Abstract
Pelvic abscesses are usually the end stage in the progression of an infection. They may occur from surgical complications, generalized abdominal infections such as appendicitis or diverticulitis, or from localized infections such as pelvic inflammatory disease or inflammatory bowel disease. Although surgery has been considered as the treatment of choice by some authors, pelvic abscesses can be managed by non-invasive methods such as ultrasound and computed tomography-guided drainage. The development of therapeutic linear echoendoscopes has allowed the endoscopist to perform therapeutic procedures. Recently, endoscopic ultrasonography (EUS)-guided drainage of pelvic collections has been demonstrated to be feasible, efficient and safe. It allows the endoscopist to insert stents and drainage catheters into the abscess cavity which drains through the large bowel. This article reviews technique, current results and future prospects of EUS-guided drainage of pelvic lesions.
Keywords: Endoscopic ultrasound, Abscess, Collection, Pelvis, Drainage
INTRODUCTION
Since its introduction some years ago, endoscopic ultrasonography (EUS) has been demonstrated to be a highly valuable technique for the diagnosis and management of both luminal and extraluminal lesions of the mediastinum, retroperitoneum and pelvis. The advent of linear echoendoscopes have allowed the endoscopist to perform fine-needle aspiration (FNA) and therapeutic procedures such as celiac plexus neurolysis[1,2], pseudocysts drainage[3-5] and stent placement[6-8]. EUS-guided pelvic examinations are usually related to colorectal cancer staging[9]. However, a great variety of lesions can also be found outside the rectum such as peritoneal tumors or collections, lymph node metastases, gynecological lesions and urinary tract neoplasias[10-12]. Pelvic abscesses are usually the end stage in the progression of an infection. They may occur from surgical complications, generalized abdominal infections such as appendicitis or diverticulitis, or from localized infections such as pelvic inflammatory disease or inflammatory bowel disease[13-16]. Pelvic abscesses may present a clinical challenge for physicians because their location is usually surrounded by the pelvis, urinary bladder, rectum, prostate, vagina and/or uterus. Moreover, since pelvic abscesses are considered a life threatening condition, they require intensive medical management including the use of broad-spectrum antibiotics, drainage or even surgery, when patients develop persistent fever, ileus or abscess rupture with septic shock. Although surgery has been considered as the treatment of choice by some authors[13,17-18], pelvic abscesses can be managed by non-invasive methods. In fact, ultrasound (US) and computed tomography (CT)-guided percutaneous drainage of pelvic fluid collections have been used for many years with excellent results[19-23]. However, these techniques have some limitations: (1) Some lesions, due to their location, are not accessible to CT or US probe. The route of CT-guided drainage is usually transabdominal (anterior route) or transgluteal (posterior route) which sometimes do not offer the optimal window due to the presence of organs and structures such as the small bowel, large bowel, prostate, urinary bladder, uterus, nerves and vessels. On the other hand, US-guided drainage routes are transrectal and transvaginal that easily avoid the exposition of the organs and structures mentioned above. However, only lesions within the reach of the US probe (limited in size) can be drained; (2) Depending on the selected route, some patients may experience pain at the puncture site. This is more frequent with the transvaginal approach but can also happen with the transabdominal and transgluteal approaches; and (3) Most of these procedures do not allow deploying stents but drainage catheters that may be uncomfortable and painful for patients, especially those placed using transgluteal and transvaginal routes.
EUS-GUIDED PROCEDURE
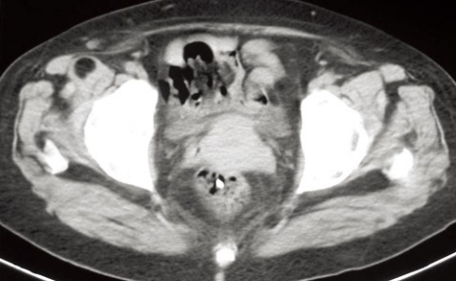
As stated before, EUS is a valuable imaging method that offers an excellent approach to pelvic lesions. Since pelvic abscesses are frequently located close to the rectum and left colon wall, they are easily and safely reached by EUS and EUS-FNA. The procedure is quite simple in experienced hands and can be summarized as follows: (1) Firstly, every patient undergoing EUS-guided drainage of a pelvic collection should be treated with prophylactic systemic antibiotics, i.e. 2 gr. of amoxicillin plus clavulanic acid before the procedure. After EUS-guided procedure, antibiotic therapy should be continued orally for 3-5 d; (2) An adequate colon preparation in EUS-guided management of pelvic collections is highly recommended. Water enemas, phosphates, polyethylene glycol, alone or combined, can be administered for that purpose; (3) The target lesion should be well defined by pelvic cross-sectional studies (CT or MRI) prior to EUS-guided drainage (Figure 1A). EUS procedure should be performed under conscious sedation. Propofol, midazolam and meperidine are some of the drugs which are usually administered. Initially conventional EUS study can be performed. Radial echoendoscopes could add valuable information regarding lesion size, location and relationships with pelvic organs and structures; (4) A therapeutic linear echoendoscope is then used. After the target lesion is located (Figure 1B), color Doppler is employed to ensure the absence of vessels at the puncture site (Figure 1C); (5) Once the puncture site is selected, a 19-gauge needle is introduced into the abscess cavity and then aspiration is performed (Figure 1D). Optionally, the abscess cavity can be flushed with normal saline solution (10-20 mL) which makes the aspiration process easier. The aspirate obtained must be sent to the microbiologist for Gram determination and culture in order to optimize the antibiotic therapy; (6) With the needle still placed into the abscess there are 3 options to continue the drainage process: (a) a 0.035 inch guide wire is passed and coiled into the cavity. Then, the tract between the rectum and the abscess is dilated, firstly using a 5F endoscopic retrograde cholangiopancreatography (ERCP) cannula or a needle knife and secondly, using an 8 mm over-the-wire biliary balloon; (b) the needle is withdrawn and the abscess cavity is punctured with a needle knife. Then, the metal part of the needle knife is withdrawn leaving the Teflon catheter into the cavity. A 0.035 inch guide wire is passed through the Teflon catheter and coiled into the abscess cavity. Over the wire, the tract between the rectum and the abscess is dilated with an 8 mm biliary balloon; and (c) the needle is withdrawn and one-step drainage can be performed using the NWOA system designed by Giovannini (Cook Endoscopy®, Winston-Salem, NC, USA). It consists of a 0.035 inch needle-wire suitable for cutting current, a 5.5F dilator and an 8.5 or 10F stent preassembled on the same catheter (Figure 1E-G); (7) Once the tract between the rectum and the abscess cavity has been dilated, straight or double-pigtail stents (up to 10F) combined or not with a 10-F drainage catheter can be deployed into the lesion (Figures 1H and 2); and (8) After four-six weeks, a control CT is performed and if resolution of the abscess is confirmed the stents are endoscopically extracted (Figure 3).
Figure 1.
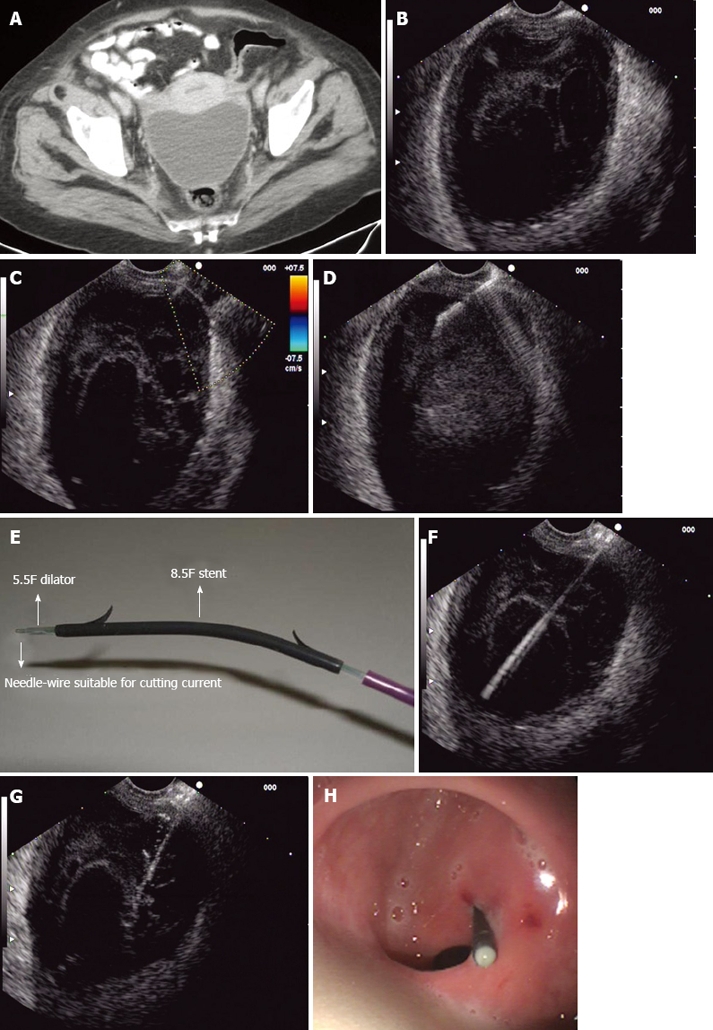
Endoscopic ultrasound (EUS)-guided pelvic abscess drai nage procedure. A: CT scan showing a pelvic collec tion at the Douglas pouch; B: Pelvic abscess detected by linear EUS; C: Color-Doppler showing no vessels between the abscess and the puncture site; D: Fineneedle aspiration with a 19-G needle; E: NWOA system for one-step drainage of fluid collections; F: 0.035-inch needle-wire into the abs cess cavity (NWOA system); G: An 8.5-F stent inserted into the abscess (NWOA system); H: Successful drainage.
Figure 2.
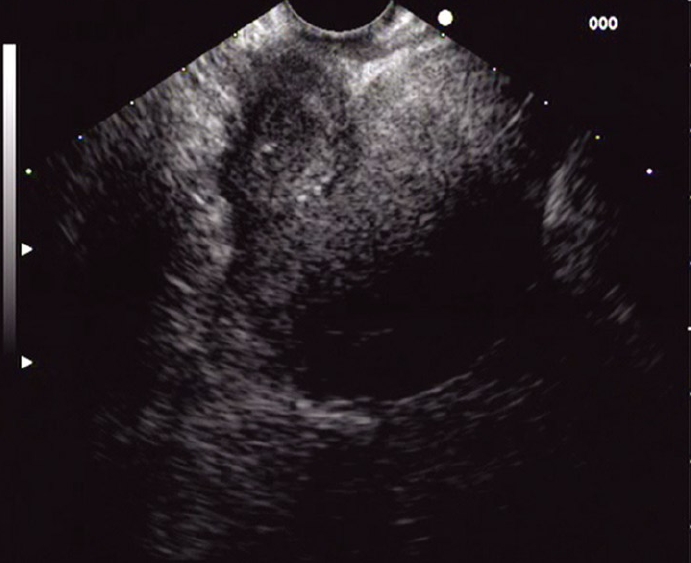
Virtual cavity close to the uterus after 3 wk.
Figure 3.
CT scan showing complete resolution of the abscess 4 wk later.
OUTCOME
EUS-guided drainage of pelvic abscesses has been previously well described by Giovannini[24], Varadarajulu[25] and Trevino[26]. Both groups demonstrated that this procedure is feasible, effective and safe and may be an excellent alternative to surgery or CT and US-guided drainage techniques. Results of available studies are summarized in Table 1. The first description of the EUS-guided technique was in 2003 by Giovannini and contributors[24]. They included 12 patients with perirectal abscesses (mean longest axis of 48.9 mm) which were secondary to abdominal surgery in 11 cases. Stent insertion was performed successfully in 9 out of 12 patients (75%) and in 3 patients only aspiration was performed. A straight 8.5F stent was inserted in 5 patients, a 10F double-pigtail stent was inserted in 3 patients and 2 stents (8.5 and 10F) were inserted in 1 patient. The mean duration of stent placement was 4.3 mo. Complete drainage with no relapse was achieved in 8 out of 9 patients with stents (mean follow-up of 10.6 mo) and in 1 out of 3 patients with aspiration. No procedure-related complications were observed. In 2007, Varadarajulu et al[25] published their experience in 4 patients with pelvic abscesses which were secondary to diverticulitis and colorectal surgery in 1 and 3 patients respectively. The mean longest size of lesions was 73.8 mm. They successfully inserted single-pigtail drains (10F and 80 cm) in all 4 patients. Early abscess resolution (mean time until resolution of 6 d) was achieved in 3 out of 4 patients (mean follow-up of 3 mo) and no procedure-related complications were observed. In 2008, the same group published a modified technique for EUS-guided drainage of pelvic abscesses[26]. It is a combined technique which uses drainage catheters as well as 7F stents. They included 4 patients with pelvic abscesses due to colorectal surgery in 2 cases, ischemic colitis in 1 case and endocarditis in 1 case. The mean longest axis diameter was greater than 90 mm. All patients received 1 drainage catheter and at least 1 stent (2 patients received 2 stents). An early resolution of abscesses was achieved in all 4 patients (mean follow-up of 221 d) and drainage catheters were discontinued after 2 d. No complications were registered in any patients.
Table 1.
Results of EUS-guided drainage of abscesses in published studies
| Author | n | Size | Technique | Complete drainage rate (follow-up) | Complications |
| Giovannini[24] | 12 | 48.9 mm | Aspiration and Stents (8.5 and 10F) | 75% (10.6 mo) | None |
| Varadarajulu[25] | 4 | 73.8 mm | Drainage catheters (10F and 80 cm) | 75% (3 mo) | None |
| Trevino[26] | 4 | 92.3 mm | Drainage catheters (10F and 80 cm) Stents (7F and 4 cm) | 100% (7 mo) | None |
DISCUSSION
Pelvic collections and abscesses are a frequent complication of colorectal surgery but are also an outcome of abdominal infections[13-16,18,20]. Since pelvic abscesses localization is usually complex (surrounded by structures such as rectum, urinary bladder, uterus, vagina and prostate), they present a clinical challenge for endoscopists, radiologists and surgeons. Current “non-invasive” approaches for pelvic abscesses include ultrasound and CT-guided drainage. These procedures are usually performed with high success rates[19-23]. However, they have some drawbacks and limitations such as patient discomfort and early drainage catheter dislodgement. EUS-guided drainage procedure has been demonstrated to be an efficient alternative to US and CT-guided procedures[24-26]. In fact, it has been demonstrated to be simple, efficient and safe but published long-term data still remain limited. Although there are no comparative studies, the main advantage of EUS-guided procedure over US and CT-guided procedures is that the distance between the probe and the abscess is usually very small (< 1 cm). Therefore there are no organs interposed between the needle and the abscess cavity that are then easily punctured. On the other hand, one or more stents and drainage catheters can be deployed into the abscess for a long time without patient discomfort and no major complications derived from EUS-guided technique have been reported in the literature. Taking into account available published data, a drainage catheter and 1 or 2 stents for each lesion seems to be the best endoscopic approach. The main limitation of the EUS-guided drainage procedure is that only abscesses located close to the rectum and left colon can be treated. Moreover, it is recommended that the distance between the colon and the abscess should be less than 2 cm. However, new echoendoscope prototypes such as the forward-viewing one are being developed. They have been used for pancreatic collections and other abdominal therapeutic interventions and could have an important role in the management of pelvic lesions. Its easier maneuverability could be helpful to reach those lesions such as appendicular collections that are more proximally located. In addition, forward echoendoscopes can overcome the main limitation of the curvilinear echoendoscopes which is that they access the targeted lesions at an acute angle. This sometimes means that it is impossible to insert guide wires, catheters and stents into the targeted lesions and can also mean that the position of the echoendoscopes is lost. On the other hand, whether or not fully covered self expandable metallic stents could be helpful in these situations should be analyzed by prospective and randomized trials. These stents could be beneficial in these lesions; firstly, avoiding early occlusions and secondly minimizing the risk of peritoneal leaks. In conclusion, EUS-guided drainage of pelvic collections has been demonstrated to be a feasible and safe procedure. However, some points such as timing and optimal indications of EUS-guided procedure, type of material to be used (plastic stents, metallic stents or drainage catheters; straight, single or double pigtail stents; fully or non-covered metallic stents, catheter diameter etc) and the role of echoendoscope prototypes (forward-viewing) should still be addressed by prospective and comparative studies involving larger cohorts of patients.
Footnotes
Peer reviewers: Brian Michael Yan, MD, Clinical Assistant Professor, Division of Gastroenterology, University of Calgary, Rm 6D16, Teaching Research and Wellness Building, 3280 Hospital Drive N.W., Calgary, Alberta T2N 4N1, Canada; Kyosuke Tanaka, MD, PhD, Assistant professor, Department of Endoscopic Medicine, Mie University Hospital, 2-174 Edobashi, Tsu, Mie 514-8507, Japan
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409–416. doi: 10.1111/j.1572-0241.2001.03551.x. [DOI] [PubMed] [Google Scholar]
- 2.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 3.Gerolami R, Giovannini M, Laugier R. Endoscopic drainage of pancreatic pseudocysts guided by endosonography. Endoscopy. 1997;29:106–108. doi: 10.1055/s-2007-1004083. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema MJ, Baron TH, Chari ST. Endosonography-guided pseudocyst drainage with a new large-channel linear scanning echoendoscope. Gastrointest Endosc. 2001;53:811–813. doi: 10.1067/mge.2001.113272. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–477. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 6.Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340–342. doi: 10.1055/s-2007-995485. [DOI] [PubMed] [Google Scholar]
- 7.Larghi A, Lecca PG, Mutignani M, Costamagna G. EUS-directed transpapillary self-expandable metallic stent placement after successful interventional EUS-guided cholangiography. Gastrointest Endosc. 2008;67:996–998. doi: 10.1016/j.gie.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Kwan V, Eisendrath P, Antaki F, Le Moine O, Devière J. EUS-guided cholecystenterostomy: a new technique (with videos) Gastrointest Endosc. 2007;66:582–586. doi: 10.1016/j.gie.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–265. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 10.Moparty B, Gomez G, Bhutani MS. Large solitary ovarian metastasis from colorectal cancer diagnosed by endoscopic ultrasound. World J Gastroenterol. 2008;14:5096–5097. doi: 10.3748/wjg.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmulewitz N, Hawes R. Ureteral metastasis from an appendiceal carcinoma and diagnosis by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2004;36:447–449. doi: 10.1055/s-2004-814302. [DOI] [PubMed] [Google Scholar]
- 12.Artifon EL, Sakai P, Ishioka S, Silva AF, Maluf F, Chaves D, Matuguma S, Pompeo A, Lucon AM, Srougi M, et al. EUS for locoregional staging of prostate cancer--a pilot study. Gastrointest Endosc. 2007;65:440–447. doi: 10.1016/j.gie.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Granberg S, Gjelland K, Ekerhovd E. The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol. 2009;23:667–678. doi: 10.1016/j.bpobgyn.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Lareau SM, Beigi RH. Pelvic inflammatory disease and tubo-ovarian abscess. Infect Dis Clin North Am. 2008;22:693–708, vii. doi: 10.1016/j.idc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Saleem MM. Scrotal abscess as a complication of perforated appendicitis: A case report and review of the literature. Cases J. 2008;1:165. doi: 10.1186/1757-1626-1-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsujinaka S, Kawamura YJ, Konishi F, Maeda T, Mizokami K. Pelvic drainage for anterior resection revisited: use of drains in anastomotic leaks. ANZ J Surg. 2008;78:461–465. doi: 10.1111/j.1445-2197.2008.04535.x. [DOI] [PubMed] [Google Scholar]
- 17.Remzi FH, Fazio VW, Kirat HT, Wu JS, Lavery IC, Kiran RP. Repeat pouch surgery by the abdominal approach safely salvages failed ileal pelvic pouch. Dis Colon Rectum. 2009;52:198–204. doi: 10.1007/DCR.0b013e31819ad4b6. [DOI] [PubMed] [Google Scholar]
- 18.Favuzza J, Friel JC, Kelly JJ, Perugini R, Counihan TC. Benefits of laparoscopic peritoneal lavage for complicated sigmoid diverticulitis. Int J Colorectal Dis. 2009;24:797–801. doi: 10.1007/s00384-009-0641-2. [DOI] [PubMed] [Google Scholar]
- 19.McGahan JP, Wu C. Sonographically guided transvaginal or transrectal pelvic abscess drainage using the trocar method with a new drainage guide attachment. AJR. 2008;191:1540–1544. doi: 10.2214/AJR.07.3830. [DOI] [PubMed] [Google Scholar]
- 20.Saokar A, Arellano RS, Gervais DA, Mueller PR, Hahn PF, Lee SI. Transvaginal drainage of pelvic fluid collections: results, expectations, and experience. AJR. 2008;191:1352–1358. doi: 10.2214/AJR.07.3808. [DOI] [PubMed] [Google Scholar]
- 21.Laganà D, Carrafiello G, Mangini M, Ianniello A, Giorgianni A, Nicotera P, Fontana F, Dionigi G, Fugazzola C. Image-guided percutaneous treatment of abdominal-pelvic abscesses: a 5-year experience. Radiol Med. 2008;113:999–1007. doi: 10.1007/s11547-008-0320-3. [DOI] [PubMed] [Google Scholar]
- 22.Soyer P, Fargeaudou Y, Boudiaf M, Hamzi L, Rymer R. [Percutaneous abdominopelvic interventional procedures using real-time CT fluoroscopy guidance at 21 mAs: an analysis of 99 consecutive cases] J Radiol. 2008;89:565–570. doi: 10.1016/s0221-0363(08)71482-3. [DOI] [PubMed] [Google Scholar]
- 23.Singh B, May K, Coltart I, Moore NR, Cunningham C. The long-term results of percutaneous drainage of diverticular abscess. Ann R Coll Surg Engl. 2008;90:297–301. doi: 10.1308/003588408X285928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannini M, Bories E, Moutardier V, Pesenti C, Guillemin A, Lelong B, Delpéro JR. Drainage of deep pelvic abscesses using therapeutic echo endoscopy. Endoscopy. 2003;35:511–514. doi: 10.1055/s-2003-39673. [DOI] [PubMed] [Google Scholar]
- 25.Varadarajulu S, Drelichman ER. EUS-guided drainage of pelvic abscess (with video) Gastrointest Endosc. 2007;66:372–376. doi: 10.1016/j.gie.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 26.Trevino JM, Drelichman ER, Varadarajulu S. Modified technique for EUS-guided drainage of pelvic abscess (with video) Gastrointest Endosc. 2008;68:1215–1219. doi: 10.1016/j.gie.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Larghi A, Lecca PG, Ardito F, Rossi ED, Fadda G, Nuzzo G, Costamagna G. Evaluation of hilar biliary strictures by using a newly developed forward-viewing therapeutic echoendoscope: preliminary results of an ongoing experience. Gastrointest Endosc. 2009;69:356–360. doi: 10.1016/j.gie.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Trevino JM, Varadarajulu S. Initial experience with the prototype forward-viewing echoendoscope for therapeutic interventions other than pancreatic pseudocyst drainage (with videos) Gastrointest Endosc. 2009;69:361–365. doi: 10.1016/j.gie.2008.09.021. [DOI] [PubMed] [Google Scholar]