells and increases cell death[24,45-49]. Unfortunately, heat alone at such a low clinically applicable temperature cannot eradicate cancer cells because of a mechanism known as thermal tolerance that acts via the up regulation of heat shock protein[46]. However, hyperthermia enhances chemotherapy efficacy and the combination of heat and anti-neoplastic drugs frequently results in increased cytotoxicity. Some chemotherapeutic agents augment cytotoxicities in combination with mild hyperthermia. Such effects have been reported for mitomycin C, cisplatinum, docetaxel, gemcitabine and irinotecan[47-49]. An additional factor in vivo is increased drug penetration which is observed at temperatures above 39-42°C[49]. Los et al[17] reported that CBDA and cisplatinum penetrated 2-3 mm from the surface of experimental PC in rats but that penetration was limited to within 1-2 mm without hyperthermia.
Drug selection is very important when combined with hyperthermia. In HIPEC, a direct cytotoxic agent is needed. Anti-metabolites are not suitable because the duration of exposure is too short. Agents with large molecular weights have more favorable pharmacokinetics because of the delay in absorption resulting from the maintenance of high loco-regional concentrations in the peritoneal cavity. Rapid renal clearance may decrease side effects. Drugs that act synergistically with hyperthermia should be chosen. In HIPEC for gastric cancer, mitomycin C and CDDP, which have synergistic effects when used with hyperthermia, are typically used.
According to pharmacokinetic studies, approximately 70% of the administered mitomycin C is eliminated from the perfusate after 2 h of HIPEC[50]. With cisplatinum, 75% is lost from the perfusion fluid after a dwelling time of 90 min[51] and only 20% of the cisplatinum reaches the systemic circulation. The targeted tumor nodules may thus absorb a high proportion of this drug after 90 min of HIPEC. However, 30 min of HIPEC is probably too short for the optimal absorption of cisplatinum by the tumor nodules. Accordingly, 90-120 min might be more beneficial.
However, a long duration of HIPEC may increase the operation time and the incidence of morbidity. Yan et al[52] reported that a meta-analysis did not show a significant difference in the incidence of perioperative mortality between the HIPC and control groups. However, the meta-analysis did show a significant increase in the incidence of intra-abdominal abscess and neutropenia in the HIPEC group.
To date, intraperitoneal chemotherapy and hyperthermia have been investigated as possible treatment options for PC from ovarian, colorectal and gastric cancer. For advanced ovarian cancer, intraperitoneal chemotherapy in combination with CRS has been declared the standard practice[53]. For colorectal carcinomatosis, a randomized trial demonstrated a superior survival rate in patients receiving CRS and HIPEC, compared with traditional systemic chemotherapy and CRS[54,55]. In addition, the combination of CRS plus HIPEC has been suggested as the standard care recommended for PC from appendiceal cancer and mesothelioma. In gastric cancer, two randomized clinical trials (RCTs) have reported the prevention of peritoneal recurrence after curative resection[56,57]. A recent meta-analysis of RCTs for gastric cancer indicated that HIPEC with CRS is associated with an improved overall survival[52].
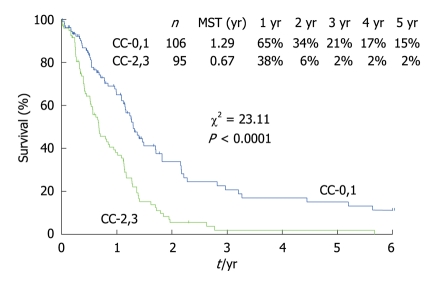
Figure 11 shows the survival curves for 211 patients with PC from gastric cancer who had been treated with CRS and HIPEC. Patients who received a CC-0 or CC-1 cytoreduction survived significantly longer than those who had received a CC-2 or CC-3 CRS. Before performing HIPEC, surgeons should remove as many PC nodules as possible. Because the depth of drug penetration is limited to 1-3 mm, the residual tumor burden should correspond to a status of CC-0 or CC-1. These results strongly suggest that HIPEC just after CRS can improve the survival of patients who have received a CC-0 or CC-1 cytoreduction. Gastric cancer spreads not only via transcoelomic routes, but also via lymphatic and hematogenous routes. Patients with both PC and distant lymph node metastasis, such as para-aortic lymph nodes or hematogenous metastasis, should be excluded from the indications for CRS and HIPEC.
Figure 11.
Survival curves of gastric cancer patients with PC after cytoreductive surgery and HIPEC using the CC score. The assessment of the CC is classified into 3 categories. CC-0: The complete cytoreduction with no residual macroscopic nodule; CC-1: No macroscopic tumor but positive margin histologically or suspicious residual nodules less than 5 mm; CC-2: Apparent macroscopic residual tumors greater than 5 mm but up to 5 cm; and CC-3: Residual PC greater than 5 cm in diameter.
As shown in Table 5, a multivariate analysis revealed that both NIPS and CC-0 or CC-1 were independent prognostic factors of a good prognosis after CRS plus HIPEC. The best indications for CRS + HIPEC are localized PC (PCI less than 6) from resectable gastric cancer that has been removed completely during a peritonectomy.
Table 5.
Multivariate survival analysis of 90 patients with PC
| Clinicopathologic factors | χ2 | P | Relative risk | 95% CI levels |
| Sex (male vs female) | 3.87 | 0.049 | 0.64 | 0.401-1.020 |
| Age (≤ 65 vs > 65) | 0.08 | 0.653 | 0.74 | 0.098-5.595 |
| CC (CC-0,1 vs CC-23) | 7.96 | 0.004 | 2.32 | 1.004-3.638 |
| NIPS (done vs not done) | 5.28 | 0.016 | 3.06 | 1.008-4.046 |
| PCI (≤ 6 vs ≥ 7) | 0.80 | 0.802 | 0.91 | 0.444-1.872 |
| Histology (diff. vs poorly diff.) | 0.59 | 0.442 | 0.62 | 0.252-4.399 |
From gastric cancer, treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) (cox proportional hazard model). PCI: Peritoneal cancer index.
EARLY POSTOPERATIVE INTRAPERITONEAL CHEMOTHERAPY (EPIC)
EPIC is started during the early postoperative period as soon as the patient’s physical condition allows. It is started at the time of minimal residual tumor burden before the residual cancer cells can become entrapped in postoperative fibrin deposits[11,58]. EPIC regimens with cell-cycle dependent drugs in 500 mL of saline are administered on postoperative days 1-5 through a catheter. Jeung et al[11] started EPIC on the day of operation using 5-FU (500 mg/m2) and cisplatinum (40 mg/m2, days 1-3) administered over a four weeks interval. The predominant toxicity was neutropenia and nausea/vomiting. The authors recommended EPIC for the treatment of patients with resectable gastric cancer with PC who had a good performance status (PS-0 of PS-1). Yu et al[59] performed an RCT consisting of 248 advanced gastric cancer patients treated with surgery plus EPIC (mitomycin-C plus 5-FU) and surgery alone. The surgery plus EPIC group had a superior overall survival, compared with the surgery alone group. In a subgroup analysis, the improvement in the survival rate was found to be statistically significant for patients with gross serosal invasion and lymph node metastasis. The authors recommended the use of EPIC for the treatment of stage 3 or 4 advanced gastric cancer patients with T3 or N+.
FAILURE ANALYSIS OF RECURRENT DISEASE FOLLOWING CRS AND PERIOPERATIVE INTRAPERITONEAL CHEMOTHERAPY
After complete cytoreduction and perioperative intraperitoneal chemotherapy for PC from colorectal cancer, about two thirds of patients experienced recurrences. The most common type of recurrence was a localized intra-abdominal recurrence and the median time for progression was 9 mo.
Unfortunately, approximately 75% of the patients who underwent a complete cytoreduction and HIPEC developed recurrences. In the 111 gastric cancer patients with PC who received CC-0 or CC-1 CRS and HIPEC, 65 patients experienced recurrences. The median survival period was 9.5 mo. Thirty-one (48%) and 51 (78%) of the patients died from recurrences at one and two years after CRS + HIPEC respectively. Four patients died of peritoneal (3 patients) or bone (one patient) recurrences 5-7 years after CRS + HIPEC. Thirty-seven patients had diffuse intraperitoneal recurrences, 5 had bone metastasis, 2 had lymph node recurrences and one had a skin recurrence.
For the treatment of localized intraperitoneal recurrences of colorectal cancer, surgical treatment, such as a second CRS, or intraperitoneal chemotherapy can result in long-term survival. In gastric cancer patients however, almost all recurrences are diffuse intraperitoneal recurrences. To prevent recurrence and prolong the survival of gastric cancer patients after CRS and HIPEC, EPIC and late systemic chemotherapy are mandatory.
TREATMENTS FOR PATIENTS WITH A P0/CY1 STATUS
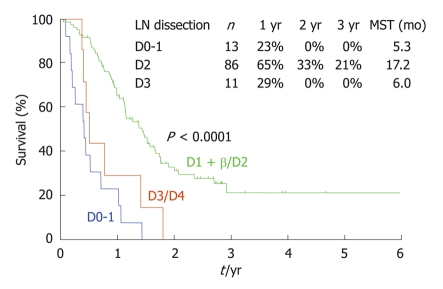
As already mentioned, a P0/Cy1 status means the absence of macroscopic PC but a positive cytological examination of peritoneal washing fluid. The survival of P0/Cy1 patients after gastrectomy is very poor, with a 5-year survival rate of less than 5% because of the persistence of micrometastases outside the surgical field. Since no effective chemotherapy regimens for PC have been reported, some Japanese surgeons do not recommend performing a gastrectomy for P0/Cy1 patients. In general however, a gastrectomy is believed to improve the survival of these patients. No reports describing the efficacy of lymph node dissection in P0/Cy1 patients have been made. As shown in Figure 12, patients who received a D2 dissection showed a superior survival outcome when compared with patients who received a D1 or D3 dissection. Furthermore, patients with D number ≥ pN showed a significantly better survival outcome than those with D < pN. These results may indicate that lymph node dissection improves the survival of P0/Cy1 patients[35].
Figure 12.
Survival curves of P0/Cy1 patients without distant metastasis, according to the extent of lymph node dissection.
A bursectomy, used to resect peritoneal deposits within the omental bursa, is considered an essential procedure for gastric cancer surgery. Yamamura et al[60] studied the PFCCs and CEA or cytokeratin 20 mRNA signals in the omentum and other peritoneal zones in the same patients. CEA/cytokeratin 20 mRNA signals could be detected simultaneously in the omental bursa and zones other than the omentum. Accordingly, P0/Cy1 patients cannot be cured by a gastrectomy + omento-bursectomy because invisible viable cancer cells persist after the omentectomy. They proposed that a routine bursectomy could be omitted from radical gastrectomy even for curable gastric cancer patients.
If P0/Cy1 patients are treated with NIPS (Figure 2), the PFCCs can be eradicated in two-thirds of the patients. Accordingly, P0/Cy1 patients should undergo NIPS followed by CRS.
As shown in Table 6, peritoneal recurrence is the main site of recurrence after curative resection for P0/Cy1 patients. Accordingly, a gastrectomy combined with chemotherapy to control peritoneal recurrences should be performed.
Table 6.
recurrence patterns after curative resection of P0/Cy1 patients n (%)
| Peritoneum | Lymph node | Liver | The others | |
| Negative | 42/92 (45.6) | 20/92 (21.7) | 23/92 (25.0) | 7/92 (7.6) |
| cytological status | ||||
| Positive | 22/27 (81.4) | 2/27 (7.4) | 2/27 (7.4) | 1/27 (3.7) |
| cytological status |
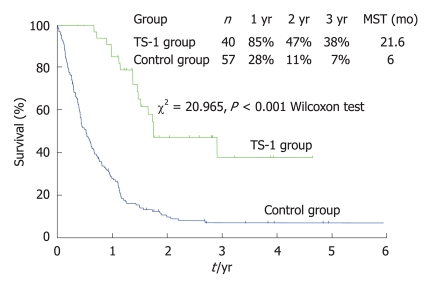
For the multimodal therapy of P0/Cy1 patients, Yonemura et al[61] reported the use of a combination therapy comprised of radical gastrectomy and postoperative S-1 therapy. After radical gastrectomy, 35 patients were treated with oral S-1 (80 mg/m2) for 28 consecutive days followed by a 14-d rest. This schedule was repeated every 6 wk (S-1 group). The other 66 patients did not receive any chemotherapy (control group). The patients in the S-1 group survived significantly longer than those in the control group (Figure 13) (P < 0.0001). The two-year survival rates of the control and S-1 groups were 9% and 53%, respectively. Recurrences were not observed in 15 patients (43%) in the S-1 group and 3 patients (5%) in the control group. Peritoneal recurrences after S-1 treatment and in the control group were observed in 11 (31%) and 34 (52%) patients respectively (P < 0.05).
Figure 13.
Survival of P0, Cy1 patients treated with gastrectomy+ postoperative TS-1 therapy and gastrectomy alone.
The Cox proportional hazard model showed that S-1 treatment was an independent prognostic factor and the relative risk of the S-1 treatment group was 0.17-fold lower than that of the control group. Major adverse reactions included myelosuppression and gastrointestinal toxicities but these effects were generally mild and no treatment-related deaths occurred. Thus, S-1 treatment appears to be a safe and effective postoperative chemotherapy treatment for patients with a P0/Cy1 status.
Yonemura et al[35] reported an effect of HIPEC in P0/Cy1 patients and the 5-year survival rate of 15 P0/Cy1 patients after gastrectomy plus HIPEC was 42%.
FUTURE PROSPECTS FOR THE TREATMENT OF PC FROM GASTRIC CANCER
The development of effective intraperitoneal chemotherapy regimens and new regimens of systemic chemotherapy is awaited. Recently, new molecules with important roles in the formation of PC have been reported and molecular targeting strategies for these molecules will soon be exploited. Surgeons should combine CRS and perioperative chemotherapy using new anticancer agents for patients with PC.
Footnotes
Peer reviewer: Ugur Coskun, MD, Associate Professor, Department of Medical Oncology, Gazi University Medical School, Besevler, Ankara 06500, Turkey
S- Editor Li LF L- Editor Roemmele A E- Editor Lin YP
References
- 1.Yonemura Y, Kawamura T, Bandou E, Tsukiyama G, Endou Y, Miura M. The natural history of free cancer cells in the peritoneal cavity. In: Gonzalez-Moreno S, editor. Advances in peritoneal surface oncology. Berlin: Springer; 2007. pp. 11–23. [DOI] [PubMed] [Google Scholar]
- 2.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000;58:96–107. doi: 10.1159/000012086. [DOI] [PubMed] [Google Scholar]
- 4.Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Endou Y, Sasaki T. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am. 2003;12:635–648. doi: 10.1016/s1055-3207(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald JS, Schein PS, Woolley PV, Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R, et al. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med. 1980;93:533–536. doi: 10.7326/0003-4819-93-4-533. [DOI] [PubMed] [Google Scholar]
- 6.Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, Conroy T, Fickers M, Leyvraz S, Buyse M. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9:827–831. doi: 10.1200/JCO.1991.9.5.827. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA. Optimizing docetaxel chemotherapy in patients with cancer of the gastric and gastroesophageal junction: evolution of the docetaxel, cisplatin, and 5-fluorouracil regimen. Cancer. 2008;113:945–955. doi: 10.1002/cncr.23661. [DOI] [PubMed] [Google Scholar]
- 8.Jacquet PH. Sugarbaker, Peritoneal-plasma barrier. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Principles of Management. Boston: Kluwar Academic Publisher; 1996. pp. 53–63. [Google Scholar]
- 9.Markman M. Intraperitoneal chemotherapy. Semin Oncol. 1991;18:248–254. [PubMed] [Google Scholar]
- 10.Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Mizuno M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009;100:311–316. doi: 10.1002/jso.21324. [DOI] [PubMed] [Google Scholar]
- 11.Jeung HC, Rha SY, Jang WI, Noh SH, Chung HC. Treatment of advanced gastric cancer by palliative gastrectomy, cytoreductive therapy and postoperative intraperitoneal chemotherapy. Br J Surg. 2002;89:460–466. doi: 10.1046/j.0007-1323.2001.02048.x. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–2212. doi: 10.1038/sj.bjc.6601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabusaki H, Nashimoto A, Tanaka O. [Evaluation of TS-1 combined with cisplatin for neoadjuvant chemotherapy in patients with advanced gastric cancer] Gan To Kagaku Ryoho. 2003;30:1933–1940. [PubMed] [Google Scholar]
- 14.Matsuzaki T, Yashiro M, Kaizaki R, Yasuda K, Doi Y, Sawada T, Ohira M, Hirakawa K. Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer. Cancer Sci. 2009:Epub ahead of print. doi: 10.1111/j.1349-7006.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10:112–122. doi: 10.1634/theoncologist.10-2-112. [DOI] [PubMed] [Google Scholar]
- 16.Baron MA. Structure of the intestinal peritoneum in man. Am J Anat. 1941;69:439–497. [Google Scholar]
- 17.Los G, Mutsaers PH, van der Vijgh WJ, Baldew GS, de Graaf PW, McVie JG. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res. 1989;49:3380–3384. [PubMed] [Google Scholar]
- 18.Ozols RF, Locker GY, Doroshow JH, Grotzinger KR, Myers CE, Young RC. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res. 1979;39:3209–3214. [PubMed] [Google Scholar]
- 19.Yonemura Y, Endou Y, Tochiori S, Bando E, Kawamura T, Shimada T, Miyamoto K, Tanaka M, Sasaki T. [Effect of intraperitoneal chemotherapy on experimental peritoneal dissemination of gastric cancer] Gan To Kagaku Ryoho. 2005;32:1635–1639. [PubMed] [Google Scholar]
- 20.Wasaburo K, Tanabe S, Higuchi K, Sasaki T, Nakayama N, Mihara S, Nakatani K, Nishimura K, Shimoda T, Azuma M, et al. [Clinical development of S-1 (TS-1) for advanced gastric cancer] Gan To Kagaku Ryoho. 2006;33 Suppl 1:57–63. [PubMed] [Google Scholar]
- 21.Yonemura Y, Endou Y, Bando E, Kuno K, Kawamura T, Kimura M, Shimada T, Miyamoto K, Sasaki T, Sugarbaker PH. Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett. 2004;210:189–196. doi: 10.1016/j.canlet.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, Sugarbaker PH. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol. 2006;32:661–665. doi: 10.1016/j.ejso.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Esquivel J, Vidal-Jove J, Steves MA, Sugarbaker PH. Morbidity and mortality of cytoreductive surgery and intraperitoneal chemotherapy. Surgery. 1993;113:631–636. [PubMed] [Google Scholar]
- 24.Van der Speeten K, Stuart OA, Sugarbaker PH. Using pharmacologic data to plan clinical treatments for patients with peritoneal surface malignancy. Curr Drug Discov Technol. 2009;6:72–81. doi: 10.2174/157016309787581084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata S, Yamamoto H, Kim BS, Matsuki N, et al. Operative morbidity and mortality after D2 and D4 extended dissection for advanced gastric cancer: a prospective randomized trial conducted by Asian surgeons. Hepatogastroenterology. 2006;53:389–394. [PubMed] [Google Scholar]
- 26.Inokuchi M, Yamashita T, Yamada H, Kojima K, Ichikawa W, Nihei Z, Kawano T, Sugihara K. Phase I/II study of S-1 combined with irinotecan for metastatic advanced gastric cancer. Br J Cancer. 2006;94:1130–1135. doi: 10.1038/sj.bjc.6603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba H, Yamamoto M, Endo K, Ikeda Y, Toh Y, Kohnoe S, Okamura T. Clinical efficacy of S-1 combined with cisplatin for advanced gastric cancer. Gastric Cancer. 2003;6 Suppl 1:45–49. doi: 10.1007/s10120-003-0222-y. [DOI] [PubMed] [Google Scholar]
- 28.Japanese research Society for Gastric Cancer. The general rules for the gastric cancer study in surgery and pathology. 12th ed. Tokyo: Kanehara Shuppan; 1993. [Google Scholar]
- 29.Yonemura Y, Bando E, Kawamura T, Ito H, Endo Y, Miura M, Kiyosaki K, Sasaki T. Cytoreduction and intraperitoneal chemotherapy for carcinomatosis from gastric cancer. Cancer Treat Res. 2007;134:357–373. doi: 10.1007/978-0-387-48993-3_23. [DOI] [PubMed] [Google Scholar]
- 30.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Yonemura Y, Bandou E, Kawamura T, Endou Y, Sasaki T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol. 2006;32:602–606. doi: 10.1016/j.ejso.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, François Y, Vignal J, Gilly FN. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20–26. doi: 10.1001/archsurg.139.1.20. [DOI] [PubMed] [Google Scholar]
- 33.Yan TD, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg. 2008;248:829–835. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 34.Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–262. doi: 10.1016/s0002-9610(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 35.Yonemura Y, Shinbo M, Hagiwara A. Shimada S, Nakajima T, Ikeda S, Pkamura H, Hirano M, Mizuno M, Endou Y, Miura M, Mizumoto Y. Treatment for potentially curable gastric cancer patients with intraperitoneal free cancer cells. Gastroenterological Surgery. 2008;31:802–812. [Google Scholar]
- 36.Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, Lupo PJ, Pisters PW, Feig B, Mansfield P. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15:2684–2691. doi: 10.1245/s10434-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 37.Sugarbaker TA, Chang D, Koslowe P, Sugarbaker PH. 1996. Patterns of spread of recurrent intraabdominal sarcoma. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Principles of management. Boston: Kluwer Academic; pp. 65–78. [DOI] [PubMed] [Google Scholar]
- 38.Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–333. doi: 10.1245/s10434-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43 Suppl:S15–S25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 41.Berthet B, Sugarbaker TA, Chang D, Sugarbaker PH. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;35:413–419. doi: 10.1016/s0959-8049(98)00375-x. [DOI] [PubMed] [Google Scholar]
- 42.Yang QM, Bando E, Kawamura T, Tsukiyama G, Nemoto M, Yonemura Y, Furukawa H. The diagnostic value of PET-CT for peritoneal dissemination of abdominal malignancies. Gan To Kagaku Ryoho. 2006;33:1817–1821. [PubMed] [Google Scholar]
- 43.Yang QM, Kawamura T, Itoh H, Bando E, Nemoto M, Akamoto S, Furukawa H, Yonemura Y. Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology. 2008;55:782–785. [PubMed] [Google Scholar]
- 44.Valle M, Garofalo A. Laparoscopic staging of peritoneal surface malignancies. Eur J Surg Oncol. 2006;32:625–627. doi: 10.1016/j.ejso.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am. 2003;12:689–701. doi: 10.1016/s1055-3207(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 46.Lepock JR. How do cells respond to their thermal environment? Int J Hyperthermia. 2005;21:681–687. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- 47.Kusumoto T, Holden SA, Ara G, Teicher BA. Hyperthermia and platinum complexes: time between treatments and synergy in vitro and in vivo. Int J Hyperthermia. 1995;11:575–586. doi: 10.3109/02656739509022491. [DOI] [PubMed] [Google Scholar]
- 48.Barlogie B, Corry PM, Drewinko B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res. 1980;40:1165–1168. [PubMed] [Google Scholar]
- 49.Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–468. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Sayag-Beaujard AC, Francois Y, Glehen O, Sadeghi-Looyeh B, Bienvenu J, Panteix G, Garbit F, Grandclément E, Vignal J, Gilly FN. Intraperitoneal chemo-hyperthermia with mitomycin C for gastric cancer patients with peritoneal carcinomatosis. Anticancer Res. 1999;19:1375–1382. [PubMed] [Google Scholar]
- 51.Panteix G, Beaujard A, Garbit F, Chaduiron-Faye C, Guillaumont M, Gilly F, Baltassat P, Bressolle F. Population pharmacokinetics of cisplatin in patients with advanced ovarian cancer during intraperitoneal hyperthermia chemotherapy. Anticancer Res. 2002;22:1329–1336. [PubMed] [Google Scholar]
- 52.Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–2713. doi: 10.1245/s10434-007-9487-4. [DOI] [PubMed] [Google Scholar]
- 53.Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006:CD005340. doi: 10.1002/14651858.CD005340.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 55.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 56.Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer. 1994;73:2048–2052. doi: 10.1002/1097-0142(19940415)73:8<2048::aid-cncr2820730806>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 57.Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776–1782. [PubMed] [Google Scholar]
- 58.Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, Oliff L, Schlag P. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. 1990;50:5790–5794. [PubMed] [Google Scholar]
- 59.Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985–990. doi: 10.1007/s00268-001-0067-7. [DOI] [PubMed] [Google Scholar]
- 60.Yamamura Y, Ito S, Mochizuki Y, Nakanishi H, Tatematsu M, Kodera Y. Distribution of free cancer cells in the abdominal cavity suggests limitations of bursectomy as an essential component of radical surgery for gastric carcinoma. Gastric Cancer. 2007;10:24–28. doi: 10.1007/s10120-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 61.Yonemura Y, Endou Y, Bando E, Kawamura T, Tsukiyama G, Takahashi S, Sakamoto N, Tone K, Kusafuka K, Itoh I, et al. The usefulness of oral TS-1 treatment for potentially curable gastric cancer patients with intraperitoneal free cancer cells. Cancer Therapy. 2006;4:135–142. [Google Scholar]