Abstract
Rhizobial lipopolysaccharide (LPS) is required to establish an effective symbiosis with its host plant. An exo5 mutant of Rhizobium leguminosarum RBL5523, strain RBL5808, is defective in UDP-glucose (Glc) dehydrogenase that converts UDP-Glc to UDP-glucuronic acid (GlcA). This mutant is unable to synthesize either UDP-GlcA or UDP-galacturonic acid (GalA) and is unable to synthesize extracellular and capsular polysaccharides, lacks GalA in its LPS and is defective in symbiosis (Laus MC, Logman TJ, van Brussel AAN, Carlson RW, Azadi P, Gao MY, Kijne JW. 2004. Involvement of exo5 in production of surface polysaccharides in Rhizobium leguminosarum and its role in nodulation of Vicia sativa subsp. nigra. J Bacteriol. 186:6617–6625). Here, we determined and compared the structures of the RBL5523 parent and RBL5808 mutant LPSs. The parent LPS core oligosaccharide (OS), as with other R. leguminosarum and Rhizobium etli strains, is a Gal1Man1GalA3Kdo3 octasaccharide in, which each of the GalA residues is terminally linked. The core OS from the mutant lacks all three GalA residues. Also, the parent lipid A consists of a fatty acylated GlcNGlcNonate or GlcNGlcN disaccharide that has a GalA residue at the 4′-position, typical of other R. leguminosarum and R. etli lipids A. The mutant lipid A lacks the 4′-GalA residue, and the proximal glycosyl residue was only present as GlcNonate. In spite of these alterations to the lipid A and core OSs, the mutant was still able to synthesize an LPS containing a normal O-chain polysaccharide (OPS), but at reduced levels. The structure of the OPS of the mutant LPS was identical to that of the parent and consists of an O-acetylated →4)-α-d-Glcp-(1→3)-α-d-QuipNAc-(1→ repeating unit.
Keywords: lipopolysaccharide, Rhizobium leguminosarum, structure, symbiosis
Introduction
The outer leaflet of the outer membrane of the Gram-negative bacterial cell envelope is built of lipopolysaccharide (LPS; Raetz and Whitfield 2002). The LPS consists of three distinct structural regions: a rather conserved lipid A, a more variable core oligosaccharide (OS) and an even more variable O-chain polysaccharide (OPS). LPSs are well known as virulent determinants in, which all three structural regions play important roles in interacting with the defense mechanism of the host cells. Depending on the structure, lipid A is non-reactive and either activates or inhibits activation of the host's innate immune response (Raetz and Whitfield 2002; Raetz et al. 2007). The core OS structure affects the stability of the bacterial membrane (Frirdich and Whitfield 2005) and is also a determinant of OPS ligation (Heinrichs et al. 1998, 1999; Raetz and Whitfield 2002; Kaniuk et al. 2004). The OPS is required for the virulence of a bacterial pathogen and its presence often enables the bacterium to counteract host defense mechanisms (for reviews, see Raetz and Whitfield 2002; King et al. 2009).
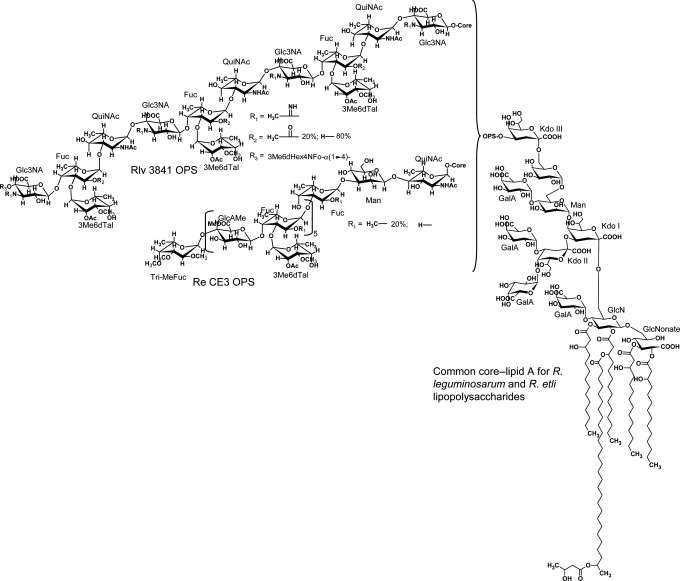
Rhizobium leguminosarum biovar viciae 5523, the topic of this paper, is a symbiotic nitrogen-fixing member of the Rhizobiales. The LPSs from strains of R. leguminosarum and Rhizobium etli, as with bacterial animal pathogens, have three structural regions as described in the previous paragraph, and the OPS and lipid A regions are important in the interaction with their legume hosts (for reviews, see Kannenberg et al. 1998; Price 1999; De Castro et al. 2008; Carlson et al. 2010). Figure 1 shows the structure of the LPSs from R. leguminosarum bv. viciae 3841 and R. etli CE3 (Forsberg et al. 2000; Forsberg and Carlson 2008). The LPSs from R. leguminosarum and R. etli species share a common core–lipid A structure, vary in their OPS structures and have a number of unusual features compared with structures observed for enteric bacterial species (Carlson et al. 2010).
Fig. 1.
Structures of the LPSs reported for R. etli CE3 (Forsberg et al. 2000) and R. leguminosarum biovar viciae 3841 (Forsberg and Carlson 2008). The core–lipid A portion of the LPS is identical in structure in these strains and the structure of each OPS is as shown.
Rhizobial mutants having LPSs that lack or are deficient in the level of the OPS are symbiotically defective (Carlson et al. 1987; Cava et al. 1989; Stacey et al. 1991; Carlson and Krishnaiah 1992; Perotto et al. 1994; Noel et al. 2000; Forsberg et al. 2003). Furthermore, in the case of R. leguminosarum and R. etli, subtle changes occur to the OPS during symbiosis (Kannenberg and Carlson 2001; Noel et al. 2004; D'Haeze et al. 2007). These OPSs are linked to the external Kdo (Kdo III) residue of the core OS. Thus, when the OPSs are prepared from the LPSs by mild acid hydrolysis, they are obtained without being attached to the core OS. Genetic regions required for the biosynthesis of OPSs have been identified in the genomic sequences (Gonzalez et al. 2006; Young et al. 2006) for both R. leguminosarum bv. viciae 3841 (RL0794–RL0826) and R. etli CE3 (RHE_CH00745–RHE_CH00772, also known as the lpsα region; Cava et al. 1989, 1990; Duelli et al. 2001). Interestingly, even though both these OPSs are heteropolysaccharides, the synthesis of R. etli CE3 OPS likely occurs by a wzy-independent mechanism as its genetic region lacks wzy and, instead, contains wzm and wzt, which encode the ABC transporter, the mutation of, which results in a mutant that lacks OPSs (Lerouge et al. 2001). The mechanism by, which R. leguminosarum bv. viciae 3841 OPS is synthesized is not yet known because neither wzm/wzt nor wxy/wzx homologs are present in its OPS genetic region.
It has been determined that structural changes occur to R. leguminosarum bv. viciae 3841 and R. etli CE3 OPSs during symbiosis of their respective hosts (Kannenberg and Carlson 2001; Noel et al. 2004; D'Haeze et al. 2007). The R. leguminosarum bv. viciae 3841 OPS is altered in its O-acetylation and methylation (Kannenberg and Carlson 2001), whereas the R. etli CE3 OPS adds a single methyl group to O2 of a fucosyl residue in one of its repeat units (Noel et al. 2004; D'Haeze et al. 2007). The gene encoding this methyl transferase was originally identified as lpeM and is now known as wreM. It encodes a protein, WreM, which contains both the methylation (WreMα) and glycosyl transferase (WreMβ) activities (Ojeda et al. 2010). The symbiotic phenotype of the wreMα mutant is a delay in nodulation and a reduction in nodule number at early times after inoculation, which is corrected at later times (Noel et al. 2004; Ojeda et al. 2010). Thus, the additional 2-O-methylation of a fucosyl residue facilitates, but is not required for symbiosis. In the case of R. leguminosarum bv. viciae 3841, the LPS becomes hydrophobic during symbiosis as does the entire bacteroid (Kannenberg and Carlson 2001). It also produces a second polysaccharide that is composed of xylose, mannose (Man) and glucose (Glc) (Kannenberg and Carlson 2001; Forsberg and Carlson 2008). Thus, it is apparent that the presence of the OPS and the structural changes that occur are important for symbiosis.
During LPS synthesis in Gram-negative bacteria, the core OS structure is an important determinant for ligation of the OPS. Although changes are reported to occur to fatty acylation pattern of the lipid A region of R. leguminosarum bv. viciae 3841 and R. etli CE3 LPSs during symbiosis (Kannenberg and Carlson 2001; D'Haeze et al. 2007), it has been shown that the core OS region of R. leguminosarum and R. etli LPSs (see Figure 1) is not modified during symbiotic infection (Kannenberg and Carlson 2001; D'Haeze et al. 2007). Unlike the core OS from the enteric LPS, the core region of R. leguminosarum and R. etli lacks heptosyl residues as well as phosphorylated substituents such as phosphoethanolamine. Instead, the core OS from R. leguminosarum and R. etli strains has a common structure consisting of an octasaccharide containing one Man, one galactose (Gal), three GalA and three 3-deoxy-d-manno-2-octulosonic acid (Kdo) residues (Carlson et al. 1995; Forsberg and Carlson 1998; Kannenberg et al. 1998). All of the GalA residues are terminally linked: one to the Man and two to the branching internal Kdo residue (Kdo II in Figure 1). The most recently identified genes that encode enzymes for the synthesis of the core OS are those encoding the transferases responsible for the addition of the GalA residues: rgtA, rgtB and rgtC (Kanjilal-Kolar et al. 2006). Interestingly, the GalA donor for these transferases is dodecaprenyl-P-GalA and not UDP-GalA (Kanjilal-Kolar and Raetz 2006). We have hypothesized that the unusual structural features of the core OS, particularly the terminal GalA residues, are important for establishing a fully effective symbiosis with the host and, possibly, for LPS biosynthesis. Core mutants that lack the OPS form defective Fix− nodules and, therefore, any mutant that is defective in the addition of the Man, Gal or the external Kdo would lack OPS and be Fix−. In fact, a mutant that is defective in the synthesis of UDP-Gal (an exoB mutant) has been shown to lack the OPS (Laus et al. 2004).
It has been shown from the above that the core OS structure is a determinant for ligation of the OPS for various enteric bacterial species. The importance of the terminal GalA residues for OPS ligation to the core–lipid A and symbiosis in R. leguminosarum strains is not known. Here, we present structural studies on LPSs isolated from R. leguminosarum strain RBL5523 and its exo5 mutant RBL5808 that is defective in UDP-Glc dehydrogenase and unable to synthesize UDP-GlcA and, therefore, UDP-GalA. As reported by Laus et al. (2004), this single gene mutation resulted in multiple phenotypic changes: (i) the loss of extracellular polysaccharide (EPS) which contains GlcA as an integral part of the repeating unit, (ii) the absence of GalA in the LPS, (iii) a reduced level of OPSs and (iv) as with other EPS minus mutants, is symbiotically defective (i.e. it is impaired in the colonization of infection threads). The lack of the LPS GalA residues allowed us to determine how their absence affects the biosynthesis of the LPS by characterizing the structures of the OPS, core OS and lipid A from the parent and exo5 mutant.
Results
Initial LPS analysis
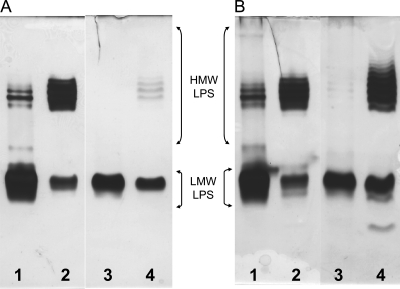
Deoxycholate-polyacrylamide gel electrophoresis (DOC-PAGE) analysis of phenol/water-extracted LPS is shown in Figure 2. For both the mutant and the parent, phenol/water extraction results in a high high-molecular weight/low-molecular weight (HMW/LMW) LPS ratio preparation in the phenol layer and a low ratio in the water layer material. The HMW LPS for both the parent and the mutant shows a compact ladder banding pattern, indicating variation, although limited, in the number of OPS repeat units in these LPSs. The mutant HMW LPS seems to have greater variation in that it has a slightly broader banding pattern than that of the parent LPS. In addition, the mutant LPS has a lower HMW/LMW LPS band intensity ratio in both the water and phenol phase layer material, indicating that it contains reduced amounts of LPSs with OPSs as reported by Laus et al. (2004). However, this effect is not noticeable in the phenol layer LPS when silver staining includes the use of Alcian blue in the fixing solution (compare well 4 of panel A with well 4 of panel B in Figure 2). Alcian blue is a cationic dye that presumably binds to anionic polymers and, therefore, its increased binding to the mutant 5808 LPS, which has reduced anionic character due to the loss of GalA residues was unexpected. The chemical basis for this increased binding requires further investigation. A faint, fast migrating band in 5808 LPS extracted into the phenol layer (Panel B, Lane 4) was also observed, which is likely due to trace amounts of free lipid A. This band was observed in both the parent and mutant LPSs when the samples were separated in the presence of DOC (see Figure 3). The presence of a larger amount of HMW LPSs in the phenol layer than in the water layer for both the parent and the mutant indicates that these LPSs contain rather hydrophobic OPSs. As, for both the parent and mutant strains, this LPS fraction is enriched in HMW LPSs that contains OPSs, it was used for structural analysis of the OPS.
Fig. 2.
DOC-PAGE analysis of R. leguminosarum LPSs extracted with hot phenol/water and purified by ultracentrifugation. Samples (2 µg each) were loaded onto gel and stained as follows. The gel was fixed in the absence (A) or presence (B) of Alcian blue, followed by silver staining. Lanes: R. leguminosarum biovar viciae 5523 LPS obtained from (1) the water layer and (2) the phenol layer; mutant 5808 LPS obtained from (3) the water layer and (4) the phenol layer.
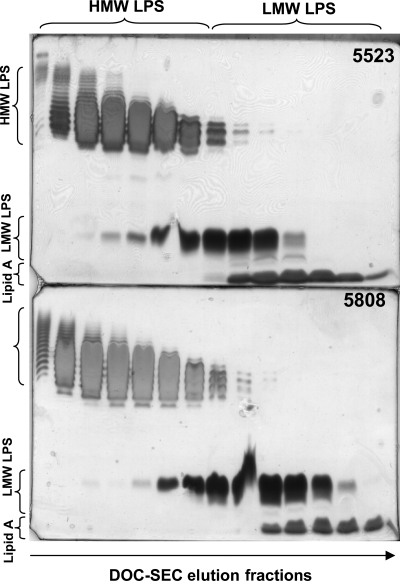
Fig. 3.
DOC-PAGE analysis of the R. leguminosarum biovar viciae 5523 (top) and the mutant 5808 LPS (bottom) fractions eluting during S100 gel filtration in the presence of DOC buffer. HMW LPS, high-molecular weight LPS that contains the OPS; LMW LPS, low-molecular weight LPS that lacks or may contain truncated OPS.
Composition analysis of the LPS revealed that, in contrast to the parent RBL5523, the mutant RBL5808 LPS does not contain GalA, which corroborated results previously reported by Laus et al. (2004). This result is inconsistent with the fact that the mutant is defective in the synthesis of UDP-GlcA, which is the necessary precursor for UDP-GalA (Laus et al. 2004). As GalA is a major component in the core and lipid A LPSs and in the structural regions of R. leguminosarum and R. etli LPSs (see Figure 1), this suggested that the mutant 5808 LPS should be altered in both its core and lipid A structures. Both the parent and mutant LPS preparations contain d-Man, Kdo, d-Glc and N-acetylquinovosamine d-QuiNAc, with the latter two glycoses being the major glycosyl residues. The LPSs from R. etli CE3 and R. leguminosarum bv. viciae 3841 also contain QuiNAc in their OPSs (Forsberg et al. 2000; Forsberg and Carlson 2008); however its stereochemical configuration was assigned as “l” based on nuclear Overhauser effect (NOE) observations from other glycosyl reference points (Forsberg and Carlson 2008). The assignment of the absolute “d” configuration of QuiNAc in the LPSs from R. leguminosarum bv. viciae 5523 and its exo5 5808 mutant is based on comparison of the GC retention time of its trimethylsilyl (TMS) 2-(−)-butyl glycoside with those of l-QuiNAc found in R. etli CE3 or R. leguminosarum bv. viciae 3841 LPSs (Forsberg et al. 2000; Forsberg and Carlson 2008). Characteristic lipid A components were also present, that is, d-GlcN, 2-aminogluconate (GlcNonate), β-hydroxymyristic (β-OHC14:0), β-hydroxypentadecanoic (β-OHC15:0), β-hydroxypalmitic (β-OHC16:0), β-hydroxystearic (β-OHC18:0) and the very-long-chain fatty acid (VLCFA) 27-hydroxyoctacosanoic acid (27-OHC28:0). Small amounts of C18:1 and C19:1 were detected in both LPS samples due to slight contamination by phospholipids.
The HMW and LMW LPSs for the parent and mutant preparations were separated using size exclusion chromatography (SEC) in the presence of DOC buffer. The result of this separation was confirmed by DOC-PAGE analysis and is shown in Figure 3. The indicated HMW and LMW fractions for the parent and mutant LPS preparations were collected and subjected to further analysis as described below.
The R. leguminosarum 5808 mutant produces an altered core OS
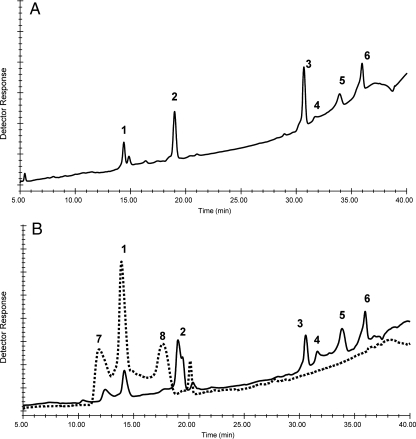
Both the parent and mutant LMW LPSs were subjected to mild acid hydrolysis, and the carbohydrate components were compared by high-performance anion exchange chromatography (HPAEC; Figure 4). The profile for R. etli CE3 is shown in Figure 4A and is typical for the core OSs from R. etli and R. leguminosarum LPSs that were characterized in previous reports (Carlson et al. 1995; Forsberg and Carlson 1998; Kannenberg et al. 1998; Kannenberg and Carlson 2001). By comparing with R. etli CE3 core components, the core OSs for Rlv RBL5523 are monomeric Kdo (peak 1), monomeric GalA (peak 2), tetrasaccharide GalA1Gal1Man1Kdo (peak 3), anhydro versions of the tetrasaccharide (peaks 4 and 5) and the trisaccharide GalA2Kdo (peak 6). These results support the conclusion that the parental RBL5523 LPS contains the same core structure as that shown in Figure 1 for the LPSs from R. leguminosarum biovar viciae 3841 and R. etli CE3. The mutant RBL5808 LPS (Figure 4B) shows only three major early eluting peaks, one of which, is monomeric Kdo, and the remaining two peaks having retention times that are different from any of the parent or other typical core OSs found in R. etli or R. leguminosarum. However, comparison with the LPS from R. etli mutant CE109 shows that mutant RBL5808 LPS contains monomeric Kdo and an OS with a retention time identical to the Gal-Man-Kdo trisaccharide reported in the CE109 core (Carlson et al. 1989). Composition analysis of the mutant LMW LPS showed only the presence of Gal, Man and Kdo as well as the lipid A components, results that are consistent with these HPAEC results. Considering the structure of the LPS core region shown in Figure 1, the lack of GalA in the core region would, on mild acid hydrolysis of the LPS, produce increased amounts of monomeric Kdo (i.e. both Kdo II and Kdo III would be released as monomeric Kdo from the mutant rather than just Kdo III) and a Gal-Man-Kdo trisaccharide in, which Kdo could also exist as an anhydro residue. Due to the lack of GalA, a Gal-Man-Kdo trisaccharide with normal and anhydro Kdo would be produced together with the monomeric Kdo, which is consistent with what we observe.
Fig. 4.
Comparative DIONEX HPAEC analysis of the OSs released by mild acid hydrolysis of the LMW LPS from R. etli CE3 (A), R. leguminosarum biovar viciae 5523 (B, solid line) and the mutant 5808 (B, dashed line). The peaks are: 1, Kdo; 2, GalA; 3, tetrasaccharide; 4 and 5, anhydroKdo versions of the Gal(GalA)ManKdo tetrasaccharide; 6, GalA(GalA)Kdo trisaccharide; 7, GalManKdo trisaccharide as reported by Carlson et al. (1989); 8, possibly an anhydroKdo version of 7.
The 5808 mutant produces a lipid A that lacks GalA and exclusively has GlcNonate as the proximal residue
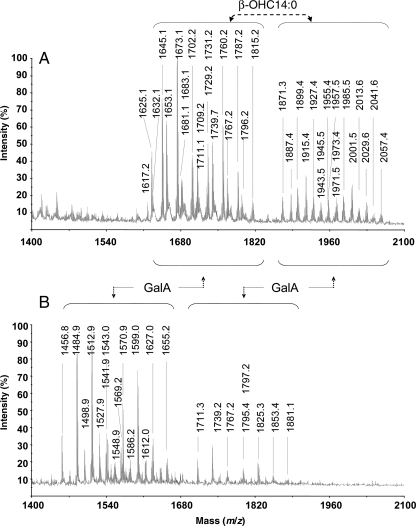
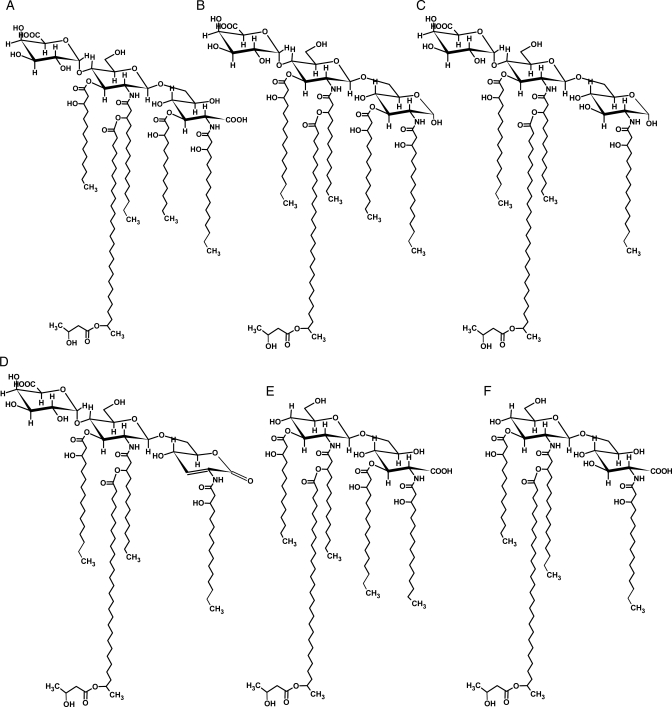
The comparative study of the lipid A compositions from the parent RBL5523 and the mutant RBL5808 was performed using fatty acid methyl esters (FAME) and matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) analyses. Similar fatty acid compositions were observed for both lipid A preparations (see Table I), where the most abundant fatty acids were β-OHC14:0, β-OHC18:0, β-OHC16:0, β-OHC15:0 and the VLCFA 27-OHC28:0. Trace amounts of C16:0 and C18:0 were also observed. Based on these results and those from previous studies on rhizobial lipid A (Bhat et al. 1991, 1994), we expected to observe size heterogeneity in the lipid A during MALDI-TOF-MS analysis. Indeed for both lipid A preparations, two major cluster of ions reflecting the presence of penta- and tetraacylated (lacking a β-OHC14:0 acyl group) lipid A species were observed (see Panels A and B in Figure 5, and Table II). Structures of the various ions observed in the 5523 parent lipid A have been previously reported for R. etli and R. leguminosarum strains (Que et al. 2000; Vedam et al. 2003). Based on these reports and on our composition and MS data (Figure 5 and Table III), structures consistent with each of the ions observed for the parent 5523 and the mutant 5808 lipid A preparations are proposed and shown in Figure 6.
Table I.
Fatty acid composition of the R. leguminosarum biovar viciae 5523 and the mutant 5808 lipid A preparations
| FAME-TMS | Distribution of fatty acids in lipid A (%) |
|
|---|---|---|
| 5523 | 5808 | |
| C16:0 | 1.1 | 1.9 |
| C18:0 | 1.7 | 0.3 |
| β-ΟHC14:0 | 42 | 37 |
| β-ΟHC15:0 | 4.6 | 3.5 |
| β-ΟHC16:0 | 18 | 21 |
| β-ΟHC18:0 | 32 | 36 |
| 27-OHC28:0 (VLCFA) | ++ | ++ |
β-ΟHC14:0, β-hydroxymyristic acid; β-ΟHC16:0, β-hydroxypalmitic acid; β-ΟHC18:0, β-hydroxystearic acid; β-ΟHC15:0, β-hydroxypentadecanoic acid. VLCFA 27-OHC28:0 (27-hydroxyoctacosanoic acid) was present in substantial amounts (i.e., the GC peak area of its fatty acid methyl ester was as large as that of β-OHC14:0) but not quantified. However, MALDI-TOF-MS analysis (see Figures 6 and 7 and Table II) showed that all lipid A structures present contain one 27-OHC28:0 moiety. Trace amounts of C16:0 and C18:0 were also detected, possibly due to slight contamination by phospholipids.
Fig. 5.
MALDI-TOF-MS analysis of lipid A isolated from the R. leguminosarum biovar viciae 5523 LPS (A) and the 5808 mutant (B). Each lipid A shows two clusters of ions. The smaller molecular weight cluster differs from the larger one due to the lack of a β-ΟHC14:0 fatty acyl residue. The ion masses for the mutant 5808 lipid A are less than those of the parent 5523 lipid A due to the lack of a GalA residue. Proposed compositions for the ions observed are given in Table II.
Table II.
The major ions observed in MALDI-TOF-MS spectrometry of lipid A of the R. leguminosarum biovar viciae 5523 parent and 5808 mutant strains with proposed compositions
| Strain | Observed [M-H]− | Calc. [M-H]− | Proposed composition |
|---|---|---|---|
| RBL5523 | |||
| Structure A | 2057.4 | 2057.9 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC18:02,27-OHC28:0,β-OHC4:0 |
| 1971.5 | 1971.8 | GalA,GlcNGlcNonate,β-OHC14:02,b-OHC18:02,27-OHC28:0 | |
| 2029.6 | 2029.8 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC18:0,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1943.5 | 1943.8 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC18:0,β-OHC16:0,27-OHC28:0 | |
| 2001.5 | 2001.8 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC16:02,27-OHC28:0,β-OHC4:0 | |
| 1915.4 | 1915.7 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC16:02,27-OHC28:0 | |
| 1973.4 | 1973.7 | GalA,GlcNGlcNonate,β-OHC14:03,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1887.4 | 1887.7 | GalA,GlcNGlcNonate,β-OHC14:03,β-OHC16:0,27-OHC28:0 | |
| 1945.5 | 1945.7 | GalA,GlcNGlcNonate,β-OHC14:02,β-OHC18:0,β-OHC16:0,27-OHC28:0 | |
| Structure B | 2041.6 | 2041.9 | GalA,GlcNGlcN,β-OHC14:02,β-OHC18:02,27-OHC28:0,β-OHC4:0 |
| 1955.5 | 1955.8 | GalA,GlcNGlcN,β-OHC14:02,β-OHC18:02,27-OHC28:0 | |
| 2013.6 | 2013.8 | GalA,GlcNGlcN,β-OHC14:02,β-OHC18:0,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1927.4 | 1927.8 | GalA,GlcNGlcN,β-OHC14:02,β-ΟHC18:0,β-OHC16:0,27-OHC28:0 | |
| 1985.5 | 1985.8 | GalA,GlcNGlcN,β-OHC14:02,β-OHC16:02,27-OHC28:0,β-OHC4:0 | |
| 1899.4 | 1899.7 | GalA,GlcNGlcN,β-OHC14:02,β-OHC16:02,27-OHC28:0 | |
| 1957.5 | 1957.8 | GalA,GlcNGlcN,β-OHC14:03,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1871.3 | 1871.7 | GalA,GlcNGlcN,β-OHC14:03,β-OHC16:0,27-OHC28:0 | |
| Structure C | 1815.3 | 1815.5 | GalA,GlcNGlcN,β-OHC14:0,β-OHC18:02,27-OHC28:0,β-OHC4:0 |
| 1729.2 | 1729.5 | GalA,GlcNGlcN,β-OHC14:0,β-OHC18:02,27-OHC28:0 | |
| 1787.3 | 1787.5 | GalA,GlcNGlcN,β-OHC14:0,β-OHC18:0,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1701.2 | 1701.4 | GalA,GlcNGlcN,β-OHC14:0,β-OHC18:0,β-OHC16:0,27-OHC28:0 | |
| 1759.2 | 1759.4 | GalA,GlcNGlcN,β-OHC14:0,β-OHC16:02,27-OHC28:0,β-OHC4:0 | |
| 1673.1 | 1673.4 | GalA,GlcNGlcN,β-OHC14:0,β-OHC16:02,27-OHC28:0 | |
| 1731.1 | 1731.4 | GalA,GlcNGlcN,β-OHC14:02,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1645.1 | 1645.3 | GalA,GlcNGlcN,β-OHC14:02,β-OHC16:0,27-OHC28:0 | |
| 1703.2 | 1703.3 | GalA,GlcNGlcN,β-OHC14:03,27-OHC28:0,β-OHC4:0 | |
| 1617.2 | 1617.2 | GalA,GlcNGlcN,b-OHC14:03,27-OHC28:0 | |
| Structure D | 1795.2 | 1795.5 | GalA,GlcNGlcNonolactone,b-OHC14:0,b-OHC18:02,27-OHC28:0,b-OHC4:0 |
| 1709.2 | 1709.4 | GalA,GlcNGlcNonolactone,b-OHC14:0,b-OHC18:02,27-OHC28:0 | |
| 1767.2 | 1767.5 | GalA,GlcNGlcNonolactone,b-OHC14:0,b-OHC18:0,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1681.1 | 1681.4 | GalA,GlcNGlcNonolactone,b-OHC14:0,b-OHC18:0,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1739.7 | 1739.4 | GalA,GlcNGlcNonplactone,b-OHC14:0,b-OHC16:02,27-OHC28:0 | |
| 1654.1 | 1653.3 | GalA,GlcNGlcNonolactone,b-OHC14:0,b-OHC16:02,27-OHC28:0 | |
| 1711.1 | 1711.4 | GalA,GlcNGlcNonolactone,b-OHC14:02,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1625.1 | 1625.3 | GalA,GlcNGlcNonolactone,b-OHC14:02,b-OHC16:0,27-OHC28:0 | |
| 1683.0 | 1683.3 | GalA,GlcNGlcNonolactone,b-OHC14:03,27-OHC28:0,b-OHC4:0 | |
| RBL5808 | |||
| Structure E | 1881.1 | 1881.8 | GlcNGlcNonate,β-OHC14:02,β-OHC18:02,27-OHC28:0,β-OHC4:0 |
| 1795.4 | 1795.4 | GlcNGlcNonate,β-OHC14:02,β-OHC18:02,27-OHC28:0 | |
| 1853.1 | 1853.7 | GlcNGlcNonate,b-OHC14:02,b-OHC18:0,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1767.2 | 1767.6 | GlcNGlcNonate,b-OHC14:02,b-OHC18:0,b-OHC16:0,27-OHC28:0 | |
| 1825.3 | 1825.7 | GlcNGlcNonate,b-OHC14:02,b-OHC16:02,27-OHC28:0,b-OHC4:0 | |
| 1739.2 | 1739.6 | GlcNGlcNonate,b-OHC14:02,b-OHC16:02,27-OHC28:0 | |
| 1797.2 | 1797.6 | GlcNGlcNonate,b-OHC14:03,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1711.1 | 1711.5 | GlcNGlcNonate,b-OHC14:03,b-OHC16:0,27-OHC28:0 | |
| Structure F | 1655.2 | 1655.4 | GlcNGlcNonate,β-OHC14:0,β-OHC18:02,27-OHC28:0,β-OHC4:0 |
| 1569.2 | 1570.3 | GlcNGlcNonate,β-OHC14:0,β-OHC18:02,27-OHC28:0 | |
| 1627.0 | 1627.4 | GlcNGlcNonate,β-OHC14:0,β-OHC18:0,β-OHC16:0,27-OHC28:0,β-OHC4:0 | |
| 1541.9 | 1541.3 | GlcNGlcNonate,b-OHC14:0,b-OHC18:0,b-OHC16:0,27-OHC28:0 | |
| 1599.0 | 1599.3 | GlcNGlcNonate,b-OHC14:0,b-OHC16:02,27-OHC28:0,b-OHC4:0 | |
| 1512.9 | 1513.2 | GlcNGlcNonate,b-OHC14:0,b-OHC16:02,27-OHC28:0 | |
| 1570.9 | 1571.3 | GlcNGlcNonate,b-OHC14:02,b-OHC16:0,27-OHC28:0,b-OHC4:0 | |
| 1484.9 | 1485.2 | GlcNGlcNonate,b-OHC14:02,b-OHC16:0,27-OHC28:0 | |
| 1543.0 | 1543.2 | GlcNGlcNonate,b-OHC14:03,27-OHC28:0,b-OHC4:0 | |
| 1456.8 | 1457.1 | GlcNGlcNonate,b-OHC14:03,27-OHC28:0 | |
Table III.
The proton and carbon chemical shift assignments for the de-O-acetylated OPS oligosaccharide in the R. leguminosarum RBL5523 parent and 5808 mutant strains
| Strain | Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a,b/C6 |
|---|---|---|---|---|---|---|---|
| RBL5523 | α-Glc (A) | 5.02/99.56 | 3.47/71.67 | 3.74/71.67 | 3.55/76.32 | 3.74/71.67 | 3.66,3.74/59.06 |
| α-QuiNAc (B) | 4.86/96.90 | 4.13/53.75 | 3.74/78.31 | 3.30/73.67 | 4.19/67.69 | 1.26 (CH3)/15.09 | |
| RBL5808 | α-Glc (A) | 5.01/99.56 | 3.47/71.67 | 3.74/71.67 | 3.55/76.32 | 3.74/71.67 | 3.66,3.74/59.39 |
| α-QuiNAc (B) | 4.86/97.57 | 4.13/53.75 | 3.73/78.31 | 3.30/73.99 | 4.18/68.36 | 1.26 (CH3)/16.57 |
The observed results indicate similar structures and are in agreement with results from methylation analysis and MALDI-TOF-MS analysis.
Residues are given in bold.
Fig. 6.
The proposed structures of the major ions observed in MALDI-TOF-MS analysis of the lipid A from the R. leguminosarum biovar viciae 5523 (structures A–D) and its exo5 mutant, strain 5808 (structures E and F). Proposed compositions of all the ionic species are given in Table II.
When the lipid A of the parent is compared with that of the mutant, the major penta- and tetraacylated ion clusters in the mutant lipid A were reduced by a mass of 176.5 amu, which is in consistent with the lack of a GalA residue in the mutant lipid A. The parent lipid A preparation shows ions consistent with pentacylated and tetraacylated lipid A in, which the proximal glycosyl residue can be either GlcNonate, GlcNonolactone (see Figure 6, structures A and D) or GlcN (Figure 6, structures B and C). Structure D is likely a result of lactone formation and acid-catalyzed β-elimination of the β-OHC14:0 acyl group during preparation of the lipid A by mild acid hydrolysis. The ions observed for the lipid A of mutant strain 5808 (Figure 5B) were consistent with the proximal residue being present only as GlcNonate for both penta- and tetraacylated lipid A species (see structures E and F in Figure 6, and Table II). Ions consistent with structures in, which the proximal residue was GlcN or GlcNonolactone were not observed in the mutant lipid A preparation. Both lipid A preparations consist of various structures due to the presence and absence of β-hydroxybutyryl (β-OHC4:0, 86 amu) substitution of the 27-OHC28:0 and also due to variation in fatty acid chain length (28 amu).
The OPS from both the parent and mutant LPSs is a polymer of a Glc–QuiNAc repeating disaccharide
It has been previously suggested that the OPS in the R. leguminosarum RBL5523 and its exo5 mutant, 5808, LPSs consists of Glc and QuiNAc because these two sugars were present in substantial amounts (Laus et al. 2004). Our results are in agreement with this finding. To confirm that these two most abundant sugars are the OPS components, we liberated the polysaccharide from the lipid A using mild hydrolysis. Due to the unique structures of the R. leguminosarum and R. etli LPSs in which the OPS is attached directly to an external core Kdo, residue, we were able to separate the OPS from other core fragments by gel-filtration chromatography as described for other rhizobial LPSs (for a review, see Kannenberg et al. 1998). Composition analysis revealed that both parent RBL5523 and the RBL5808 mutant strains have compositions with an approximate 1:1 ratio of d-Glc and d-QuiNAc and trace levels of Kdo, which is likely the external core Kdo III residue. Methylation analysis of each OPS resulted in a 1:1 molar ratio of 4-linked Glc:3-linked QuiNAc. There were also detectable trace amounts (1–2%) of terminally linked Glc and QuiNAc.
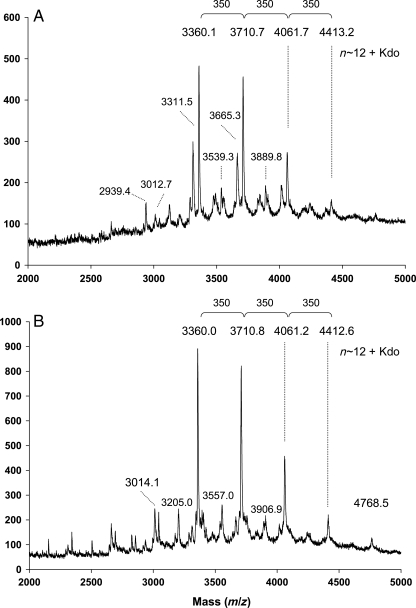
MALDI-TOF-MS analysis of the OPS showed a number of ion clusters that differed from one another by approximately 350 amu, which is in consistent with that of the Glc–QuiNAc dimeric repeat units (a calculated mass of 349.3 amu). These clusters are observed within a range from 3000 to 5000 amu and the ions within each cluster vary from one another by 42 amu signals, indicating O-acetyl groups in the O-chain. The multiple 42 amu increments were reduced after alkaline de-O-acetylation (Figure 7). Analysis of de-O-acetylated OPSs clearly showed similar distribution of major ions in the parent and the mutant OPSs with increments of 350 amu consistent with Glc–QuiNAc disaccharide. The most intense ion for both parent and the mutant OPSs was m/z 3360 with ions of lesser intensity at m/z 3711, 4061 and 4413. Considering the presence of one Kdo residue at the reducing end of each OPS molecule, the major portion of these OPSs has between 9 and 12 Glc–QuiNAc disaccharide repeat units. There were also less prominent signals in the MALDI-TOF-MS spectrum of the parent OPS that were approximately 45 amu lower than those of the major signals. These ions likely arise from decarboxylation of Kdo often observed in MALDI-TOF-MS analysis. The reason for the lack of these ions in the spectrum of the mutant OPS is not known; however, further structural analysis by nuclear magnetic resonance (NMR) spectroscopy (described in the NMR spectroscopy section) did not reveal any detectable structural differences between the parent and the mutant OPSs.
Fig. 7.
MALDI-TOF-MS analysis of de-O-acetylated OPSs isolated from the R. leguminosarum biovar viciae 5523 (A) and the 5808 mutant (B). Spectra were acquired in a negative mode.
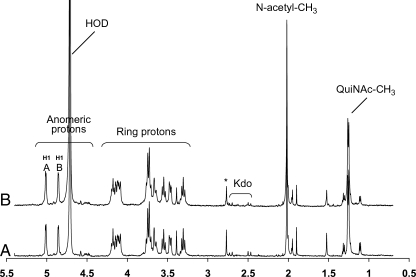
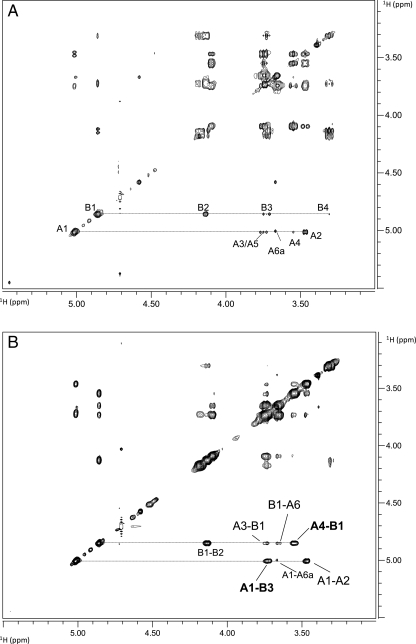
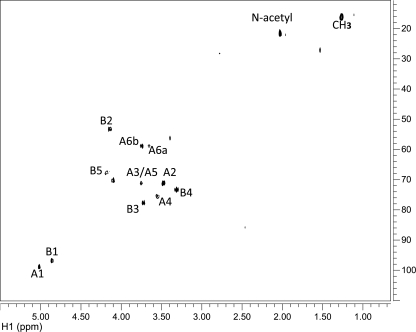
To reveal the complete structure of the OPS, one- and two-dimensional NMR spectroscopic analyses were performed on the parent and the mutant OPSs. Analysis of the parent OPS gave results that were identical to those of the mutant OPS. Due to the heterogeneity of OPS O-acetylation, the glycosyl sequence was determined using NMR analysis of the de-O-acetylated samples. The proton and carbon chemical shift values for each glycosyl residue were determined utilizing 1D proton (1H NMR) (Figure 8 compares the parent and mutant OPS spectra), 2D 1H–1H correlation spectroscopy (COSY; not shown) and total correlation spectroscopy (TOCSY; Figure 9A), and 1H–13C heteronuclear single quantum coherence (HSQC; Figure 10) experiments. These assignments are given in Table III. Two glycosyl ring systems were present with anomeric proton resonances at δ 5.02 ppm (J1,2 = 3.9 Hz) for residue A and at δ 4.86 ppm (J1,2 = 3.3 Hz) for residue B. The chemical shift values and small J1,2 coupling constants for these protons show that both residues A and B are α-anomers. The H1 A proton (δ 5.02 ppm) showed strong correlations in COSY and TOCSY with H2 (δ 3.47 ppm), H3 (δ 3.74 ppm), H4 (δ 3.55 ppm), H5 (δ 3.74 ppm) and H6 and H6′ (δ 3.66 and 3.74 ppm), and the coupling constants between these protons were large and, therefore, consistent with residue A having a gluco configuration. In the case of residue B, H1 (δ 4.86 ppm) is coupled to H2 (δ 4.13 ppm), H3 (δ 3.74 ppm), H4 (δ 3.30 ppm), H5 (δ 3.19 ppm) and H6 (δ 1.26 ppm). The chemical shift of H6 at δ 1.26 ppm shows that residue B is a 6-deoxyhexose. The chemical shift of C2 of residue B at δ 53.8 ppm shows that C2 has an attached nitrogen and, therefore, residue B is 6-deoxy-2-N-acetylaminohexose, that is, QuiNAc.
Fig. 8.
The proton spectra for the OPS from (A) the R. leguminosarum biovar viciae RBL5523 and (B) its RBL5808 mutant.
Fig. 9.
The TOCSY (A) and NOESY (B) spectra for the OPS from the R. leguminosarum biovar viciae 5523. The assignments are as indicated. The OPS from the 5808 mutant gave an identical spectrum. The proton chemical shift assignments are shown in Table III.
Fig. 10.
The HSQC spectrum for the OPS from the R. leguminosarum biovar viciae 5523. The OPS from the 5808 mutant gave an identical spectrum. The carbon chemical shift assignments are as indicated and are listed in Table III.
The combination of glycosyl composition, linkage and the above NMR results supports the conclusion that these OPSs have a →4)-α-d-Glcp-(1→3)-α-d-QuiNAcp-(1→ repeat unit. This conclusion was supported by a nuclear Overhauser effect spectroscopy (NOESY) experiment (Figure 9B). As shown in Figure 9, inter-residue interactions were observed between A H1 (δ 5.02 ppm) and B H3 (δ 3.74 ppm), and between B H1 (δ 4.86 ppm) and A H4 (δ 3.55 ppm). The downfield chemical shifts of A C4 (δ 76.3 ppm) and B C3 (δ 78.3 ppm) also support substitution at these positions.
Discussion
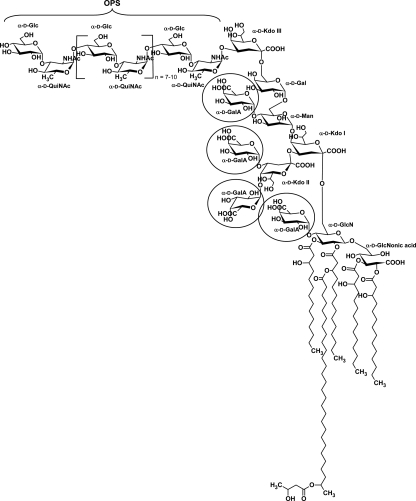
Here we show that the R. leguminosarum bv. viciae strain RBL5523 has an OPS largely consisting of nine to twelve →4)-α-d-Glcp-(1→3)-α-d-QuiNAcp-(1→ disaccharide repeat units that is likely linked to the external Kdo residue of the core region. In addition, this OPS is quite heavily O-acetylated, which is likely the reason that the HMW LPS from this strain is preferentially found in the phenol layer after hot phenol/water extraction. The core–lipid A structure of R. leguminosarum bv. viciae strain RBL5523 LPS is the same as observed for R. etli CE3 and R. leguminosarum biovar viciae 3841. Our results also show that the UDP-Glc dehydrogenase mutant of strain RBL5523, that is, strain RBL5808, has an LPS structure that differs from that of strain 5523 in that (i) it lacks all of the GalA residues in the core that consists of a Kdo-Gal-Man-(Kdo)Kdo- pentasaccharide, (ii) the lipid A lacks the GalA at the 4′-position of the distal GlcN residue and the proximal glycosyl residue consists exclusively of GlcNonate rather than having a portion of the lipid A in, which this residue is GlcN and (iii) the OPS from the RBL5808 mutant is reduced in amount but has the same structure as that of the parent RBL5523. The complete structure of the LPS from RBL5523 and mutant strain 5808 is shown in Figure 11.
Fig. 11.
The structures of the LPS from the R. leguminosarum bv. viciae RBL5523 and its mutant 5808. The OPS for both RBL5523 and 5808 have the same structure as shown. The glycosyl residue that attached the OPS to Kdo III of the core region has not been determined but, based on the R. etli CE3 structure shown in Figure 1, is hypothesized to be the QuiNAc residue. The OPS is also highly O-acetylated (the location of the O-acetyl groups has not been determined). The LPS from the mutant 5808 differs from that of 5523 in that it is devoid of all GalA residues, that is, the circled residues in the structures shown.
Laus et al. (2004) suggested that the amount of OPSs in the exo5 mutant strain was reduced due to the observation of a relative decrease in the HMW/LMW LPS ratio observed by silver staining the gel after PAGE. Using the same procedure, we obtained similar results for the mutant LPS. However, the effect of this mutation on the HMW/LMW LPS ratio may not be quite as large as initially indicated (Laus et al. 2004), because when the PAGE gel was fixed in the presence of Alcian blue prior to silver staining, we observed an HMW/LMW LPS ratio in the phenol layer material that appeared to be more similar to that of the parent RBL5523 LPS (Figure 2). Alcian blue is a cationic dye that normally binds to acidic polysaccharides; however, in the case of the mutant RBL5808, the overall charge of the LPS has been reduced due to the removal of GalA from the core region, and the OPS structure is unchanged from that of the parent and does not contained any charged glycosyl residues or substituents. The chemical basis for the apparent increase in binding of Alcian blue dye to the mutant HMW LPS requires further investigation. It may be that Alcian blue binding is increased by removing GalA residue and exposing Kdo residues and/or to the increase in GlcNonate content of the lipid A.
The HMW/LMW LPS ratio reflects the level of LPS that contains OPS because HMW LPS is the form of LPS, which has OPS ligated to the core OS. In this study, we were interested in determining whether or not the terminal GalA residues in the core region and on the lipid A of R. leguminosarum bv. viciae LPS were important for the ligation of the OPS to the core OS and also how their absence affected the structure of the LPS. This is important because these terminal GalA residues are unique to R. leguminosarum and R. etli LPSs and, therefore, may imply a common functional requirement for LPS biosynthesis and/or symbiosis with the host legume. In the case of the bean symbiont, R. etli CE3, previous work reported that phenol/water extraction of a mutant, CE358, produced an LPS that lacked the terminal GalA attached to the core Man residue and also did not contain the OPS (Forsberg and Carlson 1998). However, LPSs with some OPSs were observed in the CE358 LPS extracted with phenol/EDTA/triethylamine (Ridley et al. 2000). Thus, for mutant CE358, the missing GalA residue apparently affected OPS ligation and the extraction properties of the LPS. However, the mutation in CE358 is in the OPS lpsα gene region and not in any of the identified R. etli CE3 core GalA transferase gene orthologs (RHE_CH01318, RHE_CH01317 or RHE_CH01320). Thus, it is difficult to ascertain the relationship of the CE358 mutation with the effect on either LPS biosynthesis or symbiosis. In the case of the R. leguminosarum bv. vicae 5808 mutant, which lacks all of the GalA residues, we observed that the LPS has a lower HMW/LMW LPS ratio than the parent LPS, but the mutant clearly produces a significant level of the LPS with the OPS that is unaltered in its structure from that of the parent. Therefore, synthesis and ligation of OPSs to the core OS of R. leguminosarum 5523 LPS do not absolutely require the core and lipid A terminal GalA residues, but the level of OPS ligation to the core may be somewhat reduced by the lack of these residues.
The structure of the OPSs and the phenotypic characteristics of the mutant allow some speculation regarding functions of the various LPS structural features. The OPS structure, in which Glc is substituted at O4, explains the finding of Laus et al. (2006) that the LPS of strain RBL5523 is not a ligand for pea lectin, a host plant protein involved in rhizobial attachment. For binding of pea lectin, the C4- and C6-hydroxyls of the ligand's glucosyl residues must be unsubstituted.
The mutant core region completely lacks GalA residues going from a Kdo-Gal-(GalA)Man-[(GalA)2Kdo]Kdo octasaccharide to a Kdo-Gal-Man-(Kdo)-Kdo pentasaccharide, that is, from a core containing six to a core containing three anionic glycosyl residues. Thus, the anionic character of the core is significantly reduced. Previous work (Laus et al. 2004) showed that the mutant is more sensitive to detergents, indicating that these missing GalA residues have a role in promoting membrane stability, which could be important during the endocytotic infection and/or synchronous symbiosome membrane/bacterial division process. The mutant also contains a lipid A in which, in addition to lacking the 4′-GalA residue, the proximal glycosyl residue consists exclusively of GlcNonate rather than as a mixture of GlcN and GlcNonate. The conversion of GlcN to GlcNonate is carried out by the outer membrane monooxygenase, LpxQ. It is possible that the lack of GalA on the lipid A makes this structure a better substrate for LpxQ, and/or it may be that the mutant compensates for the loss of LPS anionic character by increased LpxQ activity, thereby increasing the anionic character of the lipid A and possibly improving membrane stability.
The symbiotic phenotype of the R. leguminosarum bv. viciae 5808 mutant was reported by Laus et al. (2004). The mutant is symbiotically defective in the colonization of the infection thread, and, when compared with the parent, the development of host root nodules is delayed. R. leguminosarum mutants that lack the OPS portion of their LPSs are also symbiotically defective; they partially colonize the root nodule, a minor portion of the host cells contain mutant bacteria, bacteroid cell division is disrupted and there is evidence for the induction of a host defense response (Perotto et al. 1994). As the mutation in the R. leguminosarum 5808 mutant is pleotropic in that it affects both EPS and LPS, it was not possible to determine what portion of the symbiotic defect, if any, may be due to the loss of the GalA residues from the lipid A and core region of the LPS. However, the recent identification of the genes that encode the core and lipid A galacturonosyl transferases (Kanjilal-Kolar et al. 2006; Kanjilal-Kolar and Raetz 2006) now allows the specific targeting of the individual GalA residues without affecting the synthesis of other surface polysaccharides. We are currently creating specific mutants of R. leguminosarum bv. viciae 3841 in each of these genes and determining the effect on symbiosis and LPS synthesis.
Materials and methods
Bacterial strains
The R. leguminosarum bv. viciae (Rlv) strains used in this work were RBL5523, which is RBL5515(pRL1JI) Spc3::Tn1831, StrRif (van Workum et al. 2007), and its exopolysaccharide-deficient mutant RBL5808, which is RBL5523 exo5::Tn5 (EXO5), StrRif (Laus et al. 2004).
Bacteria were grown under vigorous shaking at 30°C in 30 mL of modified Vincent medium (per liter: 0.15 g MgSO4·7H2O; 0.05 g CaCl2·2H2O; 0.6 g K2HPO4; 0.0018 g FeCl3; 0.06 g NaCl, 0.02 g biotin; 0.01 g calcium pantothenate; 0.01 g thiamine HCl; 10 g mannitol; 1.1 g glutamic acid; pH 6.8). The medium was supplemented with appropriate antibiotics: 50 μg mL−1 of kanamycin and 200 μg mL−1 of spectinomycin. After 72 h of growth, the 30 mL cultures were transferred into 300 mL, cultured for 72 h, and then transferred to 3000 mL of fresh medium and grown for 72 h. Finally, cells were harvested by centrifugation at 3500 × g and then washed with sterile 0.9% (m/v) NaCl solution until free from the remaining medium and any EPS (present in case of the parent Rlv5523 culture), followed by washing with distilled water.
Isolation of LPS
Crude LPSs were isolated from bacteria using the hot phenol/water extraction procedure (Westphal and Jann 1965). Dialyzed phenol and water phases were freeze-dried, and the LPS preparations were suspended in deionized water and further purified by ultracentrifugation at 100,000 × g at 4°C for 6 h. The resulting LPS pellets were suspended in a small volume of deionized water, and lyophilized.
Deoxycholate-polyacrylamide gel electrophoresis
LPS was analyzed by PAGE using an 18% acrylamide gel with deoxycholic acid (DOC) as the detergent (Krauss et al. 1988). The LPS bands were visualized by fixing the gel in the presence or absence of Alcian blue dye (Corzo et al. 1991) followed by silver staining or only by silver staining (Tsai and Frisch 1982).
Isolation of lipid A
Lipid A was released from the LPS by mild hydrolysis in 1% (v/v) acetic acid at 100°C for 1.5 h (Ryan and Conrad 1974). The lipid A precipitate was collected by centrifugation for 25 min at 3500 × g at 4°C, and washed three times with nanopure water and extracted with chloroform:methanol:water (2:2:1.8, v/v/v). The organic phase was collected, reduced in volume and subjected to chemical and structural analyses.
Isolation of the OPS
The HMW LPS was separated from the LMW LPS by gel-filtration chromatography using a Sephacryl S-100 (Amersham Bioscience, piscataway, NJ) column (120 × 1.5 cm) in the presence of DOC (Reuhs et al. 1993, 1994). The eluting fractions were recorded with a Shimadzu Refractive Index Detector (RID-10A) and analyzed by DOC-PAGE. The OPS was liberated from the HMW LPS using 1% acetion acid hydrolysis at 100°C for 2 h and separated from lipid A by centrifugation for 25 min at 3500 × g. The OPS was separated from core OSs on a Biogel P2 (fine) gel-filtration chromatography column (120 × 1 cm) eluted with 1% HOAc (Carlson et al. 1989). The eluting fractions were recorded using a Shimadzu Refractive Index Detector (RID-10A). For analytical purposes, a portion of the OPS was de-O-acetylated with 10 mM NaOH at 4°C for 10 h (Forsberg et al. 2000). The de-O-acetylated sample was then neutralized with HCl, and salts were removed by Biogel P2 gel-filtration chromatography prior to further analyses.
Core OS isolation
The LMW LPS was treated with 1% acetic acid at 104°C for 2.5 h followed by centrifugation for 30 min at 3500 × g to remove the precipitated lipid A, and the aqueous supernatant was extracted with 1 vol of chloroform to further remove any lipid A traces. The supernatant containing the OSs was lyophilized.
HPAEC chromatography
Prior to analysis, the core OS samples were dissolved in deionized water and filtered through a 0.2 μm nylon filter. They were then analyzed by HPAEC using a CarboPack PA 10 (4 × 250 mm) anion-exchange analytical column (Dionex Corp., Sunnyvale, CA) and eluted with a gradient of 3–90% 1 M sodium acetate in 100 mM NaOH at a flow rate of 1 mL min−1 over 50 min. Separation was detected using a pulsed amperometric detector as previously described (Carlson et al. 1995; Forsberg and Carlson 1998). The separation and identification of the core OSs were accomplished by comparison with monomeric Kdo, GalA and previously characterized OS structures from R. etli CE3 (Carlson et al. 1995; Forsberg and Carlson 1998), and a mutant, CE109, that contains a major core OS lacking the GalA residue attached to the core Man residue (Carlson et al. 1989).
Chemical analyses
The composition of the various LPS and LPS-derived fractions was determined by the preparation of TMS methylglycosides after methanolysis with 1 M methanolic HCl at 80°C for 18 h in the presence of an internal standard of inositol and analyzed by combined gas chromatography–mass spectrometry (GC-MS; York et al. 1985). This method also allows the identification and quantification of fatty acid components as their FAME (Bhat et al. 1994). Glycosyl composition was also determined in some instances by the preparation and GC-MS analysis of partially methylated alditol acetates (PMMA) as previously described (York et al. 1985). Stereochemical configuration of the glycosyl residues was done following the method of Gerwig et al. (1979). Glycosyl linkages were determined by the preparation and GC-MS analysis of PMMA (Ciucanu and Kerek 1984).
Mass spectrometric analysis of the OPS and lipid A
Intact and de-O-acetylated OPS and lipid A fractions were analyzed by MALDI-TOF-MS using Applied Biosystems 4700 Proteomics Analyzer. The OPS samples were dissolved in nanopure water and mixed with a 0.5 M 2,5-dihydroxybenzoic acid matrix in methanol. Samples, 1 μL, were loaded onto a stainless-steel target, and spectra were acquired in either positive or negative modes. Lipid A samples were dissolved in 3:1 chloroform:methanol solution and mixed with 0.5 M 2,4,6-trihydroxyacetophenone matrix in methanol in a 1:1 ratio. Mass calibration was performed with the chemically synthesized lipid A, based on Escherichia coli lipid A (a gift obtained from Geert-Jan Boons at the Complex Carbohydrate Research Center) of defined mass (MW = 1714.21). Analysis of the lipid A samples was performed in the negative reflectron mode.
NMR spectroscopy
Prior to NMR analyses, the purified OPS fractions were lyophilized from D2O (99.999 at.% D) twice and dissolved in 0.7 mL of D2O 100 at.% D (Cambridge Isotope Laboratories, Andover, MA). Proton NMR spectra were acquired on a Varian Inova 500 and 600 MHz spectrometer (Varian, Palo Alto, CA) at 30°C using standard Varian software. TOCSY experiments were recorded using a mixing time of 60 ms and two sets of 256 time increments at 32 scans per increment and with a 1 s relaxation delay. Proton–carbon HSQC experiments were recorded with an acquisition time of 0.2 s and two sets of 128 time increments with 96 scans per increment and with a 1.3 s relaxation delay. NOESY experiments were recorded with a mixing time of 0.3 s, two sets of 256 increments with 32 scans per increment, an acquisition time of 0.18 s and a 1 s relaxation delay. The data sets were processed using MestReC software (Mestrelab Research, Santiago de Compostela, Spain).
Funding
This work was supported by grants from the NIH (GM39583 to R.W.C.) and from the DOE (DE-FG02-98ER20307).
Conflict of interest statement
None declared.
Abbreviations
β-OHC4:0, β-hydroxybutyryl; β-OHC14:0, β-hydroxymyristic; β-OHC15:0, β-hydroxypentadecanoic; β-OHC16:0, β-hydroxypalmitic; β-OHC18:0, β-hydroxystearic; COSY, homonuclear correlation spectroscopy; CPS, capsular polysaccharide; DOC, deoxycholate; EPS, extracellular polysaccharide; FAME, fatty acid methyl esters; Gal, galactose; GC-MS, gas chromatography-mass spectrometry; gHSQC, gradient heteronuclear single quantum coherence; Glc, glucose; GlcNonate, 2-aminogluconate; HMW, high-molecular weight; HPAEC, high-performance anion exchange chromatography; Kdo, 3-deoxy-d-manno-2-octulosonic acid; LPS, lipopolysaccharide; LMW, low-molecular weight; MALDI-TOF-MS, matrix-assisted laser desorption ionization-time-of-flight mass spectrometry; Man, mannose; NMR, nuclear magnetic resonance; NOESY, nuclear Overhauser effect spectroscopy; OPS, O-chain polysaccharide; OS, oligosaccharide; PAGE, polyacrylamide gel electrophoresis; PMAA, partially methylated alditol acetates; SEC, size exclusion chromatography; TMS, trimethylsilyl; TOCSY, total correlation spectroscopy; VLCFA, very-long-chain fatty acid.
Acknowledgements
The authors acknowledge Scott Forsberg and Biswa Choudhury (CCRC) for their assistance with DIONEX and NMR analyses. The authors also thank Elmar Kannenberg and Scott Forsberg for their helpful comments.
References
- Bhat UR, Forsberg LS, Carlson RW. Structure of lipid A component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide: unique non-phosphorylated lipid A containing 2-amino-2-deoxy-gluconate, galacturonate, and glucosamine. J Biol Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]
- Bhat UR, Mayer H, Yokota A, Hollingsworth RI, Carlson RW. Occurrence of lipid A variants with 27-hydroxyoctacosanoic acid in lipopolysaccharides from members of the family Rhizobiaceae. J Bacteriol. 1991;173:2155–2159. doi: 10.1128/jb.173.7.2155-2159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Forsberg LS, Kannenberg EL. Lipopolysaccharides in Rhizobium-legume symbioses. In: Quinn PJ, Wang X, editors. Subcellular Biochemistry. Endotoxins: Structure, Function and Recognition. vol. 53. Secaucus, NJ: Springer; 2010. pp. 339–386. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Garci F, Noel D, Hollingsworth R. The structures of the lipopolysaccharide core components from Rhizobium leguminosarum biovar phaseoli CE3 and two of its symbiotic mutants, CE109 and CE309. Carbohydr Res. 1989;195:101–110. doi: 10.1016/0008-6215(89)85092-x. doi:10.1016/0008-6215(89)85092-X. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Kalembasa S, Turowski D, Pachori P, Noel KD. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987;169:4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Krishnaiah BS. Structures of the oligosaccharides obtained from the core regions of the lipopolysaccharides of Bradyrhizobium japonicum 61A101c and its symbiotically defective lipopolysaccharide mutant, JS314. Carbohydr Res. 1992;231:205–219. doi: 10.1016/0008-6215(92)84020-s. doi:10.1016/0008-6215(92)84020-S. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Reuhs B, Chen TB, Bhat UR, Noel KD. Lipopolysaccharide core structures in Rhizobium etli and mutants deficient in O-antigen. J Biol Chem. 1995;270:11783–11788. doi: 10.1074/jbc.270.20.11783. doi:10.1074/jbc.270.20.11783. [DOI] [PubMed] [Google Scholar]
- Cava JR, Elias PM, Turowski DA, Noel KD. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989;171:8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava JR, Tao H, Noel KD. Mapping of complementation groups within a Rhizobium leguminosarum CFN42 chromosomal region required for lipopolysaccharide synthesis. Mol Genet Genomics. 1990;221:125–128. doi:10.1007/BF00280377. [Google Scholar]
- Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. doi:10.1016/0008-6215(84)85242-8. [Google Scholar]
- Corzo J, Pérez-Galdona R, Le¢n-Barrios M, Gutiérrez-Navarro AM. Alcian blue fixation allows silver staining of the isolated polysaccharide component of bacterial lipopolysaccharides in polyacrylamide gels. Electrophoresis. 1991;12:439–441. doi: 10.1002/elps.1150120611. doi:10.1002/elps.1150120611. [DOI] [PubMed] [Google Scholar]
- De Castro C, Molinaro A, Lanzetta R, Silipo A, Parrilli M. Lipopolysaccharide structures from Agrobacterium and Rhizobiaceae species. Carbohydr Res. 2008;343:1924–1933. doi: 10.1016/j.carres.2008.01.036. doi:10.1016/j.carres.2008.01.036. [DOI] [PubMed] [Google Scholar]
- D'Haeze W, Leoff C, Freshour G, Noel KD, Carlson RW. Rhizobium etli CE3 bacteroid lipopolysaccharides are structurally similar but not identical to those produced by cultured CE3 bacteria. J Biol Chem. 2007;282:17101–17113. doi: 10.1074/jbc.M611669200. doi:10.1074/jbc.M611669200. [DOI] [PubMed] [Google Scholar]
- Duelli DM, Tobin A, Box JM, Kolli VSK, Carlson RW, Noel KD. Genetic locus required for antigenic maturation of Rhizobium etli CE3 lipopolysaccharide. J Bacteriol. 2001;183:6054–6064. doi: 10.1128/JB.183.20.6054-6064.2001. doi:10.1128/JB.183.20.6054-6064.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg LS, Bhat UR, Carlson RW. Structural characterization of the O-antigenic polysaccharide of the lipopolysaccharide from Rhizobium etli strain CE3: a unique O-acetylated glycan of discrete size, containing 3-O-methyl-6-deoxy-l-talose and 2,3,4-tri-O-methyl-l-fucose. J Biol Chem. 2000;275:18851–18863. doi: 10.1074/jbc.M001090200. doi:10.1074/jbc.M001090200. [DOI] [PubMed] [Google Scholar]
- Forsberg LS, Carlson RW. The structures of the lipopolysaccharides from Rhizobium etli strains CE358 and CE359—the complete structure of the core region of R. etli lipopolysaccharides . J Biol Chem. 1998;273:2747–2757. doi: 10.1074/jbc.273.5.2747. doi:10.1074/jbc.273.5.2747. [DOI] [PubMed] [Google Scholar]
- Forsberg LS, Carlson RW. Structural characterization of the primary O-antigenic polysaccharide of the Rhizobium leguminosarum 3841 lipopolysaccharide and identification of a new 3-acetimidoylamino-3-deoxyhexuronic acid glycosyl component: a unique O-methylated glycan of uniform size, containing 6-deoxy-3-O-methyl-d-talose, N-acetylquinovosamine, and rhizoaminuronic acid (3-acetimidoylamino-3-deoxy-d-gluco-hexuronic acid) J Biol Chem. 2008;283:16037–16050. doi: 10.1074/jbc.M709615200. doi:10.1074/jbc.M709615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg LS, Noel KD, Box J, Carlson RW. Genetic locus and structural characterization of the biochemical defect in the O-antigenic polysaccharide of the symbiotically deficient Rhizobium etli mutant, CE166: replacement of an N-acetylquinovosamine with its hexosyl-4-ulose precursor. J Biol Chem. 2003;278:51347–51359. doi: 10.1074/jbc.M309016200. doi:10.1074/jbc.M309016200. [DOI] [PubMed] [Google Scholar]
- Frirdich E, Whitfield C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J Endotoxin Res. 2005;11:133–144. doi: 10.1179/096805105X46592. [DOI] [PubMed] [Google Scholar]
- Gerwig GJ, Kamerling JP, Vliegenthart JFG. Determination of the absolute configuration of monosaccharides in complex carbohydrates by capillary g.l.c. Carbohydr Res. 1979;77:1–7. doi: 10.1016/s0008-6215(00)83788-x. doi:10.1016/S0008-6215(00)83788-X. [DOI] [PubMed] [Google Scholar]
- Gonzalez V, Santamaria RI, Bustos P, Hernandez-Gonzalez I, Medrano-Soto A, Moreno-Hagelsieb G, Janga SC, Ramirez MA, Jimenez-Jacinto V, Collado-Vides J, et al. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci. 2006;103:3834–3839. doi: 10.1073/pnas.0508502103. doi:10.1073/pnas.0508502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DE, Yethon JA, Amor PA, Whitfield C. The assembly system for the outer core portion of R1- and R4-type lipopolysaccharides of Escherichia coli. J Biol Chem. 1998;273:29497–29505. doi: 10.1074/jbc.273.45.29497. doi:10.1074/jbc.273.45.29497. [DOI] [PubMed] [Google Scholar]
- Heinrichs DE, Yethon JA, Whitfield C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol Microbiol. 1999;30:221–232. doi: 10.1046/j.1365-2958.1998.01063.x. doi:10.1046/j.1365-2958.1998.01063.x. [DOI] [PubMed] [Google Scholar]
- Kaniuk NA, Vinogradov E, Whitfield C. Investigation of the structural requirements in the lipopolysaccharide core acceptor for ligation of O antigens in the genus Salmonella. J Biol Chem. 2004;279:36470–36480. doi: 10.1074/jbc.M401366200. doi:10.1074/jbc.M401366200. [DOI] [PubMed] [Google Scholar]
- Kanjilal-Kolar S, Basu SS, Kanipes MI, Guan Z, Garrett TA, Raetz CRH. Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyltransferases. J Biol Chem. 2006;281:12865–12878. doi: 10.1074/jbc.M513864200. doi:10.1074/jbc.M513864200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjilal-Kolar S, Raetz CRH. Dodecaprenyl phosphate-galacturonic acid as a donor substrate for lipopolysaccharide core glycosylation in Rhizobium leguminosarum. J Biol Chem. 2006;281:12879–12887. doi: 10.1074/jbc.M513865200. doi:10.1074/jbc.M513865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannenberg EL, Carlson RW. Lipid A and O-chain modifications cause Rhizobium lipopolysaccharides to become hydrophobic during bacteroid development. Mol Microbiol. 2001;39:379–392. doi: 10.1046/j.1365-2958.2001.02225.x. doi:10.1046/j.1365-2958.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- Kannenberg EL, Reuhs BL, Forsberg LS, Carlson RW. Lipopolysaccharides and K-antigens: their structures, biosynthesis, and function. In: Spaink HP, Kondorosi A, Hooykaas PJJ, editors. The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria. Dordrecht, Boston, London: Kluwer Academic Publishers; 1998. pp. 119–154. [Google Scholar]
- King JD, Kocincova D, Westman EL, Lam JS. Review: lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Innate Immun. 2009;15:261–312. doi: 10.1177/1753425909106436. doi:10.1177/1753425909106436. [DOI] [PubMed] [Google Scholar]
- Krauss JH, Weckesser J, Mayer H. Electrophoretic analysis of lipopolysaccharides of purple non-sulfur bacteria. Int J Syst Bacteriol. 1988;38:157–163. doi:10.1099/00207713-38-2-157. [Google Scholar]
- Laus MC, Logman TJ, Lamers GE, Van Brussel AAN, Carlson RW, Kijne JW. A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol Microbiol. 2006;59:1704–1713. doi: 10.1111/j.1365-2958.2006.05057.x. doi:10.1111/j.1365-2958.2006.05057.x. [DOI] [PubMed] [Google Scholar]
- Laus MC, Logman TJ, van Brussel AAN, Carlson RW, Azadi P, Gao MY, Kijne JW. Involvement of exo5 in production of surface polysaccharides in Rhizobium leguminosarum and its role in nodulation of Vicia sativa subsp. nigra. J Bacteriol. 2004;186:6617–6625. doi: 10.1128/JB.186.19.6617-6625.2004. doi:10.1128/JB.186.19.6617-6625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge I, Laeremans T, Verreth C, Vanderleyden J, van Soom C, Tobin A, Carlson RW. Identification of an ABC transporter for export of the O-antigen across the inner membrane in Rhizobium etli based on the genetic, functional and structural analysis of an lps mutant deficient in O-antigen. J Biol Chem. 2001;276:17190–17198. doi: 10.1074/jbc.M101129200. doi:10.1074/jbc.M101129200. [DOI] [PubMed] [Google Scholar]
- Noel KD, Box JM, Bonne VJ. 2-O-Methylation of fucosyl residues of a rhizobial lipopolysaccharide is increased in response to host exudate and is eliminated in a symbiotically defective mutant. Appl Environ Microbiol. 2004;70:1537–1544. doi: 10.1128/AEM.70.3.1537-1544.2004. doi:10.1128/AEM.70.3.1537-1544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel KD, Forsberg LS, Carlson RW. Varying the abundance of O antigen in Rhizobium etli and its effect on symbiosis with Phaseolus vulgaris. J Bacteriol. 2000;182:5317–5324. doi: 10.1128/jb.182.19.5317-5324.2000. doi:10.1128/JB.182.19.5317-5324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda KJ, Box JM, Noel KD. Genetic basis for Rhizobium etli CE3 O-antigen O-methylated residues that vary according to growth conditions. J Bacteriol. 2010;192:679–690. doi: 10.1128/JB.01154-09. doi:10.1128/JB.01154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotto S, Brewin NJ, Kannenberg EL. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol Plant Microbe Interact. 1994;7:99–112. [Google Scholar]
- Price NPJ. Carbohydrate determinants of Rhizobium-legume symbioses. Carbohydr Res. 1999;317:1–9. doi: 10.1016/s0008-6215(99)00075-0. doi:10.1016/S0008-6215(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Que NLS, Lin SH, Cotter RJ, Raetz CRH. Purification and mass spectrometry of six lipid A species from the bacterial endosymbiont Rhizobium etli—demonstration of a conserved distal unit and a variable proximal portion. J Biol Chem. 2000;275:28006–28016. doi: 10.1074/jbc.M004008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. doi:10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. doi:10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuhs BL, Carlson RW, Kim JS. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuhs BL, Kim JS, Badgett A, Carlson RW. Production of cell-associated polysaccharides of Rhizobium fredii USDA205 is modulated by apigenin and host root extract. Mol Plant Microbe Interact. 1994;7:240–247. doi: 10.1094/mpmi-7-0240. [DOI] [PubMed] [Google Scholar]
- Ridley BL, Jeyaretnam BS, Carlson RW. The type and yield of lipopolysaccharide from symbiotically deficient Rhizobium lipopolysaccharide mutants vary depending on the extraction method. Glycobiology. 2000;10:1013–1023. doi: 10.1093/glycob/10.10.1013. doi:10.1093/glycob/10.10.1013. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Conrad HE. Structural heterogeneity in the lipopolysaccharide from Salmonella newington. Arch Biochem Biophys. 1974;162:530–535. doi: 10.1016/0003-9861(74)90213-6. doi:10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- Stacey G, So JS, Roth LE, Bhagya Lakshmi SK, Carlson RW. A lipopolysaccharide mutant from Bradyrhizobium japonicum that uncouples plant from bacterial differentiation. Mol Plant Microbe Interact. 1991;4:332–340. doi: 10.1094/mpmi-4-332. [DOI] [PubMed] [Google Scholar]
- Tsai C, Frisch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. doi:10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- van Workum WAT, van Slageren S, van Brussel AAN, Kijne JW. Role of exopolysaccharides of Rhizobium leguminosarum bv. viciae as host plant-specific molecules required for infection thread formation during nodulation of Vicia sativa. Mol Plant Microbe Interact. 2007;11:1233–1241. doi:10.1094/MPMI.1998.11.12.1233. [Google Scholar]
- Vedam V, Kannenberg EL, Haynes JG, Sherrier DJ, Datta A, Carlson RW. A Rhizobium leguminosarum acpXL mutant produces lipopolysaccharide lacking 27-hydroxyoctacosanoic acid. J Bacteriol. 2003;185:1841–1850. doi: 10.1128/JB.185.6.1841-1850.2003. doi:10.1128/JB.185.6.1841-1850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O, Jann K. Bacterial lipopolysaccharides. Meth Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. Isolation and characterization of plant cell walls and cell wall components. Meth Enzymol. 1985;118:3–40. doi:10.1016/0076-6879(86)18062-1. [Google Scholar]
- Young JP, Crossman L, Johnston A, Thomson N, Ghazoui Z, Hull K, Wexler M, Curson A, Todd J, Poole P, et al. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 2006;7:R34. doi: 10.1186/gb-2006-7-4-r34. doi:10.1186/gb-2006-7-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]