Abstract
Cytomegalovirus (CMV) is the most common viral pathogen that negatively impacts on the outcome of liver transplantation. CMV cause febrile illness often accompanied by bone marrow suppression, and in some cases, invades tissues including the transplanted allograft. In addition, CMV has been significantly associated with an increased predisposition to allograft rejection, accelerated hepatitis C recurrence, and other opportunistic infections, as well as reduced overall patient and allograft survival. To negate the adverse effects of CMV on outcome, its prevention, whether through antiviral prophylaxis or preemptive therapy, is regarded as an essential component to the medical management of liver transplant patients. Two recent guidelines have suggested that antiviral prophylaxis or preemptive therapy are similarly effective in preventing CMV disease in modest-risk CMV-seropositive liver transplant recipients, while antiviral prophylaxis is the preferred strategy over preemptive therapy for the prevention of CMV disease in high-risk recipients [CMV-seronegative recipients of liver allografts from CMV-seropositive donors (D+/R-)]. However, antiviral prophylaxis has only delayed the onset of CMV disease in many CMV D+/R- liver transplant recipients, and at least in one study, such occurrence of late-onset primary CMV disease was significantly associated with increased mortality after liver transplantation. Therefore, optimized strategies for prevention are needed, and aggressive treatment of CMV infection and disease should be pursued. The standard treatment of CMV disease consists of intravenous ganciclovir or oral valganciclovir, and if feasible, one should also reduce the degree of immunosuppression. In one recent controlled clinical trial, valganciclovir was found to be as effective and safe as intravenous ganciclovir for the treatment of mild to moderate CMV disease in solid organ (including liver) transplant recipients. In this article, the authors review the current state and the future perspectives of prevention and treatment of CMV disease after liver transplantation.
Keywords: Cytomegalovirus, Outcome, Hepatitis, Transplantation, Valganciclovir, Prophylaxis, Treatment
INTRODUCTION
Cytomegalovirus (CMV) is the single most common viral pathogen that influences the outcome of liver transplantation[1,2]. CMV is a ubiquitous herpes virus that, depending on the population studied, infects 60%-100% of humans[1,2]. Primary CMV infection in immune competent individuals presents most commonly as an asymptomatic illness or a benign febrile infectious mononucleosis-like syndrome. When CMV infection occurs in individuals with compromised immunity, such as liver transplant recipients, clinical disease with high morbidity may develop and, in some cases, this may lead to death[1,2].
The outcome of primary CMV infection is latency in various cells, which ensures persistence throughout the life of the host[1,2]. This characteristic of the virus plays a very important role in how liver transplant recipients develop CMV infection. Firstly, cellular sites of viral latency become reservoirs for reactivation during periods of stress and cytokine release (such as during allograft rejection, allogeneic stimulation, and critical illness). Secondly, cellular sites of viral latency (which is widespread in the human host) serve as vehicles for transmission to susceptible hosts (i.e. during blood transfusions and transplantation of liver allografts latently infected with virus). Moreover, the pharmacologically-induced impairment of immunity in liver transplant recipients markedly limits the ability of the patients to effectively control “endogenously-reactivated” or “allograft-transmitted” CMV, leading in the short-term to febrile and tissue-invasive diseases, and in the long-term to poor allograft and patient survival[1-5].
CLINICAL IMPACT OF CMV ON LIVER TRANSPLANTATION
Direct CMV effects
The classic illness caused by CMV after transplantation is manifested as fever, bone marrow suppression, and organ-invasive diseases (Table 1)[1]. These have been traditionally categorized either as CMV syndrome (fever with bone marrow suppression) and tissue-invasive CMV disease (which may involve virtually any organ system)[6]. The most common organ system involved during CMV disease is the gastrointestinal tract (in the form of CMV gastritis, esophagitis, enteritis, and colitis), accounting for over 70% of tissue-invasive CMV disease cases in solid organ transplant recipients[7]. The transplanted liver also seems to be more predisposed to develop tissue-invasion by CMV such that CMV hepatitis occurs more frequently in liver transplant recipients than in other solid organ transplant recipients. The involvement of the transplanted allograft is often manifested by symptoms that may be clinically indistinguishable from acute allograft rejection[8]. The availability of sensitive tests for the rapid detection of CMV in the blood may obviate the need for liver biopsy to differentiate CMV disease from organ rejection. However, in many cases, a liver biopsy is needed to differentiate or to demonstrate the co-existence of CMV disease and allograft rejection.
Table 1.
Direct and indirect clinical effects of cytomegalovirus after solid organ transplantation
| Direct effects | Indirect effects |
| CMV syndrome | Acute allograft rejection |
| Fever | |
| Myelosuppression | |
| Malaise | |
| Tissue-invasive CMV disease1 | Chronic allograft rejection |
| Gastrointestinal disease (colitis, esophagitis, gastritis, enteritis) | Vanishing bile duct syndrome Chronic ductopenic rejection |
| Hepatitis | Hepatitis C virus recurrence |
| Pneumonitis | Allograft hepatitis, fibrosis and allograft failure |
| CNS disease | |
| Retinitis | |
| Mortality | Opportunistic and other infections |
| Fungal superinfection | |
| Nocardiosis | |
| Bacterial superinfection | |
| Epstein-Barr virus and PTLD | |
| HHV-6 and HHV-7 infections | |
| Vascular thrombosis | |
| Mortality |
PTLD: post-transplant lymphoproliferative disease; HHV: human herpes virus; CMV: cytomegalovirus.
Any organ system may be affected by CMV.
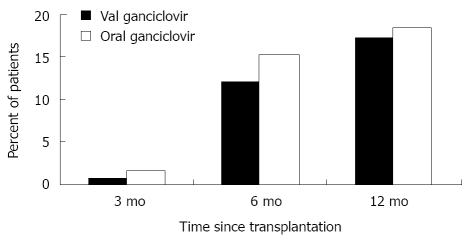
Among liver transplant recipients who are not receiving effective antiviral prophylaxis, the direct effects of CMV are observed most commonly during the first 3 mo after liver transplantation[6]. Overall, it is estimated that 18%-29% of all liver transplant recipients will develop CMV disease (Table 2)[4,5,9-11]. However, this incidence varies depending upon donor and recipient CMV serologic status; it may be as high as 44%-65% in CMV D+/R-, or as low as 8%-19% in CMV-seropositive (CMV R+) liver transplant recipients[4,9,11]. The incidence is markedly reduced in liver transplant recipients who received 3 mo of prophylaxis with valganciclovir or oral ganciclovir. Recent studies have reported CMV disease incidence rates of 12%-30% in CMV D+/R-, and < 10% in CMV R+ liver transplant recipients who received 3 mo of antiviral prophylaxis[3,4,9,11-13]. The onset of disease in these patients occurs most commonly during first 3 mo after completing antiviral prophylaxis; hence, the term “delayed-onset (also termed late-onset) CMV disease” to indicate that the onset has been delayed by antiviral prophylaxis (Figure 1)[3].
Table 2.
Estimated incidence of cytomegalovirus disease during the first 12 mo after liver transplantation
|
Use of anti-cytomegalovirus prophylaxis |
||
| Yes1 (%) | No (%) | |
| CMV D+/R- | 12-30 | 44-65 |
| CMV D+/R+ | 2.70 | 18.20 |
| CMV D-/R+ | 3.90 | 7.90 |
| CMV D-/R- | 0.00 | 0.00 |
| All patients | 4.80 | 18-29 |
D: donor; R: recipient; CMV: cytomegalovirus.
Most cases occur as delayed-onset CMV disease. CMV disease occurs rarely during prophylaxis with oral valganciclovir. Data adapted from references [4,5,77].
Figure 1.
Time to the onset of cytomegalovirus disease in solid organ transplant recipients who received three mo of oral ganciclovir or valganciclovir prophylaxis. Data obtained from the study by Paya and colleagues[5].
Indirect CMV effects
The clinical impact of CMV after liver transplantation extends beyond its direct effects to numerous indirect outcomes that are believed to be mediated by the ability of CMV to modulate the immune system (Table 1)[1,2]. CMV is known to be a potent up-regulator of alloantigens, which increases the risk of acute rejection and chronic allograft dysfunction[14]. CMV has been associated with vanishing bile duct syndrome and ductopenic rejection that leads to chronic cholestasis and eventually to allograft failure[15-17]. A higher incidence of vascular and hepatic artery thrombosis has also been reported in a few studies of liver transplant recipients with CMV disease, and this effect is postulated to result from infection of the vascular endothelial cells[18,19]. The immunomodulatory effects of CMV have been suggested to account for a higher predisposition to develop infections due to other opportunistic infections including fungi, other viruses, and bacteria such as Nocardia sp[20,21]. CMV-infected transplant recipients are also more likely to develop Epstein-Barr virus associated post-transplant lymphoproliferative disorders (PTLD), or develop co-infections with other viruses such as the human herpesviruses HHV-6 and HHV-7[20,22]. There is a well-described interaction between members of the beta-herpes group of viruses, known as β-herpesvirus syndrome, as exemplified by observations that reactivations of HHV-6 and HHV-7 are significantly associated with an increased predisposition to CMV disease after liver transplantation[23-25]. Similarly, a significant association between CMV and hepatitis C virus (HCV) is also described after liver transplantation[26-31]. This is clinically manifested as an accelerated clinical course of HCV recurrence among patients who have developed CMV infection and disease. In one study of 92 HCV-infected liver transplant recipients, there was a 4-fold higher risk of allograft failure and mortality among those who developed CMV infection and disease. Three years after liver transplantation, 48% of patients who developed CMV disease had allograft loss or had died, compared to 35% of patients with asymptomatic CMV infection, and 17% of patients who did not develop CMV infection[29,31].
Impact on mortality
Through direct, indirect and possibly immunomodulatory mechanisms, CMV is an important predictor of mortality after transplantation[20,32,33]. Prior to the availability of intravenous (IV) and oral ganciclovir, CMV was a major cause of mortality after liver transplantation. With the use of these effective antiviral drugs for prevention and treatment, death due to CMV disease has been remarkably reduced. Indeed, several meta-analyses have demonstrated that the use of anti-CMV drugs, either through antiviral prophylaxis or preemptive therapy, is associated with significant reductions in mortality after transplantation[20,34-36].
Despite these improvements in outcome with the widespread use of antiviral drugs, CMV disease occurring at a delayed onset after prophylaxis remains a common problem, and notably, remains significantly associated with increased risk of mortality after liver transplantation[33]. In an analysis of 437 liver transplant recipients, CMV disease occurred in 37 patients (8.5%) and its occurrence was independently associated with a 5-fold increased risk of all-cause mortality, and an 11-fold increased risk of infection-related mortality[33].
RISK FACTORS FOR CMV DISEASE AFTER LIVER TRANSPLANTATION
Lack of pre-existing CMV-specific immunity
The most important risk factor for the occurrence of CMV disease after liver transplantation is a lack of effective CMV-specific immunity. Specifically, CMV D+/R- patients are at highest risk of CMV disease[4,20], while CMV R+ patients have modest risk and CMV D-/R- have the lowest risk of CMV disease after liver transplantation (Table 3).
Table 3.
Selected traditional and novel factors associated with increased risk of cytomegalovirus disease after liver transplantation
| Traditional factors | Recently identified factors |
| CMV D+/R- > CMV R+ | Toll-like receptor gene polymorphism |
| Allograft rejection | Mannose binding lectin deficiency |
| High viral replication | Chemokine and cytokine defects (IL-10, MCP-1, CCR5) |
| Mycophenolate mofetil | Deficiency in CMV-specific CD4+ T cells |
| Muromonab-CD3 | Deficiency in CMV-specific CD8+ T cells |
| Anti-thymocyte globulin | Expression of immune evasion genes |
| Alemtuzumab | Programmed cell death 1 expression |
| Basiliximab | |
| Human herpesvirus-6 | |
| Human herpesvirus-7 | |
| Renal insufficiency | |
| Others1 |
D: donor; R: recipient; IL-10: interleukin-10; MCP-1: monocyte chemotactic protein-1; CCR5: chemokine (C-C motif) receptor 5; CMV: cytomegalovirus.
Others include re-transplantation, volume of blood transfusion, sepsis and other factors associated with high tumor necrosis factor-α secretion.
Drug-induced immunodeficiency
Severe pharmacologic immunosuppression impairs the ability of liver transplant recipients to mount an effective immune response against CMV, thereby predisposing to higher risk of CMV disease[4,20]. The severity of immune dysfunction is particularly intense with the use of lymphocyte-depleting drugs, as either induction or rejection therapy, such as muromonab-CD3 (OKT3) and anti-thymocyte globulin[37,38]. When alemtuzumab, an anti-CD52 lymphocytic antibody, is used for short-course induction therapy only, the risk of developing CMV disease is low[39,40]. However, when patients receive alemtuzumab as rejection therapy, the risk of developing CMV disease is higher, suggesting that rejection per se also increases the risk[40]. Basiliximab and daclizumab are also used for induction therapy and they act as anti-CD25 directed non-depleting antibodies (interleukin-2 receptor antagonist). Transplant recipients receiving basiliximab induction therapy have a lower incidence of infection but a greater incidence of CMV disease than those receiving anti-thymocyte globulin[41].
Drugs used for maintenance immunosuppression have also been associated with CMV disease, particularly with high-doses of mycophenolate mofetil[31,42]. More recently, the use of newer maintenance immunosuppressive drugs such as sirolimus and everolimus [mammalian target of rapamycin (mTOR) inhibitor] has been found to be associated with lower risk of CMV disease[43,44]. These observations have generated special interest in the use of the mTOR agents for patients at high risk of CMV disease. However, it is very likely that not only do the specific immunosuppressive drugs predispose to CMV disease, but that the net state of combined pharmacologic immunosuppression increases the risk of CMV disease after liver transplantation[1,2,20].
Defects in innate and CMV-specific cell-mediated immunity
Inherent defects in innate immunity, such as mutations in innate immunity-associated genes, increase the risk of CMV disease after liver transplantation (Table 3). In our study of 92 liver transplant recipients, a specific genetic polymorphism in the Toll-like receptor (TLR)-2 gene, which resulted in the substitution of arginine to glutamine at position 753 in the protein-receptor, was significantly associated with a higher degree of CMV replication and a higher incidence of CMV disease. TLR2 is a pattern recognition receptor expressed in innate immune cells, and it functions to sense the glycoprotein B of CMV, thereby signaling the immune cells to produce antiviral peptides and other cytokines. Our in vitro data suggests that this specific genetic polymorphism causes in impairment of cellular recognition of CMV by TLR2-expressing cells[45].
CMV-specific T cells are necessary for the adequate control of CMV after liver transplantation[46], and the detection of these pathogen-specific cells after transplantation appear to confer protection against the development of CMV disease. In one study, secretion of interferon-γ by CD8+ T cells during in vitro stimulation with a pool of CMV peptides was significantly associated with a lower incidence of late onset CMV disease in a cohort of solid organ transplant recipients who received valganciclovir prophylaxis[47]. However, data from other studies do not yet support the potential clinical utility of CMV-specific T cells as prognostic markers of CMV disease predisposition[46]. There are ongoing studies in this field that will further clarify the prognostic role of CMV-specific T cell assays in stratifying CMV disease risk after liver transplantation.
Other immune measures, such as programmed death-1 expression[48], mannose binding lectin levels or gene mutation, and immune evasion genes[49] have also been assessed as prognostic indicators of CMV disease after transplantation. In one study, programmed death-1 receptor up-regulation was significantly associated with incipient and overt CMV disease and with CMV viremia[48].
Allograft rejection
Allograft rejection is often associated with CMV reactivation, and thus it is considered as a significant risk factor for CMV disease after liver transplantation[13]. It is hypothesized that cytokines that are released during episodes of acute rejection, particularly tumor necrosis factor-α[50], could transactivate CMV from its state of latency[51,52]. Subsequent therapy for allograft rejection with intensified immunosuppression further enhances the risk of CMV disease by enhancing viral replication and by impairing the generation of an effective CMV-specific cell-mediated immunity[53]. Conversely, CMV induces allogeneic stimulation thereby increasing the risk of allograft rejection, and creating a bidirectional relationship between CMV and allograft rejection[14].
Virus-to-virus interactions
Virus-virus interactions have been proposed to enhance the risk of CMV disease after liver transplantation[22,23,27-31]. HHV-6 has been associated with an increased predisposition to develop CMV disease after liver transplantation[22,23,25]. Likewise, HCV-infected liver transplant patients have a higher incidence of CMV disease[54], although the advent of valganciclovir prophylaxis has allowed mitigation of this phenomenon[26].
Other factors
The risk of CMV disease after liver transplantation is associated, in direct proportion, with the degree of CMV replication, which is partly a function of over-immunosuppression[9,24,55,56]. Other factors associated with CMV disease after liver transplantation include cold ischemia time, bacterial and fungal infections and sepsis, the amount of blood loss, fulminant hepatic failure as the indication for liver transplantation, age, female gender, Hispanic race, and renal insufficiency[2,3,20,57].
PREVENTION OF CMV DISEASE AFTER LIVER TRANSPLANTATION
There are two major strategies for CMV disease prevention after liver transplantation: (1) preemptive therapy (wherein patients are monitored for CMV replication by sensitive assays such as PCR and pp65 antigenemia, and upon the detection of asymptomatic CMV replication, antiviral therapy is administered preemptively to prevent progression to symptomatic clinical disease); and (2) antiviral prophylaxis (wherein antiviral drugs such as valganciclovir are administered to all patients at risk of CMV disease after liver transplantation)[20]. Both of these prevention strategies are considered similarly effective in preventing CMV disease after liver transplantation[4,5,58-61]. According to the current American Society of Transplantation (AST) and The Transplantation Society (TTS) guidelines, preemptive therapy may be an option in CMV D+/R- liver transplant recipients, although many authorities prefer to use antiviral prophylaxis and reserve preemptive therapy for lower-risk populations[62,63]. The main reason for this is the rapidity of CMV replication in CMV-naïve CMV D+/R- liver recipients, which may escape detection with once weekly CMV surveillance. Indeed, antiviral prophylaxis is used by the majority of American and European transplant centers in preventing primary CMV disease in high-risk CMV D+/R- liver transplant recipients[64,65]. Moreover, primary antiviral prophylaxis has the added benefit of reduction in bacterial and fungal opportunistic infections and mortality[34,35].
Preemptive therapy
The basic principle of preemptive therapy is to detect the presence of CMV replication prior to the onset of clinical symptoms, so that antiviral therapy is administered early in order to prevent the progression of asymptomatic infection to full-blown clinical disease[56,58,59,61,66]. Preemptive therapy has the potential advantage of targeting therapy to the highest risk patients and thereby decreasing drug costs and toxicity. The success of this approach relies on several aspects including: (1) the optimal laboratory test and frequency and duration of monitoring; (2) selection of the appropriate population for preemptive therapy; and (3) choosing the type, dose and duration of an antiviral drug.
Either the CMV pp65 antigenemia assay or quantitative PCR may be used for the preemptive approach. The pp65 antigenemia assay is a semi-quantitative fluorescence assay based on detection of CMV pp65 antigen present in infected cells in the peripheral blood. This assay is comparable in sensitivity to CMV PCR[67] and remains a valuable test for centers managing small numbers of specimens. However, because the pp65 antigenemia assay needs processing of samples within 6-8 h of blood collection, a large sample volume, subjective interpretation that leads to poor standardization, and is labor-intensive, many laboratories are moving towards quantitative PCR. PCR has significant potential power for patient surveillance in the preemptive approach. However, there is lack of CMV PCR standardization across different laboratories and each center needs to validate their own threshold values for preemptive therapy[62,63]. The optimal interval and duration of monitoring is unknown, but testing approximately once weekly for 12 wk after transplant is suggested. If a patient shows viremia above an institutionally-derived threshold during the surveillance, therapy should be initiated and continued until CMV viremia is no longer detectable[62,63].
Several studies have reported the success of IV or oral ganciclovir or valganciclovir in the preemptive treatment of CMV reactivation in liver transplant recipients, including high-risk CMV D+/R- patients[60,66]. However, some studies have indicated that preemptive therapy may not be completely effective in CMV D+/R- liver transplant recipients since the replication kinetics of CMV in immune-deficient individuals is so rapid[55] that it may escape detection with once weekly PCR or antigenemia assay, and this could result in clinical illness prior to its laboratory detection[9,58]. Indeed, in our clinical experience, nearly 25% of CMV D+/R- liver transplant recipients who developed CMV disease were not identified early by a protocol-based weekly CMV PCR assay[9,58]. Accordingly, the current AST and TTS guidelines prefer antiviral prophylaxis in CMV D+/R- liver transplant recipients[62,63]. In contrast, preemptive therapy is recommended and highly effective in CMV-seropositive liver transplant recipients.
Reassuringly, clinical trials have demonstrated the efficacy of preemptive therapy in CMV disease prevention[58-60,66]. Three meta-analyses that collectively analyzed data from prospective clinical trials demonstrated the benefits of preemptive therapy in preventing CMV disease[35,36,68]. When conducted properly, preemptive therapy, with the use of oral ganciclovir, IV ganciclovir, or valganciclovir resulted in the reduction of CMV disease by about 70%[35,36,68]. Moreover, preemptive therapy is much less likely associated with late onset CMV disease (unlike in antiviral prophylaxis, as discussed below)[59,66]. Currently, valganciclovir is the most commonly used drug for preemptive therapy[64], and in one non-controlled study, it was demonstrated to be as effective in terms of clinical and virologic response, as IV ganciclovir[59,66]. In addition, preemptive therapy may be beneficial in reducing the indirect effects of CMV, although to a much lesser degree than antiviral prophylaxis. In one study, the incidence of major opportunistic infections, bacteremia, bacterial infection, HCV recurrence, and rejection were not significantly different between liver transplant patients who received preemptive therapy and those who did not have CMV reactivation[69]. An example of a preemptive algorithm is shown in Figure 2.
Figure 2.
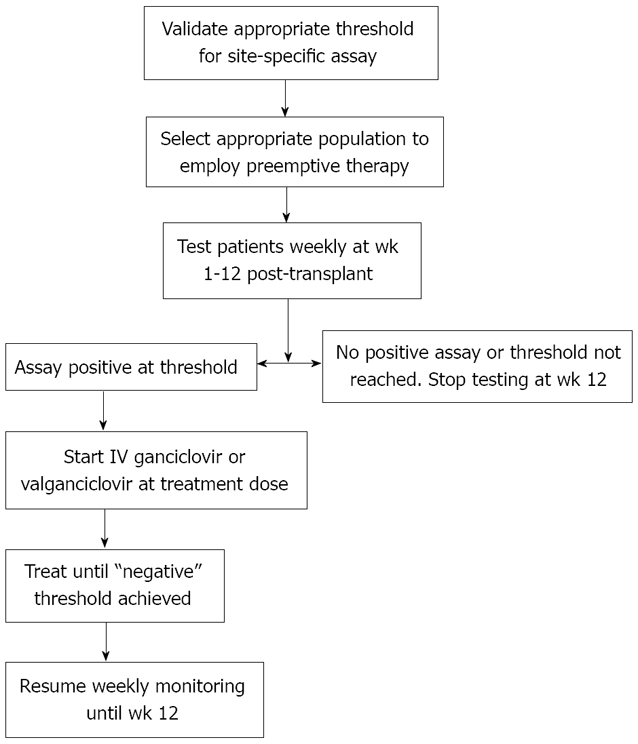
Suggested algorithm for preemptive therapy. Figure adapted from reference [62].
Antiviral prophylaxis
Antiviral prophylaxis is highly effective in preventing the direct, as well as the indirect effects of CMV after liver transplantation[4,5,35,36,68]. Compared to placebo or no treatment, patients who received antiviral prophylaxis had lower incidence of CMV disease (58%-80% reduction) and CMV infection (about 40% reduction)[68]. In one meta-analysis, a 25% reduction in the incidence of acute allograft rejection was observed[35]. In two studies, a reduction in all-cause mortality was observed[35,68], mainly due to a decline in CMV-related death[68]. A reduction in the incidence of other herpes viruses, bacterial, and protozoan infections were also observed[68]. Because of these additional benefits, liver transplant centers prefer the use of antiviral prophylaxis over preemptive therapy in the prevention of CMV disease, particularly in CMV D+/R- liver transplant recipients[64]. Table 4 shows the currently available antiviral drugs for CMV prophylaxis and treatment in liver transplant recipients.
Table 4.
Currently available antiviral drugs for cytomegalovirus prophylaxis and treatment in liver transplant recipients
| Drug | Route | Usual adult prophylaxis dose | Usual adult treatment dose | Comments on use and major toxicity |
| Ganciclovir | Intravenous | 5 mg/kg once daily | 5 mg/kg twice daily | Intravenous access; leukopenia |
| Ganciclovir | Oral | 1 g three times daily | Not applicable | Low oral bioavailability; high pill burden |
| Valganciclovir | Oral | 900 mg once daily | 900 mg twice daily | Ease of administration; leukopenia |
Ganciclovir prophylaxis
Ganciclovir-based regimen is more effective than acyclovir or immunoglobulins in reducing the incidence of CMV after liver transplantation. In one study, the administration of IV ganciclovir for 90-100 d reduced the incidence of CMV disease in CMV D+/R- liver transplant recipients to 5.4% (compared to 40% in patients who received less than 7 weeks of prophylaxis)[70]. The major drawback to IV ganciclovir was the need for long-term vascular access and its risks of thrombosis, phlebitis, and line-associated bacterial and fungal infections[38,71]. Oral ganciclovir, administered at 1000 mg PO three times daily, circumvents these limitations of intravenous therapy. In a randomized trial that compared it to placebo, oral ganciclovir for 98 days reduced the 6-mo incidence of CMV infection (51.5% vs 24.5%; P < 0.001), and CMV disease (19% vs 5%; P < 0.001) in liver transplant recipients[4], including CMV D+/R- patients (44% vs 15%; P = 0.02) and patients who received antilymphocyte antibodies (33% vs 5%; P = 0.002)[4]. Among CMV R+ liver transplant recipients, oral ganciclovir for 12 wk reduced the incidence of CMV disease to 1% (compared to 7% in patients who received acyclovir)[72]. Oral ganciclovir, however, is poorly absorbed, and its oral administration results in low systemic ganciclovir levels[73]. This factor has been implicated in the emergence of ganciclovir-resistant CMV in certain clinical settings[74,75], such as high-risk CMV D+/R- patients, and those receiving potent immunosuppressive regimens.
Valganciclovir prophylaxis
Valganciclovir, a valine ester of ganciclovir, provides systemic ganciclovir levels that are comparable to IV ganciclovir[73,76]. Pharmacokinetic studies indicate that a 900 mg dose of valganciclovir achieves a similar daily area under the concentration time curve (AUC24) as an IV dose of 5 mg/kg of ganciclovir[73]. Hence, valganciclovir (900 mg once daily) has the advantage of avoiding the cost and risks of IV ganciclovir and the pill burden and poor absorption of oral ganciclovir. The role of valganciclovir in the prevention of CMV disease after liver transplantation was evaluated in a multicenter randomized non-inferiority clinical trial that compared it with oral ganciclovir in a cohort of 364 CMV D+/R- solid organ (including liver) transplant recipients (Figure 1). The 6-mo incidence of CMV disease was 12% and 15% in the valganciclovir and oral ganciclovir groups, respectively. Follow-up at one year, demonstrated that the incidence of protocol-defined CMV disease in all patients was 17% and 18% with valganciclovir and oral ganciclovir, respectively[5]. Overall, valganciclovir was as clinically effective and well-tolerated (except for a higher incidence of neutropenia; 8% and 3%, respectively) as oral ganciclovir for CMV prevention in high-risk solid organ transplant recipients.
However, in a subgroup analysis of the 177 liver transplant recipients who participated in the clinical trial, the incidence of CMV disease was 19% in the valganciclovir group as opposed to only 12% in the ganciclovir group. There was also a higher incidence of tissue-invasive CMV disease in the valganciclovir group[5]. As a result of these findings, valganciclovir did not gain approval from the US-FDA for prophylaxis against CMV disease after liver transplantation. Although not FDA-approved for prophylaxis in liver transplant recipients, valganciclovir is the most widely used drug for the prevention of CMV disease after liver transplantation[64].
Delayed- and late- onset CMV disease
With the success of a 3-mo anti-CMV prophylaxis program (in terms of the almost complete elimination of CMV disease among individuals who are actively taking the antiviral drugs), the challenge of delayed- and late-onset CMV disease has emerged. In many high-risk CMV D+/R- individuals, the use of antiviral prophylaxis has only delayed the onset of CMV disease to 3-6 mo after liver transplantation[3-5,13]. In a retrospective study, CMV disease occurred in 14 of 54 (26%) CMV D+/R- liver transplant recipients who completed at least 3 mo of valganciclovir prophylaxis (Figure 1)[77]. In another retrospective study on 203 liver transplant recipients who received valganciclovir 900 mg daily for 3 to 6 mo, the overall incidence of CMV disease was 14%. The incidence varied among the different CMV serogroups (16% in D+/R+ group; 7% in D-/R+ group; and 26% in D+/R- group)[77]. These findings illustrate that the burden of delayed-onset CMV disease remains high, particularly in the CMV D+/R- group[5]. In our analysis of 67 CMV D+/R- liver transplant recipients who received 3 mo of oral ganciclovir and valganciclovir prophylaxis, the two year incidence of CMV disease was 29%. The incidence of delayed-onset CMV disease was not significantly different between patients who received oral ganciclovir or valganciclovir (22% vs 28%; P = 0.63)[3]. Thus, one out of every four CMV D+/R- liver transplant recipients will develop CMV disease after cessation of antiviral prophylaxis. Delayed-onset CMV disease most commonly presented as CMV syndrome, with fever and bone marrow suppression[3]. In less than half of the patients, CMV manifested as tissue-invasive disease, and frequently effected the gastrointestinal tract[3]. Factors such as age[3], female gender[3,78], renal dysfunction[78], and allograft rejection[13] predisposed to the development of delayed-onset primary CMV disease. Delayed-onset CMV disease appears to be clinically less severe, although it is associated with significant mortality after liver transplantation[33].
Because of the negative effect of late onset CMV disease on overall outcome, a better method for CMV prevention is needed among CMV D+/R- liver transplant recipients. The current AST and TTS guidelines suggest that the duration of antiviral prophylaxis may be prolonged from the standard 3 mo to 6 mo in CMV D+/R- liver transplant recipients[62,63]. This recommendation is based on a trial that investigated this approach in CMV D+/R- kidney transplant recipients. It is emphasized that this duration has not yet been studied in the liver transplant recipients, and that valganciclovir is not FDA-approved for the prevention of CMV disease after liver transplantation. Nonetheless, in this study of kidney transplant recipients, the incidence of CMV disease was reduced from 36.8% in patients who received 3 mo of valganciclovir prophylaxis to 16.1% in those who received the drug for 6 mo. While this represents a significant reduction in the incidence of CMV disease, the data also highlights the continued risk in some patients despite the prolonged prophylaxis (in this case, 16% still developed CMV disease despite 6 months of valganciclovir prophylaxis). In addition, there are theoretical concerns about ganciclovir resistance and drug toxicity particularly with leukopenia during prolonged prophylaxis, although these were not demonstrated in the clinical trial. The cost of the use of prolonged prophylaxis will need to be evaluated. Another strategy that is gaining interest is an aggressive minimization of immunosuppression, including the use of prednisone-free regimens. Many liver transplant programs (including ours) have adapted this approach, and have minimized immunosuppression gradually so that patients are maintained on tacrolimus monotherapy beyond the 4th mo after liver transplantation. In a retrospective analysis, we observed a higher incidence of CMV disease among liver transplant recipients who were still receiving mycophenolate mofetil and prednisone at the time they discontinue antiviral prophylaxis[14].
TREATMENT OF CMV DISEASE AFTER LIVER TRANSPLANTATION
The first line treatment of CMV disease after liver transplantation is IV ganciclovir or valganciclovir[62,71,79]. In contrast, oral ganciclovir should not be used for the treatment of CMV disease because of its poor bioavailability[20]. In addition, the degree of pharmacologic immunosuppression should be reduced if possible[20]. In a multi-center non-inferiority trial, 321 solid organ (including liver) transplant recipients with non-severe CMV disease were randomized to valganciclovir (900 mg twice daily) or IV ganciclovir (5 mg/kg twice daily) for a fixed 21-d course, followed by valganciclovir (900 mg once daily) maintenance treatment for 4 wk. The proportion of patients with viral eradication at 21 and 49 d were comparable in the IV ganciclovir and valganciclovir groups (Figure 3)[79]. The overall time to viral eradication was 21 d with valganciclovir and 19 d with IV ganciclovir. The calculated viral decay was 11.5 d with valganciclovir and 10.4 d with IV ganciclovir. Likewise, clinical resolution was not different between the two groups. It was noted that patients enrolled into this trial were mostly CMV-seropositive, the majority were kidney recipients (although there were good number of liver transplant recipients), and patients with severe CMV disease were excluded. Despite these limitations, this pivotal trial now supports the use of valganciclovir for oral treatment of CMV disease, at least in selected transplant patients[79]. IV ganciclovir is preferable to valganciclovir in patients with severe or life-threatening disease, or in patients who may have a problem with gastrointestinal absorption of oral drugs such as those with gastrointestinal CMV disease. In many instances, valganciclovir is used as a step-down treatment when the clinical symptoms have resolved after an initial induction treatment with IV ganciclovir.
Figure 3.
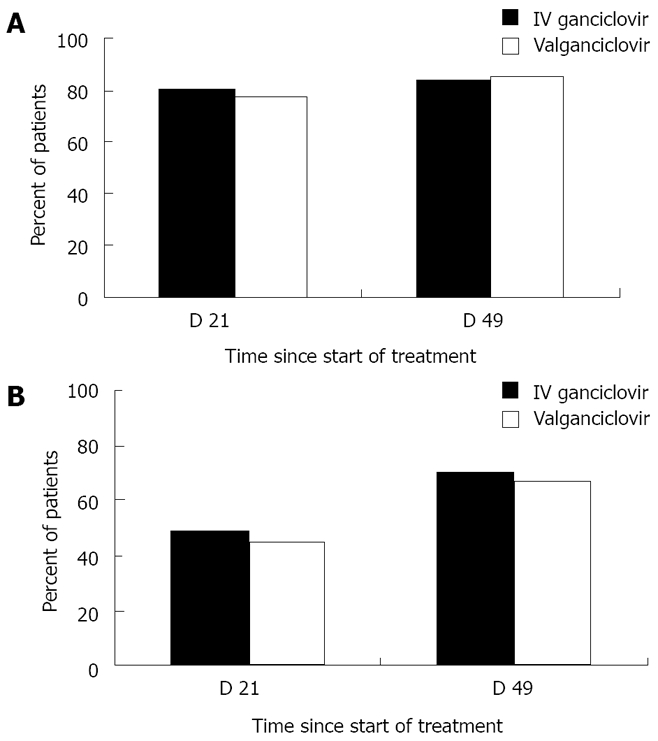
Proportion of solid organ transplant patients with resolution of clinical symptoms (panel A) and viremia eradication (panel B) at day 21 and 49 following the start of valganciclovir or intravenous ganciclovir treatment of CMV disease. Data obtained from the study by Asberg and colleagues[79].
The duration of treatment of CMV disease should be individualized[62,80]. The persistence of the virus at the end of therapy (as indicated by PCR or pp65 antigenemia) is associated with a higher risk of clinical relapse[81]. It is now generally accepted that multiple (at least two) weekly negative CMV PCR results should be obtained before antiviral therapy is discontinued. Although this may be correct for non-tissue invasive CMV syndromes, the utility of such an approach may not necessarily apply to some tissue-invasive disease, which may manifest as “compartmentalized disease”[20].
Treatment of compartmentalized CMV disease
Compartmentalized CMV disease refers to clinical syndromes wherein the virus is detected in the effected tissues but is minimally detectable or undetectable in the blood[20]. In the current era, gastrointestinal CMV disease constitutes the vast majority of tissue-invasive cases[3,7,20], and in a number of cases, especially in CMV R+ patients, this type of CMV disease is “compartmentalized.” In a retrospective study, the sensitivity of pp65 antigenemia assay (defined as detection of ≥ 1 positive cells/2 ×105 leukocytes) for diagnosis of CMV gastrointestinal disease was only 54%[82]. Such a clinical presentation is reminiscent of CMV retinitis, a very rare manifestation of tissue-invasive CMV disease after transplantation, that is often not accompanied by viremia[83,84]. This dilemma brings to the forefront the limitation of viral load monitoring in assessing duration of treatment. In our clinical practice, it is not uncommon to have negative blood PCR assays even when there remains histologic evidence of tissue invasion. Accordingly, it has been suggested that colonoscopy or upper endoscopy should be performed to document clearance of gastrointestinal CMV disease prior to discontinuation of therapy. However, our retrospective review of this practice suggests that this should not be generalized to all patients with gastrointestinal CMV disease. We observed that relapse of gastrointestinal CMV disease was significantly associated with extensive involvement of gastrointestinal tract at the time of diagnosis[85]. In contrast, CMV serologic conversion, degree of viral load, treatment duration, maintenance therapy, and endoscopic findings at the end of therapy were not significantly predictive of CMV relapse. Our experience indicates that endoscopic evidence of resolution of gastrointestinal disease may not be necessary in mild to moderate disease as long as sufficient therapy is provided[85].
Treatment of ganciclovir-resistant CMV disease
Ganciclovir-resistant CMV is now emerging as an important complication of prolonged antiviral drug use after transplantation[2,20,75]. Currently, ganciclovir-resistant CMV is very rarely seen in liver transplant recipients (while it is relatively more common after kidney-pancreas and lung transplantation). The estimated incidence of ganciclovir-resistant CMV after liver transplantation is < 0.5%[75,86]. Several studies have identified risk factors for ganciclovir resistance CMV[2,20,75], including CMV D+/R- status, high levels of viral replication, potent immunosuppressive therapy, and suboptimal ganciclovir levels. The vast majority of drug-resistant cases involve the selection of viral strains with UL97 (kinase) mutation[2,20,75,87,88]. UL97 mutation generally confers resistance to ganciclovir, although in some cases, a concomitant UL54 mutation (CMV DNA polymerase) is also observed, in which case, cross-resistance to cidofovir and/or foscarnet is likely.
Drug-resistant CMV is associated with significant morbidity and mortality, and there is a very limited number of antiviral drugs (which are often toxic) available for treatment[86]. Drug-resistant CMV should be suspected when viral load or antigenemia rises or does not decline to undetectable levels despite IV ganciclovir treatment. In our retrospective study of 225 CMV D+/R- solid organ transplant recipients who received 3 mo of valganciclovir prophylaxis, CMV disease occurred in 65 patients (29%), including four (8%) caused by drug-resistant CMV, judged by the failure of the viral load to decline to undetectable levels while on IV ganciclovir treatment. This diagnosis was confirmed by genetic analysis to demonstrate mutational changes in UL97 and UL54 genes encoding for kinase and polymerase, respectively[75,86]. In patients where foscarnet or cidofovir was used, nephrotoxicity was a major and common adverse effect[89]. Other potential drugs for treatment of multi-drug resistant CMV include the off-label use of immunoglobulins, leflunomide (an immunosuppressive drug), and artesunate (anti-malaria drug), although data supporting their use are only anecdotal[20,90]. The potential clinical utility of maribavir in treatment of resistant CMV has also been suggested. However, the clinical development of this drug is currently halted in view of disappointing results from a phase III clinical trial in bone marrow transplant recipients, which also resulted in the premature termination of the randomized clinical trial in liver transplant recipients[87,88,91,92].
CONCLUSION
Remarkable advances in molecular diagnostics and therapeutics have led to marked reduction in the incidence and severity of CMV disease after liver transplantation, and a parallel decline in associated morbidity and mortality. However, despite these improvements, CMV remains a common infectious complication and continues to negatively influence the outcome of liver transplantation. In addition to viral factors and pharmacologic immunosuppression, the role of innate and adaptive immune deficiencies is being recognized in the pathogenesis of CMV disease after liver transplantation. Such novel findings should provide additional avenues and opportunities for improving our management strategies. Prevention of CMV with antiviral prophylaxis and preemptive therapy is effective, although a well-controlled trial assessing these two strategies in a head-to-head comparison is yet to be conducted after liver transplantation. Currently, valganciclovir prophylaxis is the most common approach for the prevention of CMV disease in CMV D+/R- and R+ liver transplant recipients. The availability of predictive diagnostic tests has paved the way for the successful use of preemptive therapy in preventing the progression of CMV reactivation to clinical disease even among high-risk liver transplant patients. IV ganciclovir and oral valganciclovir are the standard drugs for treatment of established CMV disease, although valganciclovir should be limited to patients with mild to moderate CMV disease. Oral valganciclovir should be avoided as initial therapy for patients with severe CMV disease and those with questionable gastrointestinal absorption. The duration of treatment should be individualized, depending upon clinical and laboratory parameters such as the decline of CMV load in the blood as measured by rapid and sensitive molecular testing. In this context, it is generally recommended that treatment be continued until all evidence of active infection, such as positive CMV viral load, has resolved. Ganciclovir-resistant CMV and compartmentalized tissue-invasive disease (most commonly with gastrointestinal CMV disease) are emerging challenges to the management of CMV after liver transplantation. These, together with the common occurrence of late-onset CMV disease in high-risk patients, should serve as catalysts to the ongoing search for the optimal management strategy for CMV disease after liver transplantation.
Footnotes
Peer reviewer: Valentina Medici, MD, PhD, Department of Internal Medicine, University of California Davis, 4150 V Street, Suite 3500, Sacramento, CA 95817, United States
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
References
- 1.Razonable RR, Emery VC. Management of CMV infection and disease in transplant patients. 27-29 February 2004. Herpes. 2004;11:77–86. [PubMed] [Google Scholar]
- 2.Razonable RR, Paya CV. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes. 2003;10:60–65. [PubMed] [Google Scholar]
- 3.Arthurs SK, Eid AJ, Pedersen RA, Dierkhising RA, Kremers WK, Patel R, Razonable RR. Delayed-onset primary cytomegalovirus disease after liver transplantation. Liver Transpl. 2007;13:1703–1709. doi: 10.1002/lt.21280. [DOI] [PubMed] [Google Scholar]
- 4.Gane E, Saliba F, Valdecasas GJ, O’Grady J, Pescovitz MD, Lyman S, Robinson CA. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected] Lancet. 1997;350:1729–1733. doi: 10.1016/s0140-6736(97)05535-9. [DOI] [PubMed] [Google Scholar]
- 5.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 7.Fica A, Cervera C, Pérez N, Marcos MA, Ramírez J, Linares L, Soto G, Navasa M, Cofan F, Ricart MJ, et al. Immunohistochemically proven cytomegalovirus end-organ disease in solid organ transplant patients: clinical features and usefulness of conventional diagnostic tests. Transpl Infect Dis. 2007;9:203–210. doi: 10.1111/j.1399-3062.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 8.Paya CV, Hermans PE, Wiesner RH, Ludwig J, Smith TF, Rakela J, Krom RA. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989;160:752–758. doi: 10.1093/infdis/160.5.752. [DOI] [PubMed] [Google Scholar]
- 9.Razonable RR, van Cruijsen H, Brown RA, Wilson JA, Harmsen WS, Wiesner RH, Smith TF, Paya CV. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis. 2003;187:1801–1808. doi: 10.1086/375194. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Wagener MM. Strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2006;144:456–457; author reply 457. doi: 10.7326/0003-4819-144-6-200603210-00024. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transpl. 2005;11:700–704. doi: 10.1002/lt.20417. [DOI] [PubMed] [Google Scholar]
- 12.Razonable RR. Epidemiology of cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. Am J Health Syst Pharm. 2005;62:S7–S13. doi: 10.1093/ajhp/62.suppl_1.S7. [DOI] [PubMed] [Google Scholar]
- 13.Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, Jenkins G, Larson T, Hellinger WC, Spivey JR, Paya CV. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Infect Dis. 2001;184:1461–1464. doi: 10.1086/324516. [DOI] [PubMed] [Google Scholar]
- 14.Razonable RR, Paya CV. Infections and allograft rejection - intertwined complications of organ transplantation. Swiss Med Wkly. 2005;135:571–573. doi: 10.4414/smw.2005.10984. [DOI] [PubMed] [Google Scholar]
- 15.O’Grady JG, Alexander GJ, Sutherland S, Donaldson PT, Harvey F, Portmann B, Calne RY, Williams R. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988;2:302–305. doi: 10.1016/s0140-6736(88)92356-2. [DOI] [PubMed] [Google Scholar]
- 16.Noack KB, Wiesner RH, Batts K, van Hoek B, Ludwig J. Severe ductopenic rejection with features of vanishing bile duct syndrome: clinical, biochemical, and histologic evidence for spontaneous resolution. Transplant Proc. 1991;23:1448–1451. [PubMed] [Google Scholar]
- 17.Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RA. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatology. 1987;7:476–483. doi: 10.1002/hep.1840070311. [DOI] [PubMed] [Google Scholar]
- 18.Pastacaldi S, Teixeira R, Montalto P, Rolles K, Burroughs AK. Hepatic artery thrombosis after orthotopic liver transplantation: a review of nonsurgical causes. Liver Transpl. 2001;7:75–81. doi: 10.1053/jlts.2001.22040. [DOI] [PubMed] [Google Scholar]
- 19.Madalosso C, de Souza NF Jr, Ilstrup DM, Wiesner RH, Krom RA. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation. 1998;66:294–297. doi: 10.1097/00007890-199808150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Eid AJ, Razonable RR. Cytomegalovirus disease in solid organ transplant recipients: advances lead to new challenges and opportunities. Current Opinion in Organ Transplantation. 2007;12:610–617. [Google Scholar]
- 21.Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, Kwak EJ, Paterson DL. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 22.Mendez JC, Dockrell DH, Espy MJ, Smith TF, Wilson JA, Harmsen WS, Ilstrup D, Paya CV. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001;183:179–184. doi: 10.1086/317929. [DOI] [PubMed] [Google Scholar]
- 23.Dockrell DH, Prada J, Jones MF, Patel R, Badley AD, Harmsen WS, Ilstrup DM, Wiesner RH, Krom RA, Smith TF, et al. Seroconversion to human herpesvirus 6 following liver transplantation is a marker of cytomegalovirus disease. J Infect Dis. 1997;176:1135–1140. doi: 10.1086/514104. [DOI] [PubMed] [Google Scholar]
- 24.Mendez J, Espy M, Smith TF, Wilson J, Wiesner R, Paya CV. Clinical significance of viral load in the diagnosis of cytomegalovirus disease after liver transplantation. Transplantation. 1998;65:1477–1481. doi: 10.1097/00007890-199806150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Razonable RR, Rivero A, Brown RA, Hart GD, Espy MJ, van Cruijsen H, Wilson J, Groettum C, Kremers W, Smith TF, et al. Detection of simultaneous beta-herpesvirus infections in clinical syndromes due to defined cytomegalovirus infection. Clin Transplant. 2003;17:114–120. doi: 10.1034/j.1399-0012.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- 26.Humar A, Washburn K, Freeman R, Paya CV, Mouas H, Alecock E, Razonable RR. An assessment of interactions between hepatitis C virus and herpesvirus reactivation in liver transplant recipients using molecular surveillance. Liver Transpl. 2007;13:1422–1427. doi: 10.1002/lt.21266. [DOI] [PubMed] [Google Scholar]
- 27.Humar A, Kumar D, Raboud J, Caliendo AM, Moussa G, Levy G, Mazzulli T. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant. 2002;2:461–466. doi: 10.1034/j.1600-6143.2002.20511.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosen HR, Chou S, Corless CL, Gretch DR, Flora KD, Boudousquie A, Orloff SL, Rabkin JM, Benner KG. Cytomegalovirus viremia: risk factor for allograft cirrhosis after liver transplantation for hepatitis C. Transplantation. 1997;64:721–726. doi: 10.1097/00007890-199709150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Razonable RR, Burak KW, van Cruijsen H, Brown RA, Charlton MR, Smith TF, Espy MJ, Kremers W, Wilson JA, Groettum C, et al. The pathogenesis of hepatitis C virus is influenced by cytomegalovirus. Clin Infect Dis. 2002;35:974–981. doi: 10.1086/342911. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Husain S, Carrigan DR, Knox KK, Weck KE, Wagener MM, Gayowski T. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin Transplant. 2002;16:92–96. doi: 10.1034/j.1399-0012.2002.1o096.x. [DOI] [PubMed] [Google Scholar]
- 31.Burak KW, Kremers WK, Batts KP, Wiesner RH, Rosen CB, Razonable RR, Paya CV, Charlton MR. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transpl. 2002;8:362–369. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 32.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 33.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 34.Hodson EM, Barclay PG, Craig JC, Jones C, Kable K, Strippoli GF, Vimalachandra D, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2005:CD003774. doi: 10.1002/14651858.CD003774.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 36.Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869–880. doi: 10.1086/507337. [DOI] [PubMed] [Google Scholar]
- 37.Portela D, Patel R, Larson-Keller JJ, Ilstrup DM, Wiesner RH, Steers JL, Krom RA, Paya CV. OKT3 treatment for allograft rejection is a risk factor for cytomegalovirus disease in liver transplantation. J Infect Dis. 1995;171:1014–1018. doi: 10.1093/infdis/171.4.1014. [DOI] [PubMed] [Google Scholar]
- 38.Winston DJ, Imagawa DK, Holt CD, Kaldas F, Shaked A, Busuttil RW. Long-term ganciclovir prophylaxis eliminates serious cytomegalovirus disease in liver transplant recipients receiving OKT3 therapy for rejection. Transplantation. 1995;60:1357–1360. [PubMed] [Google Scholar]
- 39.Malek SK, Obmann MA, Gotoff RA, Foltzer MA, Hartle JE, Potdar S. Campath-1H induction and the incidence of infectious complications in adult renal transplantation. Transplantation. 2006;81:17–20. doi: 10.1097/01.tp.0000189713.14993.db. [DOI] [PubMed] [Google Scholar]
- 40.Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, Shutt KA, Shapiro R, Thai N, Abu-Elmagd K, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44:204–212. doi: 10.1086/510388. [DOI] [PubMed] [Google Scholar]
- 41.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 42.Sarmiento JM, Dockrell DH, Schwab TR, Munn SR, Paya CV. Mycophenolate mofetil increases cytomegalovirus invasive organ disease in renal transplant patients. Clin Transplant. 2000;14:136–138. doi: 10.1034/j.1399-0012.2000.140206.x. [DOI] [PubMed] [Google Scholar]
- 43.Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40:1407–1410. doi: 10.1016/j.transproceed.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 44.Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, Øyen O, Viljoen HG, Filiptsev P, Sadek S, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5:2521–2530. doi: 10.1111/j.1600-6143.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- 45.Kijpittayarit S, Eid AJ, Brown RA, Paya CV, Razonable RR. Relationship between Toll-like receptor 2 polymorphism and cytomegalovirus disease after liver transplantation. Clin Infect Dis. 2007;44:1315–1320. doi: 10.1086/514339. [DOI] [PubMed] [Google Scholar]
- 46.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633–644. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 47.Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, Venkataraman S, Humar A. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 48.La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, Limaye AP, Diamond DJ. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 49.Humar A, Mazzulli T, Moussa G, Razonable RR, Paya CV, Pescovitz MD, Covington E, Alecock E. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R- transplant recipients. Am J Transplant. 2005;5:1065–1070. doi: 10.1111/j.1600-6143.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 50.Warlé MC, Farhan A, Metselaar HJ, Hop WC, van der Plas AJ, Kap M, de Rave S, Kwekkeboom J, Zondervan PE, IJzermans JN, et al. In vitro cytokine production of TNFalpha and IL-13 correlates with acute liver transplant rejection. Hum Immunol. 2001;62:1258–1265. doi: 10.1016/s0198-8859(01)00321-4. [DOI] [PubMed] [Google Scholar]
- 51.Fietze E, Prösch S, Reinke P, Stein J, Döcke WD, Staffa G, Löning S, Devaux S, Emmrich F, von Baehr R. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation. 1994;58:675–680. [PubMed] [Google Scholar]
- 52.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. 2006;80:9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooks MA, Perlino CA, Henderson JM, Millikan WJ Jr, Kutner MH. Prevalence of invasive cytomegalovirus disease with administration of muromonab CD-3 in patients undergoing orthotopic liver transplantation. Ann Pharmacother. 1992;26:617–620. doi: 10.1177/106002809202600501. [DOI] [PubMed] [Google Scholar]
- 54.Singh N, Gayowski T, Wagener MM, Marino IR. Increased infections in liver transplant recipients with recurrent hepatitis C virus hepatitis. Transplantation. 1996;61:402–406. doi: 10.1097/00007890-199602150-00014. [DOI] [PubMed] [Google Scholar]
- 55.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 56.Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, Emery VC. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- 57.Singh N. Cytomegalovirus infection in solid organ transplant recipients: new challenges and their implications for preventive strategies. J Clin Virol. 2006;35:474–477. doi: 10.1016/j.jcv.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Paya CV, Wilson JA, Espy MJ, Sia IG, DeBernardi MJ, Smith TF, Patel R, Jenkins G, Harmsen WS, Vanness DJ, et al. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J Infect Dis. 2002;185:854–860. doi: 10.1086/339449. [DOI] [PubMed] [Google Scholar]
- 59.Singh N, Wannstedt C, Keyes L, Gayowski T, Wagener MM, Cacciarelli TV. Efficacy of valganciclovir administered as preemptive therapy for cytomegalovirus disease in liver transplant recipients: impact on viral load and late-onset cytomegalovirus disease. Transplantation. 2005;79:85–90. doi: 10.1097/01.tp.0000146844.65273.62. [DOI] [PubMed] [Google Scholar]
- 60.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Cytomegalovirus antigenemia directed pre-emptive prophylaxis with oral versus I.V. ganciclovir for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, controlled trial. Transplantation. 2000;70:717–722. doi: 10.1097/00007890-200009150-00002. [DOI] [PubMed] [Google Scholar]
- 61.Singh N, Yu VL. Preemptive therapy for cytomegalovirus. Liver Transpl. 2006;12:327. doi: 10.1002/lt.20676. [DOI] [PubMed] [Google Scholar]
- 62.Humar A, Snydman D. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S78–S86. doi: 10.1111/j.1600-6143.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 63.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 64.Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant. 2008;8:158–161. doi: 10.1111/j.1600-6143.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- 65.Vandecasteele E, De Waele J, Vandijck D, Blot S, Vogelaers D, Rogiers X, Van Vlierberghe H, Decruyenaere J, Hoste E. Antimicrobial prophylaxis in liver transplant patients--a multicenter survey endorsed by the European Liver and Intestine Transplant Association. Transpl Int. 2010;23:182–190. doi: 10.1111/j.1432-2277.2009.00974.x. [DOI] [PubMed] [Google Scholar]
- 66.Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, Wagener MM, Cacciarelli TV. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl. 2008;14:240–244. doi: 10.1002/lt.21362. [DOI] [PubMed] [Google Scholar]
- 67.Caliendo AM, St George K, Kao SY, Allega J, Tan BH, LaFontaine R, Bui L, Rinaldo CR. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J Clin Microbiol. 2000;38:2122–2127. doi: 10.1128/jcm.38.6.2122-2127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365:2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 69.Singh N, Wannstedt C, Keyes L, Wagener MM, Gayowski T, Cacciarelli TV. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver-transplant recipients with the use of preemptive therapy for 13 years. Transplantation. 2005;79:1428–1434. doi: 10.1097/01.tp.0000157867.98649.f5. [DOI] [PubMed] [Google Scholar]
- 70.Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet. 1995;346:69–74. doi: 10.1016/s0140-6736(95)92110-9. [DOI] [PubMed] [Google Scholar]
- 71.Paya CV, Hermans PE, Smith TF, Rakela J, Wiesner RH, Krom RA, Torres VE, Sterioff S, Wilkowske CJ. Efficacy of ganciclovir in liver and kidney transplant recipients with severe cytomegalovirus infection. Transplantation. 1988;46:229–234. doi: 10.1097/00007890-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Winston DJ, Busuttil RW. Randomized controlled trial of oral ganciclovir versus oral acyclovir after induction with intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in cytomegalovirus-seropositive liver transplant recipients. Transplantation. 2003;75:229–233. doi: 10.1097/01.TP.0000040601.60276.96. [DOI] [PubMed] [Google Scholar]
- 73.Razonable RR, Paya CV. Valganciclovir for the prevention and treatment of cytomegalovirus disease in immunocompromised hosts. Expert Rev Anti Infect Ther. 2004;2:27–41. doi: 10.1586/14787210.2.1.27. [DOI] [PubMed] [Google Scholar]
- 74.Boivin G, Goyette N, Gilbert C, Roberts N, Macey K, Paya C, Pescovitz MD, Humar A, Dominguez E, Washburn K, et al. Absence of cytomegalovirus-resistance mutations after valganciclovir prophylaxis, in a prospective multicenter study of solid-organ transplant recipients. J Infect Dis. 2004;189:1615–1618. doi: 10.1086/382753. [DOI] [PubMed] [Google Scholar]
- 75.Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35:866–872. doi: 10.1086/342385. [DOI] [PubMed] [Google Scholar]
- 76.Pescovitz MD, Rabkin J, Merion RM, Paya CV, Pirsch J, Freeman RB, O’Grady J, Robinson C, To Z, Wren K, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44:2811–2815. doi: 10.1128/aac.44.10.2811-2815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain A, Orloff M, Kashyap R, Lansing K, Betts R, Mohanka R, Menegus M, Ryan C, Bozorgzadeh A. Does valganciclovir hydrochloride (valcyte) provide effective prophylaxis against cytomegalovirus infection in liver transplant recipients? Transplant Proc. 2005;37:3182–3186. doi: 10.1016/j.transproceed.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 78.Freeman RB, Paya C, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Heaton N. Risk factors for cytomegalovirus viremia and disease developing after prophylaxis in high-risk solid-organ transplant recipients. Transplantation. 2004;78:1765–1773. doi: 10.1097/01.tp.0000142619.01510.a5. [DOI] [PubMed] [Google Scholar]
- 79.Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann A. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 80.Humar A, Paya C, Pescovitz MD, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Mueller B. Clinical utility of cytomegalovirus viral load testing for predicting CMV disease in D+/R- solid organ transplant recipients. Am J Transplant. 2004;4:644–649. doi: 10.1111/j.1600-6143.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 81.Sia IG, Wilson JA, Groettum CM, Espy MJ, Smith TF, Paya CV. Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis. 2000;181:717–720. doi: 10.1086/315242. [DOI] [PubMed] [Google Scholar]
- 82.Jang EY, Park SY, Lee EJ, Song EH, Chong YP, Lee SO, Choi SH, Woo JH, Kim YS, Kim SH. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis. 2009;48:e121–e124. doi: 10.1086/599116. [DOI] [PubMed] [Google Scholar]
- 83.Eid AJ, Bakri SJ, Kijpittayarit S, Razonable RR. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transpl Infect Dis. 2008;10:13–18. doi: 10.1111/j.1399-3062.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 84.Eid AJ, Razonable RR. Valganciclovir for the treatment of cytomegalovirus retinitis in patients with AIDS. Expert Review of Ophthalmology. 2007;2:351–361. [Google Scholar]
- 85.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2010;10:157–161. doi: 10.1111/j.1600-6143.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 86.Limaye AP. Antiviral resistance in cytomegalovirus: an emerging problem in organ transplant recipients. Semin Respir Infect. 2002;17:265–273. doi: 10.1053/srin.2002.36447. [DOI] [PubMed] [Google Scholar]
- 87.Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J Infect Dis. 2007;196:91–94. doi: 10.1086/518514. [DOI] [PubMed] [Google Scholar]
- 88.Chou S. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev Med Virol. 2008;18:233–246. doi: 10.1002/rmv.574. [DOI] [PubMed] [Google Scholar]
- 89.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant. 2008;22:162–170. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 90.Shapira MY, Resnick IB, Chou S, Neumann AU, Lurain NS, Stamminger T, Caplan O, Saleh N, Efferth T, Marschall M, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 91.Maribavir: 1263W94, Benzimidavir , GW 1263, GW 1263W94, VP41263 Drugs R D. 2007;8:188–192. doi: 10.2165/00126839-200708030-00006. [DOI] [PubMed] [Google Scholar]
- 92.Winston DJ, Young JA, Pullarkat V, Papanicolaou GA, Vij R, Vance E, Alangaden GJ, Chemaly RF, Petersen F, Chao N, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111:5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]