Abstract
As a result of having undergone computed tomography (CT), a 75-year-old woman with type-C liver cirrhosiswas shown to have two tumors on the ventral and dorsal sides of subsegment 3 (S3). The tumor on the ventral side was diagnosed as a classic hepatocellular carcinoma (HCC), while that on the dorsal side was considered atypical for a HCC. Although the indocyanine green (ICG) findings indicated poor hepatic reserve, the prothrombin time (PT) was relatively good. An operation was performed in February 2007; however, this resulted in exploratory laparotomy. Dynamic CT performed 12 mo after the operation revealed that the tumor on the dorsal side of S3 had apparently increased. The marginal portion of the tumor was shown to be in the early and parenchymal phases, while the internal portion was found to have grown only slightly in the delayed phase. We diagnosed this tumor as a cholangiocellular carcinoma (CCC). S3 subsegmentectomy was performed in April 2008. The tumor on the ventral side was pathologically diagnosed as a moderately differentiated HCC, and that on the dorsal side was diagnosed as a CCC. We can therefore report a rare case of synchronous development of HCC and CCC in the same subsegment of the liver in a patient with type-C liver cirrhosis. We also add a literature review for all the reported cases published in Japan and around the world, and summarize the features of double cancer exhibiting both HCC and CCC.
Keywords: Double cancer, Hepatocellular carcinoma, Cholangiocellular carcinoma, Synchronous, Literature review
INTRODUCTION
The incidence of synchronous development of hepatocellular carcinoma (HCC) and cholangiocellular carcinoma (CCC) (combined HCC and CCC) has been reported to be low (constituting between 0.54% and 0.70% of primary liver cancers)[1-3]. Allen and Lisa defined three types of HCC-CCC: (a) separate masses composed of either hepatocellular or cholangiocellular components (mixed type); (b) contiguous but independent masses of hepatocellular and cholangiocellular components (combined type); and (c) an intimate intermingling of hepatocellular and glandular elements (double cancer)[4]. A literature search of Japana Centra Revuo Medicina found 18 cases[5-22] of synchronous double cancer with HCC and CCC, while a search of MEDLINE found only 16 such cases[13,23-36] (including 13 cases reported in Japan). In this report, we describe a rare case of synchronous development of HCC and CCC in the same subsegment of the liver in a patient with type-C liver cirrhosis. We also review all the previously reported cases of synchronous double cancer.
CASE REPORT
A 75-year-old woman diagnosed with chronic hepatitis or liver cirrhosis (type C) had been followed as an outpatient since 1982. Although endoscopic variceal ligation and endoscopic injection sclerotherapy (EIS) had been carried out several times, the esophageal varices ruptured in December 2002. Five EIS treatments were thenfollowed, after which the condition of the patient maintained static, that is, without the esophageal varices showing red.
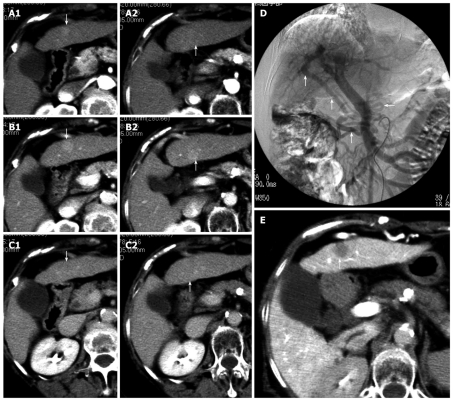
Dynamic computed tomography (CT) of the liver performed on December 18, 2006, revealed two tumors, each 1 cm or less in diameter, on the ventral and dorsal sides of S3 (Figure 1A-C). The patient was hospitalized for further examination and therapy in January 2007. We could not detect the tumors by ultrasonography and were unable to perform a tumor biopsy. With abdominal angiography, the left hepatic arterial angiogram (LHAG) showed no tumor stain on S3 and the superior mesenteric arterial angiogram (SMAG) showed a large shunt through the epigastric vein during the portal phase (Figure 1D). CT during arterial portography showed a 1 cm defect on the ventral side of S3; however, no defect was evident on the dorsal side (Figure 1E). Because CT had shown the tumor on the ventral side of S3 to be densely enhanced in the early phase and washed out in the delayed phase, we diagnosed the tumor as a classic HCC. The values for indocyanine green (ICG) dye retention at 15 min (R15) and elimination (K) were 32.0% and 0.070, respectively. However, because prothrombin time (PT) was 82.7%, indicating good hepatic reserve, the operation was performed in February 2007. Since the epigastric veins were overdeveloped, the liver appeared very cirrhotic and we were unable to detect the two masses by intraoperative ultrasonography. The first operation therefore resulted in exploratory laparotomy.
Figure 1.
Dynamic computed tomography findings at the onset of double cancer with hepatocellular and cholangiocellular carcinomas. Two tumors, ~1 cm in diameter, were detected on the ventral and dorsal sides of S3. Arrows indicate the tumors (A1, A2 arterial phase; B1, B2 parenchymal phase; C1, C2 delayed phase). Angiographic findings at the onset of double cancer; D: A superior mesenteric arterial angiogram showing a large shunt through the epigastric vein during the portal phase (the arrows); E: Computed tomography during arterial portography (CTAP) showing a ~1 cm defect on the ventral side of S3 (the arrow), which was diagnosed as a classic hepatocellular carcinoma. No defect can be seen on the dorsal side of S3.
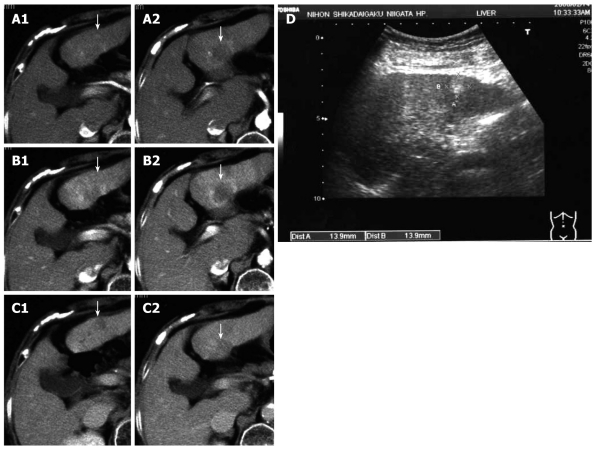
Dynamic CT of the liver performed 12 mo after the first operation revealed the tumor on the ventral side of S3 to be a classic HCC. Furthermore, the tumor on the dorsal side of S3 had apparently increased to 3 cm or less in diameter. It was discovered that the peripheral portion of the tumor showed typical signs of early and parenchymal phases, while the internal portion was slightly and gradually enhanced in the delayed phase. The peripheral portion of the tumor was typically found to be enhanced in the early and parenchymal phases, while the internal portion was found to be slightly and gradually enhanced in the delayed phase. (Figure 2A-C). Subcutaneous ultrasonography of the abdomen revealed a hyperechoic mass, 14 mm in diameter, at the site of the tumor on the ventral side, a finding characteristic of HCC rich in fat (Figure 2D). The tumor on the dorsal side of S3 was also represented by a hyperechoic mass, 30 mm or less in diameter, with irregular and unclear margins, but this finding was not typical for HCC (Figure 2D). In abdominal angiography, the LHAG showed no tumor stain and because the shunt through the epigastric vein was resected at the first operation, the SMAG did not show this shunt. 12 mo after the first operation, the patient’s platelet count was 8.4 × 104/mm3. The ICG R15 and K values were 35.9% and 0.066, respectively; however, PT was 81.3%. Although the ICG findings indicated poor hepatic reserve, PT was relatively good. We diagnosed the tumor on the ventral side of S3 as HCC and that on the dorsal side of S3 as a CCC tumor. Because of the poor hepatic reserve, lateral segmentectomy was ruled out.
Figure 2.
Dynamic computed tomography performed 12 mo after the first operation. The tumor on the ventral side of S3 appears to be a classic hepatocellular carcinoma and that on the dorsal side of S3 appears to be increased to ~3 cm in diameter. Typical findings including enhancement of the peripheral portion of the tumor in the early (A1) and parenchymal (B2) phases, and the slight and gradual enhancement of the internal portion in the delayed phase were observed (C2). Arrows indicate the tumors (A1, A2 arterial phase; B1, B2 parenchymal phase; C1, C2 delayed phase). Subcutaneous ultrasonography performed 12 mo after the first operation (D); The tumor on the ventral side of S3 is represented by a hyperechoic mass, 14 mm in diameter, a finding characteristic of hepatocellular carcinoma rich in fat. The tumor on the dorsal side of S3 is also represented by a hyperechoic lesion, ~30 mm in diameter, with irregular and unclear margins. Arrows indicate the tumors (D).
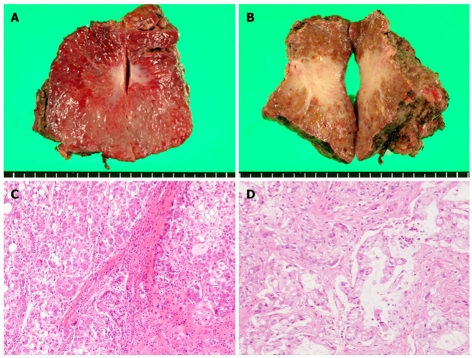
S3 subsegmentectomy was performed in April 2008 (Figure 3A and B). The tumor on the ventral side was pathologically diagnosed as a moderately differentiated HCC (with nodular, trabecular, and plate-like components) (Figure 3C) and that on the dorsal side was diagnosed as CCC (diffuse type showing a vestigial remnant of the tumor) (Figure 3D). Pathological examination also confirmed that the larger CCC tumor on the dorsal side was partially in contact with the HCC tumor on the ventral side of S3 (Figure not shown). We administered two courses of chemotherapy with gemcitabine hydrochloride (Gemzar®, Eli Lilly Japan, Kobe, Japan) followed by oral administration of S-1 (TS-1®, Taiho Pharma, Tokyo, Japan).
Figure 3.
Gross findings of the resected S3 subsegment. A: The cut surface of the tumor on the ventral side of S3; B: The cut surface of the tumor on the dorsal side of S3. Histopathological findings of the two resected tumors; C: The tumor on the ventral side was pathologically diagnosed as a moderately differentiated hepatocellular carcinoma (HCC) (with nodular, trabecular, and plate-like components); D: The tumor on the dorsal side was pathologically diagnosed as a cholangiocellular carcinoma (CCC) (diffuse type showing a vestigial remnant of the tumor).
DISCUSSION
The present report describes a case of synchronous development of HCC and CCC in the same subsegment of the liver. Only three similar cases[19,22,30] have been previously reported, and this condition is considered to be very rare. According to several earlier reports[37-39], hepatitis C virus (HCV)-related chronic hepatitis and cirrhosis are major risk factors for both HCC and CCC. The patient described in this report was found to be positive for HCV.
Earlier research has suggested the existence of amphi-potential progenitor cells that can differentiate into hepatocytes as well as cholangiocytes[40].
The following points may explain the mechanism underlying the development of combined HCC and CCC: (1) during the differentiation of the tumor into the two cancer phenotypes HCC and CCC, a trigger may cause the amphi-potential progenitor cells to turn malignant, undergo proliferation, and become tumorous in the same lesion, and (2) the cells that have already differentiated and matured into hepatocytes and cholangiocytes may become malignant and undergo proliferation in the presence of chronic liver inflammation. In the present case, although the cells involved belonged to the same subsegment of the liver, these cells could have metachronously turned malignant, undergone proliferation, and become tumorous, resulting in a synchronous double cancer (HCC-CCC) by either of the mechanisms described above.
In the present case, on performing dynamic CT in December, 2006, HCC and CCC were observed as being situated far from each other. However, we had pathologically confirmed that the larger CCC on the dorsal side was partially in contact with the HCC on the ventral side of S3 in the resected specimen, thus demonstrating that these tumors constituted a combined type of HCC-CCC, which had developed from different lesions in the liver. In this paper we have described the entire course of the two tumors involved in a combined type of HCC-CCC, making these findings valuable for research as well as clinical purposes.
The usual gross appearance of a mass-forming CCC is a large, white, firm tumor that is solid and fibrous, with a sclerotic appearance on the cut surface of the specimen, accompanied by a frequent finding of dense fibrous stranding in the central portion[41]. In most cases of mass-forming CCCs, ultrasonography reveals a hypoechoic mass with satellite nodules around the tumor[42]. However, the echo patterns are diverse and non-specific[43]. Dynamic CT and magnetic resonance imaging (MRI) reveal rim-like or band-like peripheral contrast enhancement of variable thickness around the tumor during the early phase, with progressive and concentric filling of the contrast material at a later phase[44]. CCCs appear hypointense on T1-weighted images and hyperintense on T2-weighted images[45,46].
In the present case, the size of the CCC was small (1 cm in diameter) when first discovered. We could not detect the tumor by ultrasonography because of overdeveloped epigastric veins, and were unable to identify the typical findings of an enhanced marginal portion in the early phase and the gradual enhancement of the internal portion in the delayed phase. The existence of this tumor was therefore questionable. However, abdominal ultrasonography performed 12 mo after the first operation showed a heterogeneously hyperechoic area with irregular margins on the dorsal side of S3. Dynamic CT of the liver performed during the same period showed a rim-like or band-like enhancement around the tumor in the early phase and a slight enhancement of the central portion of the tumor in the delayed phase. We preoperatively diagnosed the tumor on the dorsal side of S3 as a CCC. Early diagnosis of CCCs is very important for an improvement in prognosis. We hope that imaging studies will eventually be conducted for preoperative diagnosis of CCCs that are ≤ 1 cm in diameter.
In the present case, however, because of progressive liver cirrhosis, with poor hepatic reserve and an enlarged lateral segment of the liver, we performed an S3 subsegmentectomy. Because type-C liver cirrhosis generally results in poor hepatic reserve, it is imperative to consistently monitor the development of not only HCCs, but also CCCs, and facilitate early detection and treatment by curative hepatectomy.
Literature review of reported synchronous double cancer cases with HCC and CCC
Synchronous double cancer is particularly rare in the case of combined HCC and CCC. A literature search of Japana Centra Revuo Medicina database, version 4 (systematic literature search system through a computer web site for Japanese literature) found 18 cases[5-22] of synchronous double cancer with HCC and CCC, while a search of MEDLINE found only 16 such cases[13,23-36] (including 13 cases reported in Japan). The Japana Centra Revuo Medicina database available for analysis has a good reputation for accuracy and completeness. We review all 33 cases in the following section (Table 1). (A literature search of both Japana Centra Revuo Medicina and MEDLINE found one duplicated case, which Yoshikawa et al[13] have reported).
Table 1.
Clinical status of reported cases of synchronous double cancer with hepatocellular carcinoma and cholangiocellular carcinoma
| Clinical status | Compiled numbers |
| Age, in years (mean ± SD) (range) | 66.7 ± 7.4 (51-84) (y/o) |
| Sex | Total: 33 |
| Male | 30 (90.9%) |
| Female | 3 (9.1%) |
| Anti-HCV | Total: 33 |
| Positive | 24 (72.7%) |
| Negative | 9 (27.3%) |
| HBs antigen | Total: 33 |
| Positive | 3 (9.1%) |
| Negative | 30 (90.9%) |
| Total: 33 | |
| Neither HBV nor HCV infection | 2 (6.1%) |
| Total: 33 | |
| Both positive HBV and HCV infections | 1 (3.0%) |
| Tumor markers | The number/Total number |
| High levels of CEA (> 3.0 ng/mL) | 11/24 (45.8%) |
| High levels of CA19-9 (> 37 ng/mL) | 12/21 (57.1%) |
| High levels of AFP (> 10 ng/mL) | 25/33 (75.8%) |
| Localization of HCCs and CCCs | Total: 33 |
| In the same subsegment | 3 (9.1%) |
| In the segment of the liver accessed by the same portal vein | 5 (15.2%) |
| In a segment of the liver accessed by a different portal vein- | 22 (66.7%) |
| Mean maximum size of the tumors (mean ± SD) (range) | Total: 33 |
| HCC | 3.9 ± 2.7 (0.8-10.0) (cm) |
| CCC | 3.3 ± 3.0 (0.6-14.0) (cm) |
| Pathological diagnosis of the non-cancerous portions of the liver | Total: 33 |
| Chronic hepatitis | 19 (57.6%) |
| Liver cirrhosis | 12 (36.4%) |
| Therapeutic procedures | Total: 33 |
| Surgery | 24 (72.8%) |
| TAE | 2 (6.1%) |
| Hepatic arterial infusion therapy | 1 (3.0%) |
| Surgery plus PEIT | 1 (3.0%) |
| Surgery plus MCT | 1 (3.0%) |
| Liver transplantation | 1 (3.0%) |
| Unknown | 3 (9.1%) |
y/o: Years old; HCV: Hepatitis C virus; HB(V): Hepatitis B (virus); CEA: Carcinoembryonic antigen; AFP: α fetoprotein; HCC: Hepatocellular carcinoma; CCC: Cholangiocellular carcinoma; TAE: Transcatheter arterial embolization; PEIT: Percutaneous ethanol injection therapy; MCT: Microwave coagulation therapy.
Since Mitsui’s report in 1986[5], a total of 33 cases of synchronous double cancer with HCC and CCC have been reported in MEDLINE and Japana Centra Revuo Medicina databases. The patients were aged 66.7 ± 7.4 years (mean ± SD) and the male to female ratio was 30:3. Twenty-four out of 33 cases (72.7%) were positive for HCV and many double cancers developed in livers with HCV infections. Three out of 33 cases (9.4%) were positive for HBs antigen and about 10% of double cancers developed in livers with HBV infections. Two out of 33 cases (6.1%) had neither HBV nor HCV infection, while one had both HBV and HCV infections. Double cancer with HCC and CCC tended to develop in livers with HCV infection, followed by HBV infection, as in HCC. In contrast, Zhang et al[47] reported that 7 out of 12 cases (58.3%) of the combined type (or mixed type) of HCC and CCC were positive for HBs antigen, but no cases were positive for HCV. It is suggested that the background of double cancer is distinctly different from that of the combined (or mixed) type HCC and CCC. High levels of serum carcinoembryonic antigen (CEA) (> 3.0 ng/mL), CA19-9 (> 37 ng/mL), and α fetoprotein (AFP) (> 10 ng/mL) were detected in 11 of 24 cases (45.8%), 12 of 21 cases (57.1%), and 25 of 33 cases (75.8%) (except unknown cases), respectively. Regarding the localization of HCCs and CCCs, only 3 cases of double cancer that developed in the same subsegment of the liver have been reported. In addition, 5 out of 33 cases (15.2%) of double cancer revealed synchronous development in the segments of the liver accessed by the same portal vein (for example, in S5 and S8, or in S2 and S3). In the cases of these double cancers, it is conceivable that (1) the amphi-potential progenitor tumor cells disseminated to a different subsegment in the same portal vein-accessed segment of the liver via the portal vein before they differentiated to either HCC or CCC, and double cancer (HCC-CCC) developed in the same segment of the liver or that (2) in a different subsegment in the same portal vein-accessed segment of the liver, hepatocytes and cholangiocytes with HCV infection became cancerous and underwent proliferation. Twenty-two of 33 cases (66.7%) with double cancer exhibited synchronous development in a different portal vein-accessed segment of the liver. In these cases, it is conceivable that the second mechanism was responsible for the development of the double cancer (multicentric development of HCC). Further laboratory studies are needed to clarify and explain the mechanisms of development of synchronous double cancer.
Across the 33 previously reported cases, the mean maximum size of the HCC and CCC tumors (if the tumors were multiple) were 3.9 ± 2.7 cm and 3.3 ± 3.0 cm, respectively. According to the pathological diagnosis of the non-cancerous portions of the liver, 19 cases (57.6%) were diagnosed with chronic hepatitis and 12 (36.4%) with liver cirrhosis. Double cancers were more likely to develop from chronic hepatitis than from liver cirrhosis. We think that the cases that undergo appropriate examinations and successful treatments because of their higher hepatic reserve tended to get reported and documented. There is thus a possibility of bias in the selection of the reported cases. Most such cases underwent surgery (24 out of 30 cases or 80.0%); the other treatment techniques performed included transcatheter arterial embolization, hepatic arterial infusion therapy, surgery plus percutaneous ethanol injection therapy, and surgery plus microwave coagulation therapy and liver transplantation. The surgical procedure is planned according to the hepatic reserve; many cases of double cancer with HCC and CCC were treated surgically by curative resection.
Footnotes
Peer reviewer: Stephen Anderson Harrison, MD, Division of Gastroenterology and Hepatology, Department of Medicine, Brooke Army Medical Center, 3851 Roger Brooke Drive, Fort Sam Houston, TX 78234, United States
S- Editor Zhang HN L- Editor Herholdt A E- Editor Ma WH
References
- 1.Yamaoka Y, Ikai I, Sakai Y, Okita K, Omata M, Kojiro M, Kobayashi K, Nakanuma Y, Nikawa T, Makuuchi M. Report of the 15th nationwide follow-up survey of primary liver cancer in Japan (In Japanese) Kanzo. 2003;44:157–175. [Google Scholar]
- 2.Ikai I, Arii S, Ichida T, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, et al. Report of the 16th follow-up survey of primary liver cancer. Hepatol Res. 2005;32:163–172. doi: 10.1016/j.hepres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsui T, Makuuchi M, Kurohiji T. [Resected case of primary liver cancer with separate two tumors of hepatocellular carcinoma and cholangiocellular carcinoma.] Kanzo. 1986;27:64–69. [Google Scholar]
- 6.Okada S, Hayashi G, Matsuzaki O, Wakatsuki S, Sumida M. [A case of combined hepatocellular and cholangiocellular carcinoma with ante mortem diagnosis from image findings.] Kanzo. 1990;31:1228–1234. [Google Scholar]
- 7.Taniguchi M, Yoshikawa K, Hashimoto T, Yamaguchi T, Dousei T, Moriguchi A, Ueda H, Taketani S, Utsumi T, Shhara H, et al. [Resected case of combined type of hepatoma contained two components of hepatocellular and cholangiocellular carcinomas.] (In Japanese) Jpan J Gastroenterol Surg. 1994;27:1085–1089. [Google Scholar]
- 8.Sato Y, Ikeda Y, Hara H, Nakamura A, Morishita S, Enomoto K, Inomoto T, Kariya T, Ban K, Imanari T, et al. [A case of double cancer of hepatocellular carcinoma and cholangiocarcinoma diagnosed by follow-up examination of chronic hepatitis C.] Liver Cancer. 1995;1:59–63. [Google Scholar]
- 9.Ide Y, Hashimoto T, Yanagibashi K, Konishi Y, Tani T, Kajiwara T, Ibuki Y, Orino A, Shirane H. [A case of combined hepatocellular and cholangiocellular carcinoma.] Shokaki Gazo. 1999;1:841–846. [Google Scholar]
- 10.Oiya H, Kioka K, Nakai T, Aoki T, Kawasaki Y, Kurai O, Nebiki H, Okawa K, Oka H, Harihara S, et al. [A resected case of double cancer of hepatocellular carcinoma and cholangiocellular carcinoma associated liver cirrhosis C.] Nippon Shokakibyou Gakkai Zassshi. 2000;97:729–734. [PubMed] [Google Scholar]
- 11.Ogata S, Koike J, Maeyama S, Uchikoshi T. [Separate type of small sized combined liver cancers arising on chronic hepatitis C -A case report-.] Kanzo. 2000;41:419–424. [Google Scholar]
- 12.Kaneko T, Ito H, Sumi Y, Sawada S, Yoshida N, Suzuki M, Matsuyama R, Matsushima A, Fukazawa M, Itakura Y, et al. [A case of small cholangiocellular carcinoma found during hepatectomy for hepatocellular carcinoma.] Nihon Rinsho Geka Gakkai Zasshi. 2001;62:2510–2514. [Google Scholar]
- 13.Yoshikawa T, Hirota S, Matsumoto S, Izaki K, Fukuda T, Sugimura K, Maeda S, Kitagaki H. Double cancer, cholangiocellular and hepatocellular carcinomas, in the cirrhotic liver. Radiat Med. 2002;20:33–36. [PubMed] [Google Scholar]
- 14.Ariizumi S, Takasaki K, Otsubo T, Yamamoto M, Katsuragawa H, Katagiri S, Yoshitoshi K, Saito A, Nakano M. [Intrahepatic recurrence of hepatocellular and cholangiocellular carcinoma of the liver treated with hepatic srterial infusion chemotherapy showing efficacy only for the hepatocellular carcinoma components.] Liver Cancer. 2002;8:59–64. [Google Scholar]
- 15.Okamoto N, Teramoto K, Takamatsu S, Ochiai T, Irie T, Noguchi N, Kawamura T, Arii S, Igari T. [A case of double cancer of hepatocellular carcinoma and cholangiocellular carcinoma after disappearance of hepatitis C virus.] Jpan J Gastroenterol Surg. 2004;37:1401–1406. [Google Scholar]
- 16.Sugimura K, Murase K, Nitta T, Kondo T, Sugimoto T, Koike D, Hayashi N, Nakahori Y, Ozeki Y, Onishi Y. [A case of double cancer of hepatocellular carcinoma and cholangiocellular carcinoma.] Liver Cancer. 2004;10:189–195. [Google Scholar]
- 17.Koga Y, Beppu T, Ishiko T, Doi K, Matsuda T, Nakano S, Ikeda K, Kawano I, Hirota M, Egami H. [A longtime-survived case of double cancer in the liver treated with microwave coagulation therapy and hepatectomy.] Jpan J Gastroenterol Surg. 2005;38:502–508. [Google Scholar]
- 18.Iso Y, Shimoda M, Rokkaku K, Abe A, Sawada T, Kubota K. [A case of double cancer of hepatocellular carcinoma and cholangiocellular carcinoma with dissemination.] Liver Cancer. 2005;11:205–210. [Google Scholar]
- 19.Takagi K, Takayama T, Higaki T, Watanabe Y, Hasegawa H. Synchronous hepatocellular carcinoma and cholangiocellular carcinoma. Nihon Univ J Med. 2005;4:203–207. [Google Scholar]
- 20.Nakano M, Hayashi T, Watanabe N, Murayama Y, Ikarashi T, Shimizu H. [A case report of simultaneous of combined hepatocellular and cholangiocellular carcinoma and rectal cancer.] Nihon Rinsho Geka Gakkai Zasshi. 2006;67:2232–2237. [Google Scholar]
- 21.Ogawa T, Itamoto T, Tashiro H, Asahara T, Arihiro K, Kitamoto M. [Double cancer of hepatocellular carcinoma and intraductal growth type of intrahepatic cholangiocarcinomaassociated with hepatolithiasis.] Nihon Rinsho Geka Gakkai Zasshi. 2007;68:1528–1534. [Google Scholar]
- 22.Yukawa N, Rino Y, Kanari M, Saeki H, Wada N, Hasuo K, Oshiro H, Masuda M, Imada T. A Case of Synchronous Combined Cholangiocellular and Hepatocellular Carcinoma Observed after Resection of Intraductal Papillary-mucinous Tumor. Nihon Gekakei Rengo Gakkaishi. 2007;32:808–813. [Google Scholar]
- 23.Takayasu K, Muramatsu Y, Moriyama N, Makuuchi M, Yamazaki S, Kishi K, Yoshino M. Hepatocellular and cholangiocellular carcinoma, double cancer of the liver: report of two cases resected synchronously and metachronously. Am J Gastroenterol. 1989;84:544–547. [PubMed] [Google Scholar]
- 24.Haratake J, Hashimoto H. An immunohistochemical analysis of 13 cases with combined hepatocellular and cholangiocellular carcinoma. Liver. 1995;15:9–15. doi: 10.1111/j.1600-0676.1995.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohwada S, Yoshihiro O, Iwazaki S, Tanahashi Y, Sawada T, Takeyoshi I, Kawashima Y, Nakaura S, Iino Y, Morishita Y. Double cancer in different hepatic lobes: hepatocellular and cholangiocellular carcinoma. Hepatogastroenterology. 1995;42:411–414. [PubMed] [Google Scholar]
- 26.Imai Y, Oda H, Arai M, Shimizu S, Nakatsuru Y, Inoue T, Ishikawa T. Mutational analysis of the p53 and K-ras genes and allelotype study of the Rb-1 gene for investigating the pathogenesis of combined hapatocellular-cholangiocellular carcinomas. Jpn J Cancer Res. 1996;87:1056–1062. doi: 10.1111/j.1349-7006.1996.tb03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagafuchi Y, Okamoto K, Shono M, Higure A, Todoroki H, Itoh H, Takeda S, Katumoto F, Toyoshima S. Separate histogenesis of combined hepatocellular and cholangiocellular carcinoma in two patients. Hepatogastroenterology. 1998;45:523–527. [PubMed] [Google Scholar]
- 28.Itamoto T, Asahara T, Katayama K, Momisako H, Dohi K, Shimamoto F. Double cancer - hepatocellular carcinoma and intrahepatic cholangiocarcinoma with a spindle-cell variant. J Hepatobiliary Pancreat Surg. 1999;6:422–426. doi: 10.1007/s005340050144. [DOI] [PubMed] [Google Scholar]
- 29.Kim YW, Park YK, Park JH, Lee J, Lee SM, Hong SW, Yang MH. A case with intrahepatic double cancer: hepatocellular carcinoma and cholangiocarcinoma associated with multiple von Meyenburg complexes. Yonsei Med J. 1999;40:506–509. doi: 10.3349/ymj.1999.40.5.506. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Kuwano H, Masuda N, Hashimoto S, Saitoh T, Kanoh K, Nomoto K, Shimura T. Resected case of a double cancer, a hepatocellular carcinoma and a cholangiocellular carcinoma, and their spread to the skin. Hepatogastroenterology. 2003;50:362–365. [PubMed] [Google Scholar]
- 31.Ito Y, Fujioka H, Matsuzaki S, Yamamoto O, Okudaira S, Azuma T, Furui J, Kanematsu T. Occurrence of hepatocellular and cholangiocellular carcinoma in different hepatic lobes. Hepatogastroenterology. 2003;50:65–68. [PubMed] [Google Scholar]
- 32.Chang JY, Kim BH, Hong SW, Kim YW, Oh JH. A case report of synchronous double primary liver cancers combined with early gastric cancer. Korean J Intern Med. 2003;18:115–118. doi: 10.3904/kjim.2003.18.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuji N, Taniguchi H, Amaike H, Oka K, Tsuchihashi Y, Urasaki K, Naito K. Synchronously resected double primary hepatic cancer, hepatocellular carcinoma and cholangiocarcinoma. J Gastroenterol Hepatol. 2005;20:967–969. doi: 10.1111/j.1440-1746.2005.03806.x. [DOI] [PubMed] [Google Scholar]
- 34.Sotiropoulos GC, Molmenti EP, Frilling A, Paul A, Malamutmann E, Broelsch CE, Malago M. Liver transplantation for double primary hepatic cancer-hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Transplantation. 2006;82:718–719. doi: 10.1097/01.tp.0000234929.56209.8e. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, Hara M, Suzuki T, Kono H, Fujii H. Synchronously resected double primary hepatic cancers - hepatocellular carcinoma and cholangiolocellular carcinoma. J Hepatobiliary Pancreat Surg. 2006;13:571–576. doi: 10.1007/s00534-006-1118-0. [DOI] [PubMed] [Google Scholar]
- 36.Inaba K, Suzuki S, Sakaguchi T, Kobayasi Y, Takehara Y, Miura K, Baba S, Nakamura S, Konno H. Double primary liver cancer (intrahepatic cholangiocarcinoma and hepatocellular carcinoma) in a patient with hepatitis C virus-related cirrhosis. J Hepatobiliary Pancreat Surg. 2007;14:204–209. doi: 10.1007/s00534-006-1134-0. [DOI] [PubMed] [Google Scholar]
- 37.Tomimatsu M, Ishiguro N, Taniai M, Okuda H, Saito A, Obata H, Yamamoto M, Takasaki K, Nakano M. Hepatitis C virus antibody in patients with primary liver cancer (hepatocellular carcinoma, cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma) in Japan. Cancer. 1993;72:683–688. doi: 10.1002/1097-0142(19930801)72:3<683::aid-cncr2820720310>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, Takasaki K, Nakano M, Saito A. Minute nodular intrahepatic cholangiocarcinoma. Cancer. 1998;82:2145–2149. [PubMed] [Google Scholar]
- 39.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283–287. doi: 10.1093/jjco/hyg056. [DOI] [PubMed] [Google Scholar]
- 41.Ros PR, Buck JL, Goodman ZD, Ros AM, Olmsted WW. Intrahepatic cholangiocarcinoma: radiologic-pathologic correlation. Radiology. 1988;167:689–693. doi: 10.1148/radiology.167.3.2834769. [DOI] [PubMed] [Google Scholar]
- 42.Choi BI, Han JK, Kim TK. Diagnosi and staging of cholangiocarcinoma by computed tomography. In: Meyers MA, editor. Neoplasms of the digestive tract: imging, staging and management. Philadelphia: Lippincott-Raven; 1998. pp. 503–516. [Google Scholar]
- 43.Choi BI, Lee JM, Han JK. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging. 2004;29:548–557. doi: 10.1007/s00261-004-0188-1. [DOI] [PubMed] [Google Scholar]
- 44.Choi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540–552, table of contents. doi: 10.1128/CMR.17.3.540-552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913–922. doi: 10.1634/theoncologist.11-8-913. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]