Abstract
The thienopyridine antiplatelet agent clopidogrel is an effective drug for the prevention of vascular events. However, data has accumulated over time to suggest it is prone to significant interpatient variability. While there are several factors that contribute to this, one of the most important is variability in forming the active metabolite necessary for clopidogrel function. Several enzymes are involved in formation of this metabolite, and two, CYP2C19 and P-glycoprotein, appear to have alleles that both occur frequently in the population and have a clinically significant impact. Patients carrying these alleles can be identified, but it remains to be determined if this information is necessary or sufficient for risk stratification. Furthermore, if patients with high-risk alleles are identified, it is unclear how treatment should be adjusted.
Keywords: clopidogrel, cytochrome P4502C19, ABCB1, on-treatment platelet reactivity
Introduction
Clopidogrel has become one of the cornerstone drugs in the treatment of atherosclerotic disease. Use of this antiplatelet agent, as monotherapy, is recommended for prevention of myocardial infarction in high risk patients (eg, patients with a history of myocardial infarction (MI), stroke, or peripheral artery disease), as well as for secondary prevention of strokes.1–3 Its use, in conjunction with aspirin, is recommended to prevent acute coronary events in patients with recent stent placement or recent acute coronary syndrome.4,5 It is one of the most prescribed drugs in the world. Yet it does not work for everyone.
Some patients who receive clopidogrel nonetheless experience acute thrombotic events. In randomized controlled trials, patients undergoing PCI who receive both clopidogrel and aspirin still have an incidence of cardiovascular death, MI, or stroke in the next year of approximately 9%.6 It is, of course, unreasonable to expect a drug to work perfectly. However, the potentially fatal consequences of clopidogrel failure have lead researchers to investigate the reasons why the drug might fail. This has resulted in the concept of clopidogrel resistance.7,8
Unfortunately, there is no single, standard definition of clopidogrel resistance. Some limit their discussion to the aforementioned treatment failures, clinically significant endpoints that occur in patients taking clopidogrel. Others use the term to refer to ex vivo studies demonstrating that clopidogrel suboptimally inhibits platelet aggregation in certain patients, as measured in a variety of assays. Regardless of the approach, investigators have proposed numerous risk factors for poor outcomes in patients prescribed clopidogrel.
One of the factors identified as increasing risk for therapeutic failure is genotypic variation. Several different genes are involved in the processing of clopidogrel, and genotypic variation of these is thus a potential source of phenotypic variation. This article will review what is known about the effects of genotypic variation on clopidogrel activity, and what options are available when genotypic variations are found in a patient.
Metabolism and Mechanism of Clopidogrel
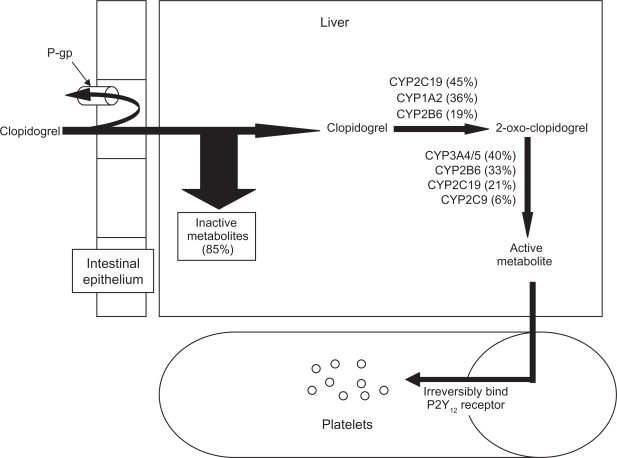
Clopidogrel is a thienopyridine pro-drug that requires bioactivation before it can achieve its antiplatelet effects9 (Fig. 1). Absorption of clopidogrel in the gut is opposed by the efflux pump P-glycoprotein, encoded by the ABCB1 gene. Once absorbed, approximately 85% of the drug is converted to an inactive metabolite by the action of esterases. The remaining 15% must undergo a two-step transformation process to become active. The first step produces 2-oxo-clopidogrel, and is catalyzed in varying proportions by the cytochromes CYP2C19, CYP1A2, and CYP2B6. The second step, which produces the reactive metabolite, can be catalyzed by CYP3A4/5, CYP2B6, CYP2C19, or CYP2C9.
Figure 1.
Activation and mechanism of action of clopidogrel. Orally administered clopidogrel is absorbed in the intestine. The efflux pump P-glycoprotein (P-gp) can return some of the clopidogrel to the intestinal lumen. Once absorbed, much of clopidogrel is inactivated through the action of esterases in the liver. The remaining clopidogrel is activated via a two step process, catalyzed by several different cytochrome P450 (CYP) enzymes. The active metabolite will irreversibly bind the P2Y12 receptor on the surface of platelets, inhibiting platelet aggregation for the life span of the platelet.
The reactive metabolite irreversibly binds to the P2Y12 receptor on the surface of platelets, inhibiting its activation for the life of the platelet. The P2Y12 receptor normally binds adenosine diphosphate (ADP), and this interaction is one of the central events in platelet activation.10 ADP binds both the P2Y1 and P2Y12 receptors. Stimulation of the former initiates platelet aggregation by activating the glycoprotein (GP) IIb/IIIa complex, but only weakly and transiently. Stimulation of P2Y12 by ADP amplifies the response, by not only potentiating the GP IIb/IIIa activation but also stimulating release of dense granules from the platelets. These granules contain more ADP, as well as other moieties that further activate GP IIb/IIIa. When GP IIb/IIIa is activated, it binds soluble fibrinogen and von Willebrand factor, triggering aggregation. The net effect is that, while P2Y12 activation is not obligately required for platelet activation, blocking ADP binding of P2Y12 with clopidogrel does markedly reduce activation and subsequent platelet aggregation.
Genomic Variants and Clopidogrel
With the multiple enzymes involved in absorption and activation of clopidogrel, it is perhaps unsurprising that there is significant population variation in the response to clopidogrel. Multiple enzymes provide multiple opportunities for genomic variation. However, variants in most potential metabolic enzymes, such as CYP3A4, CYP3A5, CYP1A2, CYP2B6, as well as genes for target proteins, such as P2RY12 (the gene for P2Y12) and ITGB3 (the gene for GP IIb/IIIa) have yielded little effect on either cardiovascular events or on platelet responsiveness when studied.11–13 Still, variants in two other enzymes, CYP2C19 and ABCB1, have suggested a potentially significant impact on clopidogrel efficacy.
CYP2C19
Over 20 allelic variants of CYP2C19 have been identified.14 The wild-type allele, following typical nomenclature, has been classified CYP2C19*1. The most common nonfunctional alleles include CYP2C19*2, and CYP2C19*3. Most of the other alleles reported are very rare and minimally functional at best. However, another common allele, CYP2C19*17, has been recently described. It is a gain-of-function allele, meaning it is associated with increased enzymatic activity. Specifically, this latter allelic variant is located upstream of the coding region, and is thought to increase the efficiency of the gene’s transcription. All of these alleles display considerable inter-ethnic variation15–18 (Table 1). Taken as a whole, Asians have a higher probability of carrying a loss-of-function allele and a lower probability of carrying a gain-of-function allele than either African Americans or Caucasians. As a consequence, Asians are much more likely to be poor metabolizers of clopidogrel.
Table 1.
Allelic frequency of common CYP2C19 variants by race.
| Race | *1 | *2 | *3 | *17 |
|---|---|---|---|---|
| African | 60%–64% | 17%–20% | <1% | 18% |
| Asian | 58%–61% | 30%–35% | 5%–10% | 2%–4% |
| Caucasian | 63%–69% | 13%–18% | <1% | 18%–20% |
The presence of variability in response to clopidogrel as measured by platelet aggregation in response to ADP was first demonstrated by Gurbel, et al.7 In this study, 15%–31% of patients could be described as clopidogrel resistant, depending on the duration of therapy. They did not attempt to ascertain why the patients had a suboptimal response. Subsequently, many groups did attempt to address that question. CYP2C19 loss-of-function alleles were first associated with poor clopidogrel responsiveness in 2006.19 Hulot, et al. treated 28 healthy patients with clopidogrel 75 mg daily for one week. The ability of each patient’s platelets to aggregate in the presence of 10 μM ADP was measured daily. Twenty of these patients had the wild-type allele, while 8 were heterozygous for the CYP2C19*2 allele. The 8 carriers of the nonfunctional allele on average did not achieve reduced platelet aggregation during the week of treatment with clopidogrel, whereas the wild-type homozygotes did. These results have since been replicated in a wide variety of patient populations, with differing acuity, and using several different assays.
One study of note was that of Kim, et al.20 The authors measured circulating clopidogrel levels in three different groups of eight Korean patients. Specifically, they compared wild-type CYP2C19 homozygotes (*1/*1) with heterozygotes (*1/*2 or *3) and with patients homozygous for nonfunctional alleles (*2/*2 or *3). The authors noted a gene dose effect in the ability to metabolize a loading dose of clopidogrel, with the nonfunctional homozygotes having 2.9 fold higher area under the curve (AUC) of the parent compound than those carrying the wild-type allele, and 1.9 fold higher AUC than those heterozygous for the allele. This then translated into a differential efficacy, with nonfunctional homozygotes having 30% and 37% less platelet inhibition after a week of maintenance clopidogrel treatment than the heterozygotes and wild-type homozygotes, respectively. Shuldiner, et al. corroborated these findings in an Amish population.21 In this study, CYP2C19*2 homozygotes (n = 9) had 35% and 42% less platelet inhibition than heterozygotes (n = 132) and wild-type homozygotes (n = 288), respectively, in response to a week of maintenance therapy with clopidogrel. Furthermore, through multivariate analysis, the authors concluded that the CYP2C19*2 allele accounted for 12% of the clopidogrel variation observed in that population.
In contrast to their findings with the CYP2C19*2 allele, Shuldiner’s group noted that carriage of the CYP2C19*17 allele did not have an effect on platelet inhibition when compared with the wild-type. However, Sibbing, et al. did find a gene-dose dependent effect of the *17 allele.22 They genotyped 1524 patients who were to receive elective coronary stent placement as treatment for their coronary artery disease. Blood was sampled after a 600 mg loading dose of clopidogrel. Patients homozygous for the *17 allele (n = 76) inhibited platelet aggregation more than those heterozygous for the allele (n = 546), who in turn had greater inhibition than wild type (n = 902). Possible reasons for the discordant results include the use of different assays to measure platelet inhibition, the use of different dosing regimens or differing disease state severity in the populations.
While effects on platelet aggregation assays are informative, these do not necessarily equate to clinical events. As a result, this has been a topic of considerable investigation. Two recent meta-analyses have looked at the aggregate data concerning the CYP2C19*2 allele.23,24 They both concluded that carriage of the nonfunctional allele was associated with an increased risk of a major adverse cardiovascular event (MACE), with an odds ratio (OR) ranging from 1.29 to 1.96. Both meta-analyses also addressed stent thrombosis, with ORs for this event ranging from 3.45 to 3.82. One of the meta-analyses also suggested a gene-dose dependence on the adverse events. Specifically, heterozygotes for the *2 allele had ORs of 1.59 (95% CI; 0.88–2.88) for MACE and 3.34 (95% CI; 1.84–5.93) for stent thrombosis when compared to patients with homozygous *1 alleles. Homozygotes of *2 had ORs of 2.05 (95% CI; 1.15–3.63) for MACE and 4.68 (95% CI; 1.55–14.11) for stent thrombosis.
Since the publication of these meta-analyses, two further substudies from large randomized trials have been published. In a substudy of the PLATO trial,25 patients with any loss-of-function allele who received clopidogrel had a higher risk of cardiovascular death, myocardial infarction, or stroke than those without a loss-of-function allele (11.2% vs. 10%). This difference was not statistically significant, though (P = 0.25). If limited to only the first 30 days, the difference was significant (OR 1.37, 95% CI; 1.04–1.82). The second study26 genotyped patients from two large trials, one targeting patients with acute coronary syndromes27 and the other patients with atrial fibrillation.28 In neither instance did the presence of loss-of-function alleles result in worsened outcomes while receiving clopidogrel when compared to those without loss of function alleles. Even patients homozygous for loss-of-function alleles did not seem to be at increased risk of clopidogrel failure. The reasons why the second study would have such starkly different outcomes when compared to the other trials are not immediately obvious. The authors did state that the rates of PCI were very low in these trials, relative to the other trials. Also, much of the data in the meta-analyses come from cohort studies. The lack of a placebo arm in those studies could yield some confounding. Still, when taken as a whole, it is not unreasonable to conclude that the presence of loss-of-function alleles, especially when homozygous, does impart some increased chance of adverse clinical events in high-risk patients taking clopidogrel.
There are fewer studies that address gain-of-function polymorphisms at the clinical level. Just as Sibbing, et al. found an increased effect of the *17 allele on platelet inhibition, as described above, they also found an increased risk of bleeding.22 However, they did not see any effect on clinical events. Similarly, Wallentin, et al. found an increased risk of bleeding in acute coronary syndrome patients with the presence of a gain-of-function allele without any change in subsequent clinical event rate.25 In contrast, Pare, et al. reported an improvement in clinical event rates in patients carrying the *17 allele, without any increase in bleeding rate.26 Tiroch et al. also suggested reduced target lesion revascularization and MACE incidence in acute MI patients who were carriers of the *17 allele, though they did not attempt to address bleeding risk.29 Thus, once again, we cannot draw hard conclusions as to whether the allele is beneficial or detrimental, but some caution seems advisable.
ABCB1
The ABCB1 (ATP-binding cassette B1) gene encodes the efflux pump, P-glycoprotein, also known as MDR1 (multi-drug resistance 1 protein). It is expressed in several tissues throughout the human body, and is a member of a large family of transporter genes, who all have broad substrate specificity. Together, they are thought to help protect the body from a wide variety of xenobiotics. Pathophysiologically, this gene can be overexpressed in many cancer cells, resulting in resistance to many chemotherapeutic agents.
Over 50 different polymorphic sequences have been identified in the ABCB1 gene.30 However, none of these are nonsense mutations. Thus all yield full length proteins. The best studied mutation is a shift from C to T at position 3435. This particular polymorphism, like CYP2C19, displays considerable inter-ethnic variation31,32 (Table 2). This is a synonymous mutation, meaning that the protein sequence is unaffected by the change in mRNA sequence. Studies to date have been conflicting, but overall suggest no significant differences in the amount of protein synthesized when either allele is translated.33 Similarly, there are conflicting data regarding mRNA expression and/or stability, but the overall evidence suggests these are unlikely to result in clinically different effects for the two alleles.33 Thus, it is quite unclear why there should be any clinical difference based on this polymorphism. Still differences have been observed.
Table 2.
Allelic frequency of common ABCB1 variants by race.
| Race | 3435C | 3435T |
|---|---|---|
| African | 79% | 21% |
| Asian | 58% | 42% |
| Caucasian | 45% | 55% |
Taubert, et al. investigated the effect of the variant alleles on the circulating levels of clopidogrel and its active metabolite.34 Sixty patients undergoing PCI were randomized to receive 300, 600, or 900 mg loading doses of clopidogrel. Those patients who were homozygous for the 3435T allele had lower concentrations of both the parent and active metabolite of clopidogrel than carriers of the 3435C allele, unless a 900 mg loading dose was used. Thus, one would expect impaired platelet reactivity and worse clinical outcomes as a consequence. Spiewak, et al. did demonstrate an increased likelihood of an impaired platelet response to clopidogrel among those acute coronary syndrome patients who were 3435T homozygotes when compared to carriers of 3435C, but did not observe any difference in clinical outcomes.35 However, a substudy of the TRITON-TIMI 38 trial, which also enrolled patients with acute coronary syndrome undergoing PCI, did report an increased risk of cardiovascular death, MI or stroke in 3435T homozygotes when compared to 3435C carriers (OR 1.72, 95% CI; 1.38–2.82).36 Interestingly, the FAST-MI investigators also reported an increased risk of cardiovascular death, MI, or stroke among 3435T homozygotes when compared to 3435C homozygotes.12 However, they reported that 3435CT heterozygotes also had increased risk relative to 3435C homozygotes. This is the opposite conclusion that the TRITON-TIMI 38 substudy observed. If this weren’t confusing enough, both Wallentin, et al. and Tiroch, et al. reported there was no effect of carrying the 3435T allele, either homozygously or heterozygously, on clinical endpoints.25,29 There are no immediately obvious reasons for the discrepant results. However, they are in keeping with the often contradictory results obtained in laboratory investigations of this polymorphism.
Non-Allelic Variation of Enzymes
Even if a patient has wild-type genes, activity of these genes can be modulated in multiple ways. Gremmel, et al. demonstrated that patients age 75 and older had higher incidences of residual platelet activity on clopidogrel than younger patients.37 They postulated that this could be secondary to generally reduced cytochrome oxidase activity, yielding a lower amount of the active metabolite. Likewise, end stage liver disease would be expected to reduce cytochrome oxidase levels and thus also significantly diminish the patient’s capacity to metabolize clopidogrel.
We can also modulate metabolic enzyme activity through use of concomitant medications. In fact, drug-drug interactions could unmask subtle effects of allelic polymorphisms. An example of such has been reported with CYP3A5. Suh, et al. reported that homozygotes for a poorly functional allele, CYP3A5*3, did not display any difference from noncarriers in platelet response to clopidogrel under baseline conditions. However, when these patients were given itraconazole, a potent CYP3A4 inhibitor, clopidogrel’s platelet inhibition was markedly reduced in the homozygotes, but unchanged in the noncarriers.38
The drug interaction that has far and away generated the most discussion and investigation is the inhibition of CYP2C19 by proton pump inhibitors (PPIs). In 2008, the American College of Gastroenterology, American College of Cardiology, and American Heart Association issued a consensus document that recommended the use of PPIs if patients were using dual antiplatelet therapy in order to reduce GI bleeding complications.39 That same year, the OCLA (Omeprazole CLopidogrel Aspirin) study was published, describing how concurrent use of omeprazole significantly diminished clopidogrel’s platelet inhibition.40 Since then, there has been a nearly constant barrage of studies on the topic, much of it contradictory.41 Reflecting the changing nature of the data, there have been four label changes to the Plavix® package insert in that time span. The most recent, and probably most applicable, data come from the COGENT trial.42 In this trial, 3761 patients were all treated with dual antiplatelet therapy, and then randomized to receive omeprazole or placebo. Use of omeprazole significantly reduced GI endpoints (OR 0.34, 95% CI; 0.18–0.63), without any apparent increase in cardiovascular events (OR 0.99, 95% CI; 0.68–1.44). The trial was stopped early due to funding issues, and did not utilize cardiovascular endpoints for their primary goals, but it does represent the largest trial to address the issue in a randomized fashion. Regardless, this topic will remain controversial for some time.
One issue yet to be investigated well is the effect of PPI therapy on clopidogrel action in patients with genomic polymorphisms. While some may assume inhibitory effects would be additive, the converse is more likely. If a patient expresses a nonfunctional protein, it cannot be further inhibited. Thus, it will be interesting to see if heterozygotes, wild-type patients, or gain-of-function carriers might have differential inhibitory effects in the presence of PPIs.
Coping with Genomic Variation
It is clear that patients with nonfunctional alleles have increased risk of residual platelet activity and of cardiovascular events, especially stent thrombosis. Patients with gain-of-function alleles may have reduced clinical events, but seem to have an increased risk of bleeding. What is less clear is whether obtaining the genotype of all potential patients, or even a significant subset, offers enough information to justify the costs involved. Genomic tests are expensive, ranging from $300–$500 per test, and typically not covered by insurance. Furthermore, they are not amenable to simple point-of-care analysis. The fastest of the current assays require at least a few hours to deliver results. Thus, any patient who requires clopidogrel acutely, such as during an emergent PCI, will not be able to obtain the results before initiating therapy.
Even for patients for whom time is not an issue, the relative importance of the information can be debated. As stated above, the CYP2C19*2 allele could only explain 12% of the variation in platelet reactivity.21 While several commercial assays do check for other loss-of-function alleles, few of the current assays detect the CYP2C19*17 allele and none detect the ABCB1 variants. Genotyping also has generally weak predictive ability. Hochholzer et al. reported that CYP2C19*2 carrier status was only 45% sensitive, and 75% specific for detecting high residual platelet activity. In fact, 53.3% of CYP2C19*2 homozygotes had normal platelet reactivity, and 22.4% of CYP2C19*1 homozygotes had impaired platelet reactivity.43 Thus, empiric strategies based solely on genotype could potentially alter therapy unnecessarily or fail to appropriately alter therapy, increasing risk of bleeding or clinical events.
It is clear that factors independent of the amount of active clopidogrel also have a large impact on the phenotypic endpoint of platelet aggregation. For example, type 2 diabetic patients are much more prone to having impaired platelet response to clopidogrel when compared to nondiabetic patients.43,44 The underlying reasons are probably multifactorial. Insulin resistance is associated with increased platelet reactivity. Likewise, obesity is highly prevalent in diabetics and has been shown independently to impair response to antiplatelet agents. Finally, elevated levels of pro-inflammatory mediators are common in diabetics, and are associated with increased platelet activation.
One other nongenotypic factor in determining clopidogrel effect worth mentioning is noncompliance. In a post-hoc analysis, Serebruany, et al. measured the levels of the main inactive metabolite of clopidogrel in over 600 patients ostensibly taking clopidogrel in the course of various clinical trials.45 Based on low levels of the detected metabolite, 22% of patients were classified as noncompliant. Obviously this does not explain away all clopidogrel resistance, as many studies have been done with directly observed loading doses. However, given the magnitude of the impact of premature discontinuation of clopidogrel on stent thrombosis (OR up to 57) and mortality (OR up to 9.0), noncompliance may dwarf any impact of genomic variation.46,47 If nothing else, any alternative strategy to normal clopidogrel dosing in order to deal with genomic variation requires patient compliance. Thus, the importance of compliance must be clearly stated to patients and reinforced at intervals.
Taking all of this information together, the use of genotyping as the sole factor in determining a patient’s risk for subsequent cardiovascular events does not seem justified. There are those who would argue that relying on the phenotypic endpoint of platelet inhibition would be a preferable strategy. These ex vivo assays are much cheaper and faster than genotyping, and theoretically should take into account both genotypic and clinical influences on platelet aggregation. However, there are several different assays available, some performed under a variety of conditions. Each assay measures, and is sensitive to, different aspects of the cascade of events associated with platelet activation. Historically their results have been presented in a wide variety of manners, eg, absolute percent reduction in platelet inhibition by clopidogrel when compared to baseline, relative percent reduction by clopidogrel, or residual platelet function after clopidogrel treatment. Only recently has the latter example, on-treatment platelet reactivity, come to be seen as the preferred measure for clinical purposes. A meta-analysis by Aradi, et al. reported that high on-treatment platelet reactivity was associated with a 3-fold increase in MI and a 4-fold increase in stent thrombosis.48 In part because of the lack of consensus, there have been few direct comparisons to see if any of the methods is superior to the others. To date, the best comparative evidence comes from the POPULAR study.49 In this study, high on-treatment platelet reactivity as measured by light transmittance aggregometry assays, the VerifyNow assay, and the Plateletworks assays all were able to predict the likelihood of a patient suffering death, MI, stent thrombosis, or stroke. The predictive accuracy was similar between the assays and modest, and none could predict the likelihood of bleeding. In contrast, Sibbing et al. did suggest that the Multiplate analyzer test could predict both stent thrombosis and bleeding.50 The authors did not compare this assay against others simultaneously to determine relative predictive ability. However, based on these results and those of several others using different assays, a working group has proposed a series of standardized cut-off values for most of the commonly used assays.51 Thus, we are only now arriving at a point where we can rely on the risk assessment by the platelet assays. That said, the positive predictive power of these assays are uniformly low. It has been suggested that combining the predictive power of genotyping and phenotyping may improve overall risk assessment.52 This seems the most probable way for genotyping to be routinely used.
The major drawback to both genotyping and platelet assays is that we don’t know how to alter treatment to account for the results. Several options have been proposed over time. In broad categories, they include 1) altering clopidogrel dose, 2) adding another agent (triple therapy), or 3) switching to a different drug (Fig. 2). Most of these strategies have not been assessed in terms of clinical outcomes, focusing instead on surrogate markers of platelet aggregation. A fourth strategy is sometimes overlooked, not using clopidogrel at all. Clopidogrel is sometimes inappropriately used for primary prevention or long-term secondary prevention of cardiac events. With the exception of use in the aspirin allergic patient, neither indication is supported by clinical evidence or guideline.
Figure 2.
Treatment options for patients with genomic variant alleles.
Several studies have investigated alterations of clopidogrel dosing. A randomized short-term doubling of the clopidogrel dose in all patients was tested in the CURRENT-OASIS 7 trial, alongside doubling the aspirin dose. In the population as a whole, there was no benefit to doubling either clopidogrel or aspirin, though there was an increase in bleeding rates with high dose clopidogrel.53 If one looked only at those undergoing PCI, there did appear to be a small benefit from a 600 mg loading dose, followed by six days of 150 mg clopidogrel.54 However, this too came at the cost of increased bleeding. The findings of benefit from a doubled loading dose are in keeping with a prior meta-analysis.55 However, they still suggest that a “one size fits all” strategy comes with tradeoffs, increased benefit with increased risk. Some investigators have tried to determine if an altered loading dose of clopidogrel can help those patients carrying loss-of-function genotypes. In a substudy of the PRINC trial, a higher loading dose of clopidogrel improved platelet inhibition as measured by the VerifyNow analyzer in carriers of loss-of-function alleles.56 Likewise, a higher maintenance dose for one week helped maintain platelet inhibition in these carriers. Bonello, et al. also reported that repeated loading doses of clopidogrel could help most patients carrying the CYP2C19*2 allele to overcome high on-treatment platelet reactivity, as measured by the VASP assay.57 However, neither study investigated long-term clinical outcomes. While there are several trials underway to repeat the above findings on platelet inhibition in a variety of populations and with a variety of assays, and a few clinical outcomes trials testing dosing strategies based on high on-treatment platelet reactivity (see Holmes, et al,58 for a discussion), there are only two current trials testing the effect of alternate dosing guided by genotype on clinical outcomes, Genotyping Infarct patients to Adjust and Normalize Thienopyridine treatment [GIANT; Clinicaltrials.gov identifier NCT01134380] and Thrombocyte Activity Reassessment and Genotyping for PCI [TARGET-PCI; NCT001177592].
Some have advocated triple therapy as the norm, irrespective of genomic status. A meta-analysis of triple therapy found that use of GP IIb/IIIa inhibitors on top of dual antiplatelet therapy offered benefits in patients with acute coronary syndromes.59 However these offer no benefits in unselected patients over the long term, and oral versions caused an increase in mortality. Thus, it is unlikely there will future studies based on genotype. Another add-on that has been studied is omega-3 fatty acids. In the OMEGA-PCI trial, their addition to dual antiplatelet therapy did improve platelet reactivity and light transmission aggregometry compared to placebo in patients undergoing elective PCI.60 While they did genotype their patients, they did not report the results based on genotype. The number of patients was also too small to determine clinical outcomes. Thus, the overall benefits in patients with loss-of-function alleles remain mostly speculative. Finally, cilostazol has been well studied as an add-on agent to dual antiplatelet therapy. It has been shown to reduce restenosis when added to clopidogrel and aspirin in both bare metal and drug-eluting stents.61 However, it does remain a question whether or not it affects MACE. There is some registry data to support a positive effect,62 but not any prospective randomized trials to date. It is also an open question as to whether patients with different genotypic variants might have a greater benefit from cilostazol. CYP2C19 is involved in metabolizing cilostazol.63 Thus, carriers of loss-of-function alleles who might not benefit as much from clopidogrel, might have higher levels and larger effects from cilostazol. As with other options, there are no clinical data with dosing determined by genotype. However, there is at least one study comparing adding cilostazol to dual antiplatelet therapy against doubling the dose of clopidogrel in patients with and without CYP2C19 variants.64 In patients without loss-of-function alleles, there was no statistical difference between the two strategies in terms of extent of platelet inhibition. In patients carrying at least one loss-of-function allele, cilostazol yielded greater platelet inhibition and lower rates of high on-treatment platelet reactivity than high-dose clopidogrel. It is worth noting that much of the research involving cilostazol was conducted in Asian nations, in populations that have much higher incidences of the different CYP2C19 loss-of-function alleles. Thus, extrapolating the benefits to other populations may not be straightforward.
Recently, newer antiplatelet agents have been compared against clopidogrel. The only one currently approved is prasugrel, also a thienopyridine. In the TRITON-TIMI 38 trial, prasugrel demonstrated superior reduction in ischemic events among acute coronary syndrome patients when compared to clopidogrel.65 However, this came at the cost of increased bleeding, including major bleeding. The bleeding was most prominent in those over the age of 75, under 60 kg, or with a history of stroke. As noted above, carriers of loss-of-function alleles of CYP2C19 or of the ABCB1 3435T allele had increased risk when treated with clopidogrel compared to noncarriers.11,36 No increased risk was documented when those same populations received prasugrel.36,66 Sorich et al. took that published data and integrated it to yield a more direct comparison. They suggested that prasugrel offered a reduced incidence of cardiovascular death, MI, or stroke when compared to clopidogrel in carriers of CYP2C19 loss-of-function alleles (OR 0.57, 95% CI; 0.39–0.83). In contrast, those without loss-of-function alleles had very similar results when compared to clopidogrel (OR 0.98, 95% CI; 0.80–1.20).67 They did not compare those with gain-of-function alleles.
A second drug under consideration by the various regulatory agencies around the world is ticagrelor. It is a direct, reversible P2Y12 receptor inhibitor. It too had reduced cardiovascular death, MI, or stroke when compared to clopidogrel, but with overall similar bleeding rates.68 Ticagrelor was numerically better than clopidogrel in both carriers and noncarriers of loss-of-function alleles, though the latter just missed statistical significance.25 Thus, ticagrelor appears to be a very promising alternative. However, ticagrelor has additional side effects than we expect from antiplatelet agents. Its use resulted in significantly higher rates of dyspnea and bradycardia, and elevations in uric acid. These may limit its use in populations such as those with chronic obstructive pulmonary disease or gout. Also, compliance may even be more of an issue with ticagrelor than with clopidogrel. With its reversible receptor inhibition and twice daily dosing, the risk of acute events may be higher with noncompliance.
Finally, with both new agents there are potential cost considerations. Prasugrel is more expensive than brand clopidogrel, and it is reasonable to expect that ticagrelor will be as well if approved. A modest cost differential may be worth it if one can achieve improved clinical outcomes. However, clopidogrel will become generic in the near future. Once it does, there may be a strong economic incentive to identify those patients who need the newer agents, and those who will do similarly on the older generic drug.
Conclusion
There is strong evidence of genotypic variation in the metabolism of clopidogrel, and that this variation has an impact on the phenotypic result of platelet inhibition. The evidence also supports an association between genotypic variants and clinical efficacy of clopidogrel. However, many factors beside genotype also influence clinical response. Consequently, genotyping cannot be used in isolation for risk stratification purposes, especially as the relative impact of heterozygous versus homozygous carrier status remains quite unclear. Since genotyping is time and labor intensive, it is therefore also costly. Thus, there is debate as to whether the incremental improvement in risk stratification is economically viable. The debate is complicated by the fact that we do not know how to alter treatment to account for genomic variations. New treatment options, such as prasugrel or ticagrelor, may render the question moot. However, current trials do not support unequivocal superiority of these agents, and a widening gap in acquisition costs may drive further efforts to define an optimal population to receive clopidogrel. Obtaining this evidence may ultimately provide a rationale to perform genotyping on a more frequent, if still selective, basis.
Footnotes
Disclosures
This manuscript has been read and approved by the author. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The author and peer reviewers of this paper report no conflicts of interest. The author confirms that they have permission to reproduce any copyrighted material.
References
- 1.Fraker TD, Jr, Fihn SD. writing on behalf of the Chronic Stable Angina Writing Committee. 2007 Chronic Angina Focused Update of the ACC/AHA 2002 Guidelines for the Management of Patients With Chronic Stable Angina: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to Develop the Focused Update of the 2002 Guidelines for the Management of Patients With Chronic Stable Angina. Circulation. 2007;116(23):2762–72. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 2.Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA Recommendations for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack. Stroke. 2008;39(5):1647–52. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The European Stroke Organisation Executive Committee, European Stroke Organization Writing Committee Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc Dis. 2008;25(5):457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 4.Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–55. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 5.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(22):2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 6.Kerr JL, Oppelt TE, Rowen RC. Role of clopidogrel in unstable angina and non-ST-segment elevation myocardial infarction: from literature and guidelines to practice. Pharmacotherapy. 2004;24(8):1037–49. doi: 10.1592/phco.24.11.1037.36135. [DOI] [PubMed] [Google Scholar]
- 7.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for Coronary Stenting: Response Variability, Drug Resistance, and the Effect of Pretreatment Platelet Reactivity. Circulation. 2003;107(23):2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 8.Oqueli E, Hiscock M, Dick R. Clopidogrel Resistance. Heart Lung Circ. 2007;16(Suppl 3):S17–28. doi: 10.1016/j.hlc.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the Human Cytochrome P450 Enzymes Involved in the Two Oxidative Steps in the Bioactivation of Clopidogrel to Its Pharmacologically Active Metabolite. Drug Metab Disp. 2010;38(1):92–9. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 10.Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113(3):340–5. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 Polymorphisms and Response to Clopidogrel. N Engl J Med. 2009;360(4):354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 12.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic Determinants of Response to Clopidogrel and Cardiovascular Events. N Engl J Med. 2009;360(4):363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 13.Momary KM, Dorsch MP, Bates ER. Genetic causes of clopidogrel nonresponsiveness: which ones really count? Pharmacotherapy. 2010;30(3):265–74. doi: 10.1592/phco.30.3.265. [DOI] [PubMed] [Google Scholar]
- 14.CYP2C19 allele nomenclature. Home Page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee. [2010 Aug 24; http://www.cypalleles.ki.se/cyp2c19.htm]. Accessed 2010 Oct 13.
- 15.Xie H-G, Kim RB, Wood AJJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Ann Rev Pharm Toxicol. 2001;41(1):815–50. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani T. PM Frequencies of Major CYPs in Asians and Caucasians. Drug Metabol Rev. 2003;35(2–3):99–106. doi: 10.1081/dmr-120023681. [DOI] [PubMed] [Google Scholar]
- 17.Verstuyft C, Simon T, Kim RB. Personalized medicine and antiplatelet therapy: ready for prime time? Eur Heart J. 2009;30(16):1943–63. doi: 10.1093/eurheartj/ehp295. [DOI] [PubMed] [Google Scholar]
- 18.Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol. 2010;69(3):222–30. doi: 10.1111/j.1365-2125.2009.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulot J-S, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–7. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 20.Kim KA, Park PW, Hong SJ, Park JY. The Effect of CYP2C19 Polymorphism on the Pharmacokinetics and Pharmacodynamics of Clopidogrel: A Possible Mechanism for Clopidogrel Resistance. Clin Pharmacol Ther. 2008;84(2):236–42. doi: 10.1038/clpt.2008.20. [DOI] [PubMed] [Google Scholar]
- 21.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of Cytochrome P450 2C19 Genotype with the Antiplatelet Effect and Clinical Efficacy of Clopidogrel Therapy. JAMA. 2009;302(8):849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 Allelic Variant, Platelet Aggregation, Bleeding Events, and Stent Thrombosis in Clopidogrel-Treated Patients With Coronary Stent Placement. Circulation. 2010;121(4):512–8. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 23.Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2010. E-published 2010 Mar 30. doi:10.1038/tpj.2010.21. [DOI] [PubMed]
- 24.Hulot J-S, Collet J-P, Silvain J, et al. Cardiovascular Risk in Clopidogrel-Treated Patients According to Cytochrome P450 2C19*2 Loss-of-Function Allele or Proton Pump Inhibitor Coadministration: A Systematic Meta-Analysis. J Am Coll Cardiol. 2010;56(2):134–43. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 25.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(9749):1320–8. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 26.Pare G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 Genotype on Outcomes of Clopidogrel Treatment. New England Journal of Medicine. 2010. E-published 2010 Aug 29. doi:10.1056/NEJMoa1008410. [DOI] [PubMed]
- 27.The Clopidogrel in Unstable angina to prevent Recurrent Events trial investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 28.ACTIVE Investigators Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation. N Engl J Med. 2009;360(20):2066–78. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 29.Tiroch KA, Sibbing D, Koch W, et al. Protective effect of the CYP2C19*17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010;160(3):506–12. doi: 10.1016/j.ahj.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 30.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. (BBA)—Proteins and Proteomics. 2009;1794(5):860–71. doi: 10.1016/j.bbapap.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang K, Wong LP, Lee EJD, Chong SS, Lee CGL. Genomic evidence for recent positive selection at the human MDR1 gene locus. Human Mol Genetics. 2004;13(8):783–97. doi: 10.1093/hmg/ddh099. [DOI] [PubMed] [Google Scholar]
- 32.Komoto C, Nakamura T, Sakaeda T, et al. MDR1 Haplotype Frequencies in Japanese and Caucasian, and in Japanese Patients with Colorectal Cancer and Esophageal Cancer. Drug Metab Pharmacokinet. 2006;21(2):126–32. doi: 10.2133/dmpk.21.126. [DOI] [PubMed] [Google Scholar]
- 33.Woodahl EL, Ho RJY. The Role of MDR1 Genetic Polymorphisms in Interindividual Variability in P-glycoprotein Expression and Function. Curr Drug Metab. 2004;5(1):11–9. doi: 10.2174/1389200043489108. [DOI] [PubMed] [Google Scholar]
- 34.Taubert D, von Beckerath N, Grimberg G, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80(5):486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Spiewak M, Malek L, Kostrzewa G, et al. Influence of C3435T multidrug resistance gene-1 (MDR-1) polymorphism on platelet reactivity and prognosis in patients with acute coronary syndromes. Kardiol Pol. 2009;67(8):827–34. [PubMed] [Google Scholar]
- 36.Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–9. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp C. Adenosine diphosphate-inducible platelet reactivity shows a pronounced age dependency in the initial phase of antiplatelet therapy with clopidogrel. J Thromb Haemost. 2010;8(1):37–42. doi: 10.1111/j.1538-7836.2009.03644.x. [DOI] [PubMed] [Google Scholar]
- 38.Suh J-W, Koo B-K, Zhang S-Y, et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ. 2006;174(12):1715–22. doi: 10.1503/cmaj.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118(18):1894–909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 40.Gilard M, Arnaud B, Cornily J-C, et al. Influence of Omeprazole on the Antiplatelet Action of Clopidogrel Associated With Aspirin: The Randomized, Double-Blind OCLA (Omeprazole CLopidogrel Aspirin) Study. J Am Coll Cardiol. 2008;51(3):256–60. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 41.Liu TJ, Jackevicius CA. Drug interaction between clopidogrel and proton pump inhibitors. Phamacotherapy. 2010;30(3):275–89. doi: 10.1592/phco.30.3.275. [DOI] [PubMed] [Google Scholar]
- 42.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without Omeprazole in Coronary Artery Disease. N Engl J Med. 2010 doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 43.Hochholzer W, Trenk D, Fromm MF, et al. Impact of Cytochrome P450 2C19 Loss-of-Function Polymorphism and of Major Demographic Characteristics on Residual Platelet Function After Loading and Maintenance Treatment With Clopidogrel in Patients Undergoing Elective Coronary Stent Placement. J Am Coll Cardiol. 2010;55(22):2427–34. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Ferreiro JL, Gomez-Hospital JA, Angiolillo DJ. Platelet abnormalities in diabetes mellitus. Diabet Vasc Dis Res. 2010. E-published 2010 Oct 4. doi:10.1177/1479164110383994. [DOI] [PubMed]
- 45.Serebruany V, Cherala G, Williams C, et al. Association of platelet responsiveness with clopidogrel metabolism: Role of compliance in the assessment of “resistance”. Am Heart J. 2009;158(6):925–32. doi: 10.1016/j.ahj.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Grines CL, Bonow RO, Casey DE, Jr, et al. Prevention of Premature Discontinuation of Dual Antiplatelet Therapy in Patients With Coronary Artery Stents: A Science Advisory From the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, With Representation From the American College of Physicians. Circulation. 2007;115(6):813–8. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- 47.Kolandaivelu K, Bhatt DL. Overcoming ‘resistance’ to antiplatelet therapy: targeting the issue of nonadherence. Nat Rev Cardiol. 2010;7(8):461–7. doi: 10.1038/nrcardio.2010.71. [DOI] [PubMed] [Google Scholar]
- 48.Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: Systematic review and meta-analysis. Am Heart J. 2010;160(3):543–51. doi: 10.1016/j.ahj.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of Platelet Function Tests in Predicting Clinical Outcome in Patients Undergoing Coronary Stent Implantation. JAMA. 2010;303(8):754–62. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 50.Sibbing D, Steinhubl SR, Schulz S, Schömig A, Kastrati A. Platelet Aggregation and Its Association With Stent Thrombosis and Bleeding in Clopidogrel-Treated Patients: Initial Evidence of a Therapeutic Window. J Am Coll Cardiol. 2010;56(4):317–8. doi: 10.1016/j.jacc.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 51.Bonello L, Tantry US, Marcucci R, et al. Consensus and Future Directions on the Definition of High On-Treatment Platelet Reactivity to Adenosine Diphosphate. J Am Coll Cardiol. 2010;56(12):919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 52.Gurbel PA, Tantry US, Shuldiner AR, Kereiakes DJ. Genotyping: One Piece of the Puzzle to Personalize Antiplatelet Therapy. J Am Coll Cardiol. 2010;56(2):112–6. doi: 10.1016/j.jacc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 53.CURRENT-OASIS 7 Investigators Dose Comparisons of Clopidogrel and Aspirin in Acute Coronary Syndromes. N Engl J Med. 2010;363(10):930–42. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 54.Mehta SR, Tanguay J-F, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 55.Lotrionte M, Biondi-Zoccai GGL, Agostoni P, et al. Meta-Analysis Appraising High Clopidogrel Loading in Patients Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2007;100(8):1199–206. doi: 10.1016/j.amjcard.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 56.Gladding P, Webster M, Zeng I, et al. The Pharmacogenetics and Pharmacodynamics of Clopidogrel Response: An Analysis From the PRINC (Plavix Response in Coronary Intervention) Trial. JACC: Cardiovasc Intervent. 2008;1(6):620–7. doi: 10.1016/j.jcin.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Bonello L, Armero S, Ait Mokhtar O, et al. Clopidogrel Loading Dose Adjustment According to Platelet Reactivity Monitoring in Patients Carrying the 2C19*2 Loss of Function Polymorphism. J Am Coll Cardiol. 2010. E-published 2010 Aug 11. doi:10.1016/j.jacc.2010.07.004. [DOI] [PubMed]
- 58.Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA “Boxed Warning”: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56(4):321–41. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Geeganage C, Wilcox R, Bath P. Triple antiplatelet therapy for preventing vascular events: a systematic review and meta-analysis. BMC Med. 2010;8(1):36. doi: 10.1186/1741-7015-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gajos G, Rostoff P, Undas A, Piwowarska W. Effects of Polyunsaturated Omega-3 Fatty Acids on Responsiveness to Dual Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Intervention: The OMEGA-PCI (OMEGA-3 Fatty Acids After PCI to Modify Responsiveness to Dual Antiplatelet Therapy) Study. J Am Coll Cardiol. 2010;55(16):1671–8. doi: 10.1016/j.jacc.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 61.Jennings DL, Kalus JS. Addition of Cilostazol to Aspirin and a Thienopyridine for Prevention of Restenosis After Coronary Artery Stenting: A Meta-Analysis. J Clin Pharmacol. 2010;50(4):415–21. doi: 10.1177/0091270009338940. [DOI] [PubMed] [Google Scholar]
- 62.Lee S-W, Park S-W, Yun S-C, et al. Triple antiplatelet therapy reduces ischemic events after drug-eluting stent implantation: Drug-Eluting stenting followed by Cilostazol treatment REduces Adverse Serious cardiac Events (DECREASE registry) Am Heart J. 2010;159(2):284–91. doi: 10.1016/j.ahj.2009.11.014. e281. [DOI] [PubMed] [Google Scholar]
- 63.Yoo H-D, Cho H-Y, Lee Y-B. Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol. 2010;69(1):27–37. doi: 10.1111/j.1365-2125.2009.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang S-J, Jeong Y-H, Kim I-S, et al. Cytochrome 2C19 Polymorphism and Response to Adjunctive Cilostazol Versus High Maintenance-Dose Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2010;3(5):450–9. doi: 10.1161/CIRCINTERVENTIONS.110.949859. [DOI] [PubMed] [Google Scholar]
- 65.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 66.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 Genetic Polymorphisms and the Response to Prasugrel: Relationship to Pharmacokinetic, Pharmacodynamic, and Clinical Outcomes. Circulation. 2009;119(19):2553–60. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 67.Sorich M, Vitry A, Ward M, Horowitz J, McKinnon R. Prasugrel versus clopidogrel for cytochrome P450 2C19 genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost. 2010;8(8):1678–84. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 68.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]