Abstract
Recently, cultured human adult skin cells were reprogrammed to induced pluripotent stem (iPS) cells, which have characteristics similar to human embryonic stem (hES) cells. Patient-derived iPS cells offer genetic and immunologic advantages for cell and tissue replacement or engineering. The efficiency of generating human iPS cells has been very low; therefore an easily and efficiently reprogrammed cell type is highly desired. Here, we demonstrate that terminally differentiated human amniotic fluid (AF) skin cells provide an accessible source for efficiently generating abundant-induced pluripotent stem (AF-iPS) cells. By induction of pluripotency with the transcription factor quartet (OCT3/4, SOX2, KLF4, and c-MYC) the terminally differentiated, cultured AF skin cells formed iPS colonies approximately twice as fast and yielded nearly a two-hundred percent increase in number, compared to cultured adult skin cells. AF-iPS cells were identical to hES cells for morphological and growth characteristics, antigenic stem cell markers, stem cell gene expression, telomerase activity, in vitro and in vivo differentiation into the three germ layers and for their capacity to form embryoid bodies (EBs) and teratomas. Our findings provide a biological interesting conclusion that these fetal AF cells are more rapidly, easily, and efficiently reprogrammed to pluripotency than neonatal and adult cells. AF-iPS cells may have a “young,” more embryonic like epigenetic background, which may facilitate and accelerate pluripotency. The ability to efficiently and rapidly reprogram terminally differentiated AF skin cells and generate induced pluripotent stem cells provides an abundant iPS cell source for various basic studies and a potential for future patient-specific personalized therapies.
Introduction
Generation of induced pluripotent stem (iPS) cells from nonembryonic tissues has been achieved by different reprogramming strategies (Jaenisch and Young, 2008). Most recently, somatic cells have been reprogrammed to iPS cells by transduction of two, three, or four specific transcription factors (OCT3/4, SOX2, KLF4, and c-MYC) (Kim et al., 2008; Meissner et al., 2007; Nakagawa et al., 2008; Takahashi et al., 2007; Yu et al., 2007), using retroviral (Park et al., 2008; Wernig et al., 2007) lentiviral vectors (Brambrink et al., 2008; Maherali and Hochedlinger, 2008), a single polycistronic vector (Carey et al., 2009), or with nonviral expression (Kaji et al., 2009; Woltjen et al., 2009). However, the efficiency of generating human iPS cells with the transcription factor quartet (OCT3/4, SOX2, KLF4, and c-MYC) has been very low (Mali et al., 2008). Because adult primary fibroblasts are more refractory to reprogramming than fetal or neonatal fibroblasts, a cocktail of six factors (the four transcription factors + hTERT and SV40 LT) has been employed with only somewhat greater success, to reprogram adult primary fibroblasts (Park et al., 2008). Such reprogrammed adult fibroblasts from individual patients have a potential for drug screening (Ebert et al., 2009; Egli et al., 2008) and regenerative therapies. Transcription factor-induced reprogramming to iPS cells has been accomplished for various cultured cell types (Aasen et al., 2008; Aoi et al., 2008; Brambrink et al., 2008; Carey et al., 2009; Egli et al., 2008; Kaji et al., 2009; Kim et al., 2008; Lowry et al., 2008; Maherali and Hochedlinger, 2008; Meissner et al., 2007; Nakagawa, et al., 2008; Sieber-Blum and Hu, 2008; Takahashi et al., 2007; Woltjen, et al., 2009; Yamanaka, 2009; Ye et al., 2009; Yu et al., 2007), but there still remains a need for an easily reprogrammable cell type (Baker, 2008; Yamanaka, 2009).
Amniotic fluid (AF) obtained in the early second trimester of pregnancy (around 15 weeks) contains different cell types (Gosden, 1983; Polgar et al., 1984, 1989; Priest et al., 1978). Approximately 1% of the amniotic fluid cells are categorized as amniotic fluid stem (AFS) cells (De Coppi et al., 2007; Fauza, 2004; Hipp and Atala, 2008; In't Anker et al., 2003; Li, et al., 2009; Prusa et al., 2003; Siegel et al., 2008). AFS cells (De Coppi et al., 2007) and hAFDCs (human amniotic fluid-derived cells) (Li et al., 2009) represent a “precursor state,” not a pluripotent stem cell state (De Coppi et al., 2007; Li et al., 2009). The “precursor state” hAFDCs could be reprogrammed rapidly (6 days after infection) and efficiently (Li et al., 2009). The rest of the cells in the amniotic fluid are terminally differentiated cells (about 99%), that were mostly desquamated from the fetal skin. Here we demonstrate that these cells can be readily reprogrammed to a pluripotent stem cell stage. In long-term culture, the fibroblast-type amniotic fluid cell predominates and is thought to originate from fibrous connective, mesenchymal tissues, and mostly from dermal fibroblasts (Prusa and Hengstschlager, 2002). In this study we report that reprogramming of cultured, terminally differentiated AF skin cells result in pluripotent stem (AF-iPS) cells that are similar to hES cells and that reprogramming those AF cells to the pluripotent stem cell stage is much easier, faster, and more efficient than reprogramming neonatal and adult cells.
Materials and Methods
Cultured AF skin cells as well as cultured neonatal and adult skin cells were induced to pluripotency with the transcription factor quartet (OCT3/4, SOX2, KLF4, and c-MYC) (Takahashi et al., 2007). iPS colonies were characterized for morphological and growth characteristics, antigenic stem cell markers, stem cell gene expression, telomerase activity, and in vivo and in vitro differentiation using previously reported methods. Immunohistochemistry and gene expression profiling used to compare AF-iPS cells, neonatal, and adult skin-derived iPS cells to undifferentiated hES cells was performed by standard methods. RT-PCR analysis was undertaken to compare a panel of key hES cell-specific markers and for the analysis of retroviral expression of the four exogenous transcription factors. Quantitative RT-PCR was used for detecting OCT3/4, SOX2, KLF4, C-MYC, TERT, NANOG, GDF3, and DPPA5 expression between the pairs of cultured parental AF skin and AF-iPS cells, cultured adult skin cells and adult skin-iPS, neonatal skin cells, and neonatal skin-iPS cells. Telomerase activity was detected with a TRAPEZE telomerase detection kit (Chemicon, Temecula, CA, USA). In vitro differentiation was evaluated by embryoid body formation and immunohistochemistry for each germ layer differentiation as described. Teratoma formation experiments were performed for analyzing in vivo differentiation using immunocompromised severe combined immunodeficiency (SCID) mice.
Cell source
AF
Approximately 20 mL of AF were received in the cytogenetic laboratory for each specimen for routine chromosome analysis. Four independent cultures were established from the fetal cells present in the fluid. At day 5 of culture the discarded supernatant from each of the four cultures was transferred to an anonymized T12.5 flask and the resulting cultures were expanded for this study. Seventeen samples were obtained as discarded, anonymous, cultured amniotic fluid cells from the Cytogenetic Laboratory at the Mount Sinai School of Medicine in New York. All cells were chromosomally normal.
Adult and neonatal skin cells
Primary skin fibroblasts were cultured from skin biopsies of eight healthy adult volunteers (aged 45–77 years) with informed consent (Staten Island Hospital). Neonatal BJ1 fibroblasts were purchased from the American Type Culture Collection (ATCC).
hES
An established HES-2 line was used for these studies (WiCell).
Cell culture
AF skin cells were cultured in AmnioMax medium (Invitrogen, Carlsbad, CA, USA). AF cells were cultured at 37°C in a humified 5% CO2 atmosphere and the media was changed twice a week. Cells were passaged at a ratio 1:4 every 5 days until they reached 80% confluence, when they were infected. For subsequent passages the media was aspirated, the cells were washed once with phosphate-buffered saline (PBS) and trypsinised with 0.05% trypsin for 5 min at 37°C.
Adult and neonatal skin cells
Adult skin (Skin) and neonatal skin (BJ) fibroblasts were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS).
hES and iPS cells
The hES cell line, HES-2, AF-iPS, SK-iPS, and BJ-iPS colonies were cultured in serum-free medium DMEM/F12, supplemented with 20% knock-out serum replacement (KSR), 0.1 mM nonessential amino acids, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, and 10 ng/mL human recombinant fibroblast growth factor (hbFGF) (all from Invitrogen) and were maintained on irradiated mouse embryonic fibroblasts (MEFs).
Retroviral infection and iPS cell generation
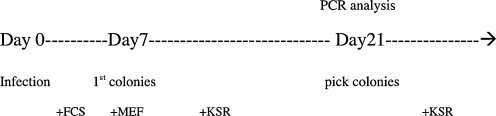
PLAT-A packaging cells were plated at 4 × 106 cells per 100-mm dish and incubated overnight. The next day they were transfected with a mixture containing equal volumes of OCT4/SOX2/KLF4/c-MYC (Takahashi et al., 2007) pMXs (Addgene, Cambridge, MA, USA) vectors with the Fugene-6 transfection reagent (Roche, Indianapolis, IN, USA). Forty-eight hours after transfection, the medium was collected as the first virus-containing supernatant and replaced with fresh medium. Twenty-four hours later, the second virus-containing supernatant was collected. The supernatants were filtered through a 0.45-μm pore-size filter and supplemented with 8 μg/mL polybrene. The human AF cells, BJ neonatal cells, and adult skin fibroblasts were infected with the first virus-containing supernatant for 4 h and the medium was replaced. Twenty-four hours later the cells were reinfected with the second supernatant. Six days after transduction the fibroblasts were harvested by trypsinization and replated on irradiated mouse MEFs feeder layer at 5 × 105 cells per 100-mm dish. The medium was replaced with DMEM/F12 with 20% KSR, 0.1 mM nonessential amino acids, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, and 10 ng/mL hbFGF (all from Invitrogen), and was changed every day. Twenty days later, colonies with hES cell morphology (iPS colonies) were picked, mechanically dissociated to small clumps, and plated on feeder cells (passage 1) for expansion. In addition to typical embryonic stem cell-like iPS colonies, a lot of partially reprogrammed pre-iPS colonies appear in the culture dish; this is not meaningful, but only useful to count the colony numbers. For measurement we used the frequency of alkaline phosphatase (AP) and NANOG-positive colonies in each group.
Infection/iPS generation time line
qRT-PCR analysis
Relative gene expression was determined using a two-step quantitative real-time PCR protocol (primer sequences) (Takashashi et al., 2007). Total RNA was isolated and purified as described in the RNeasy Isolation kit (Qiagen GmbH, Germany) with on column DNase I digestion. About 1 μg of total RNA from each sample was reverse transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Forster City, CA, USA) according to the manufacturer's protocol. Quantitative PCR reactions were performed with Power SYBR Green Master Mix (Applied Biosystems) on an ABI Prism 7500 Real-Time PCR System. Multiple transcripts were analyzed simultaneously for 35 cycles using an optimized qRT-PCR thermal profile. Data analysis was performed using Real-Time SDS software (Applied Biosystems). For each set of primers, a no template control and a no reverse amplification control were included. Postamplification dissociation curves were generated to verify the presence of a single amplification product in the absence of DNA contamination. Fold changes in gene expression were determined using the ΔΔCt method with normalization to ACTB endogenous control.
TRAPEZE® RT telomerase detection with Amplifluor® primers
Telomerase activity was detected with a TRAPEZE telomerase detection kit according to the manufacturer's instructions (Chemicon) with Platinum Taq Polymerase (Invitrogen). Briefly, protein extracts were prepared from each sample, and 200 ng of total protein was added to each reaction. If telomerase activity was present in the extract, it elongated the added primer, and the reaction product (templates) was amplified by PCR. This technique was highly sensitive and provided both qualitative (presence/absence) and quantitative analyses.
Immunofluoresence analysis
iPS cells were fixed with 4% paraformaldehyde and permeabilized with 0.05% Triton X-100. Ten percent donkey serum was used for blocking. Cells were stained with primary antibodies against SOX2 (1:200, Abcam, Cambridge, MA, USA), NANOG (1:2000, Santa Cruz, Santa Cruz, CA, USA), nestin (1:200, Chemicon), smooth-muscle actin (1:2000, Chemicon), troponin (1:2000, Chemicon), AFP SSEA4 OCT3/4, AP (1:100, R&D Systems, Minneapolis, MN, USA), followed by staining with secondary AlexaFluor-conjugated antibodies (Invitrogen).
In vitro differentiation
For the generation of EBs, iPS cells were trypsinized and plated at various densities into ultralow attachment six-well plates (Costar, Cambridge, MA, USA) in DMEM supplemented with 10% FCS, and 0.5% penicillin and streptomycin. Embryoid bodies (EBs) were maintained as a floating culture for 7 days, with medium changed every other day and then transferred into gelatin-coated plates for another 10 days. The cells were stained with nestin, α-smooth muscle actin, troponin I, and α-fetoprotein (Chemicon) for ectoderm, mesoderm, and endoderm differentiacion. Tuj1 antibody (Abcam) was used for neuron-specific staining.
In vivo differentiation—Teratoma formation
Teratoma formation was induced by injecting 2–5 × 106 lentivirally transduced (pFUGW/GFP) AF/GFP and AF-iPS/GFP cells into the subcutaneous tissue above the rear haunch of 6-week-old athymic, immunocompromised SCID mice (beige mice, Charles River Laboratories). Seven weeks postinjection, teratomas were harvested and fixed overnight at 4°C in 4% paraformaldehyde. Samples were immersed in 30% sucrose followed by embedding the tissue in O.C.T freezing compound (Tissue-Tek). Cryosections (10 μm) were incubated with the appropriate antibodies using standard protocols and analyzed by confocal microscopy for the presence of differentiation markers: Tuj1 (ectoderm; Covance, Princeton, NJ, USA); alpha-actinin (mesoderm; DAKO, Carpinteria, CA, USA), and alpha-fetoprotein (endoderm; DAKO). Nuclei were stained blue with 4′,6-diamidine-2-phenylidole dihydrochloride (DAPI). Teratomas were sectioned and hematoxylin-eosin (H&E) stained to visualize the general morphology and derivatives of the three germ layers.
Microscopy
Images were taken using Zeiss bright-field and confocal microscopes (Zeiss microscope LSM510 META). Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility.
Results and Discussion
To confirm that human AF-iPS cells were more efficiently established than iPS cells from other human somatic cells we characterized iPS cell clone formation. By induction of pluripotency with the transcription factor quartet (OCT3/4, SOX2, KLF4, and c-MYC) (Takahashi et al., 2007), the terminally differentiated, cultured AF skin cells formed iPS colonies approximately twice as fast as cultured adult skin cells (Fig. 1a). Of note, cultured AF skin cells formed approximately twice as many iPS colonies as adult cultured skin cells (Fig. 1b). In addition, we also characterized the AF-iPS colonies for morphological and growth characteristics, antigenic stem cell markers, stem cell gene expression, telomerase activity, and differentiation. Morphologically, the AF-iPS colonies were similar to hES cells and iPS colonies derived from adult skin. AF-iPS colonies formed tightly packaged, sharp-edged, flat colonies, as did hES cells and iPS cells from adult skin (Fig. 1c–j). Immunohistochemistry revealed that AF-iPS cells expressed the same markers as hES and human iPS cells from adult skin (alkaline phoshatase, OCT 3/4, SOX2, SSEA4, and NANOG) (Fig. 1k–o). In contrast, the cultured AF cells prior to reprogramming did not express immunoreactivity for any of those pluripotency markers but exhibited immunoreactivity for terminally differentiated epithelial and fibroblast cell markers.
FIG. 1.
Induction of iPS cells from cultured human amniotic fluid (AF) cells. (a) Time course of the generation of iPS colonies from AF cells (AF), neonatal (BJ), and adult skin fibroblasts (Skin). (b) Frequency of AF-iPS, BJ-iPS, and iPS colonies from adult skin generated 28 days after infection. (c, d, e). Morphology of cultured AF cells (c), BJ fibroblasts (d), and primary skin fibroblasts (e). (f, g, h, j) Morphology of an established AF-iPS colony feeder dependent (f) or feeder independent plated onto Matrigel (g), BJ-iPS colonies (h), iPS cells derived from adult skin (i), Typical image of the HES-2 human ES cell line (j). (k, l, m, n, o) Immunohistochemistry of selected AF-iPS colonies. Cells were stained with alkaline phoshatase (k) OCT 3/4 (l), SOX2 (m), SSEA4 (n), and NANOG (o).
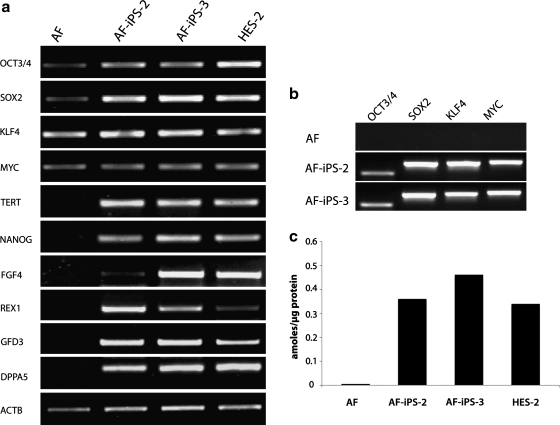
Using a panel of markers to characterize the “stemness” and the undifferentiated state of AF-iPS cells key developmentally regulated markers were highly expressed at several time points (Fig. 2). RT-PCR analysis was performed to compare the key hES cell-specific markers, including OCT3/4, SOX2, KLF4, C-MYC, TERT, NANOG, FGF4, REX1, GDF3, and DPPA5 in cultured AF cells, AF-iPS clones (AF-iPS-2, AF-iPS-3), and an hES cell line, HES-2 (Fig. 2a). Four of the retrovirally expressed transcription factors demonstrated the presence of the viral-encoded OCT3/4, SOX2, KLF4, c-MYC transcripts (Fig. 2b). Telomerase activity in AF- iPS cells was compared with that in iPS cells from adult skin fibroblasts and hES cells (Fig. 2c). Each of the iPS clones (AF-iPS-2, AF-iPS-3) expressed high telomerase activity (Fig. 2c), which was comparable to that in hES cell line, HES-2. In contrast, the cultured AF cells prior to reprogramming had negligible telomerase activity.
FIG. 2.
(a) Gene expression profile of human AF-iPS. RT-PCR analysis of key hES cell-specific markers (OCT3/4, SOX2, KLF4, c-MYC, TERT, NANOG, FGF4, REX1, GDF3, DPPA5) in AF cells, AF- iPS clones (AF-iPS-2, AF-iPS-3), and hES cell line, HES-2. Primers for OCT3/4, SOX2, KLF4, and c-MYC were specific for the 3′ untranslated region and designed to specifically amplify the endogenous genes. ACTB internal control is shown as a positive amplification and loading control. (b) Expression of exogenous factors. PCR was used for retroviral expression of the four factors. Transgene-specific primers recognize the viral-encoded transcripts of OCT3/4, SOX2, KLF4, C-MYC. (c) Telomerase activity. Each of the AF-iPS clones (AF-iPS-2, AF-iPS-3) expressed high telomerase activity, which was comparable to that of hES cell line, HES-2, whereas the cultured parental AF cells showed negligible activity.
The expression of OCT3/4, SOX2, KLF4, c-MYC, TERT, NANOG, GDF3, and DPPA5 was assessed by quantitative real-time PCR in AF-iPS and cultured AF cells (Fig. 3a), adult skin fibroblasts, and iPS cells from adult skin (Fig. 3b) and human foreskin fibroblasts (BJ line) and BJ-iPS cells (Fig. 3c). Relative levels of endogenous OCT4, SOX2, KLF4, and MYC expression in AF-iPS cells and the levels in the hES cell line, HES-2, showed similar patterns over the course of reprogramming.
FIG. 3.
(a) Quantitative real-time PCR assay for expression of OCT3/4, SOX2, KLF4, c-MYC, TERT, NANOG, GDF3, and DPPA5 in human iPS and parental AF skin cells (AF-iPS clones: AF-iPS-2, AF-iPS-3). (a) AF and AF-iPS cells, (b) human adult skin fibroblasts and iPS cells from adult skin. (c) Human foreskin fibroblasts (BJ line) and iPS cells from BJ fibroblasts. Individual PCR reactions were normalized against an internal control (ACTB) and plotted relative to the expression level in the parental AF cells. (d, e) Relative expression levels of the endogenous four transcription factors (OCT4, SOX2, KLF4, MYC) relative to the hES cell line, HES-2, over the course of reprogramming.
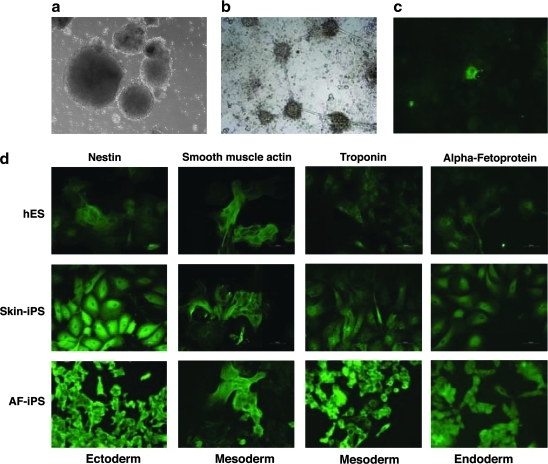
hES cells spontaneously form spheroidal embryo-like structures termed “embryoid bodies,” which consist of a core of mitotically active and differentiating hES cells and a periphery of fully differentiated cells from all three germ layers. AF-iPS cells also developed into EBs, which were similar to the EB formation of hES cells (Fig. 4a). The AF-iPS cells form EB with peripheral differentiated cells similar to that of iPS cells from adult skin. After EB formation, aggregates were transferred to gelatin-coated plates and allowed to spontaneously differentiate over 10 days. Resulting cells were differentiated into neurons (Fig. 4b–c). After EB differentiation, cells derived from AF-iPS, iPS cells from adult skin, and hES cells were stained with anti-nestin (an ectodermal marker), anti-α-smooth muscle actin, and anti-troponin I (mesodermal markers) and anti-α-fetoprotein (AFP) (an endodermal marker) (Fig. 4d). The hES, AF-iPS, and iPS cells from adult skin all had differentiated into the three germ layers.
FIG. 4.
In vitro differentiation of AF-iPS into the three germ layers. (a) Floating embryoid bodies (EB) derived from AF-iPS (AF-3 clone 10) at day 7. (b, c) After EB formation, aggregates were transferred onto gelatin-coated plates and allowed to spontaneously differentiate for 10 days. Resulting cells had differentiated into neural cells with typical morphology (b) and Tuj1-specific positive immunoreactivity (c). (d) After EB differentiation amniotic fluid cell-derived iPS cells (AF-iPS), iPS cells from adult skin (Skin-iPS), and hES (HES-2) were stained with anti-nestin (an ectodermal marker), anti-α-smooth muscle actin and anti-troponin I (mesodermal markers), and anti-α-fetoprotein (AFP) (an endodermal marker).
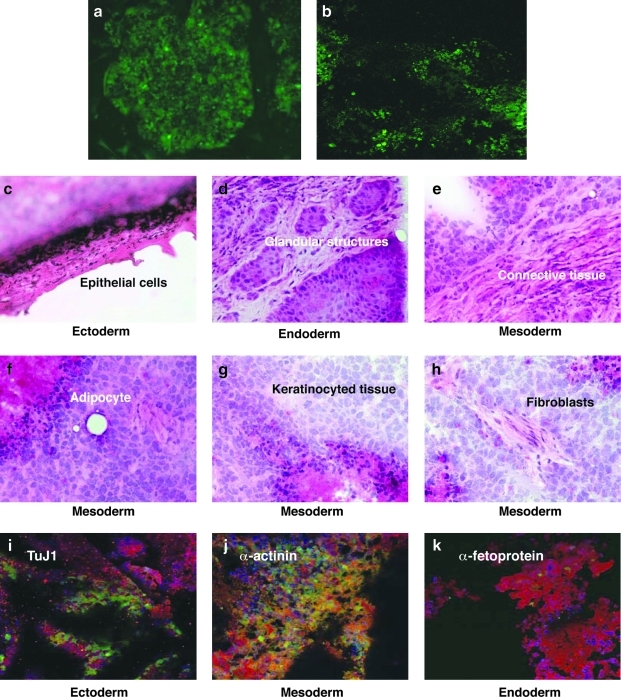
In vivo differentiation was analyzed by inducing teratoma formation by injecting 2–5 × 106 lentivirally transduced (pFUGW/GFP) AF/GFP and AF-iPS/GFP cells into the subcutaneous tissue above the rear haunch of 6-week-old athymic, immunocompromised SCID mice. Seven weeks postinjection antibody reactivity was detected for markers of all three germ layers (Fig. 5). H&E stained teratomas contained tissues representing all three germ layers: ectoderm, epidermal, and neural tissue; mesoderm, bone, and cartilage; and endoderm, respiratory epithelium, and intestinal-like epithelium (Fig. 5). Teratoma formation experiments confirmed that AF-iPS cells have the same differentiation potential as skin-iPS cells, neonatal BJ-iPS cells, and hES cells.
FIG. 5.
In vivo differentiation of AF-iPS into the three germ layers. (a) AF and AFC-iPS cells were lentivirally transduced with pFUGW/GFP vector, generating AFC/GFP and AFC-iPS/GFP. (b) The infected cells were subcutaneously injected into SCID Beige mice. Seven weeks after injection, mice transplanted with AF-iPS/GFP cells generated a tumor expressing GFP. No teratoma was detected in control mice transplanted with AF/GFP. (c–h) Hematoxylin-eosin (H&E) staining of teratoma sections of Beige SCID mice transplanted with AF-iPS cells, 7 weeks after teratoma induction. (i–k) Antibody reactivity was detected for derivatives of all three germ layers. Positive staining in teratoma sections for specific antibodies including ectoderm (Tuj1) (i), mesoderm (α-actinin) (j) and endoderm (α-fetoprotein) (k) markers. For reference, nuclei are stained with DAPI.
In summary, our results indicate that cultured AF skin cells can be reprogrammed to an undifferentiated ES cell-like state. The demonstration that these terminally differentiated cells can be reprogrammed to pluripotency more efficiently than other cell types is notable, as AF cells are easily obtainable by amniocentesis, which can provide large numbers of cells from 3–10 mL of human AF. In the United States, AF cell prenatal screening has been recommended recently from midterm gestations not only for advanced maternal age, but also for all pregnancies (ACOG-American College of Obstetricians and Gynecologists, 2007).
Terminally differentiated AF skin cells are more easily, rapidly, and efficiently reprogrammed to pluripotency than adult skin cells, and provide an abundant source for various basic studies. Efficient generation of abundant AF-iPS cells might provide a tool to study and potentially cure fatal embryonic diseases with prenatal, perinatal gene therapy. It permits investigation of prenatal cellular therapy for various recessive genetic diseases and for future patient-specific personalized therapies.
Acknowledgments
The senior author (K.P.) thanks Prof. Dr. Rudolf Jaenisch (MIT, Whitehead Institute, Boston) for his discussion, guidance, and critical comments on this article. The authors thank Drs. Hina Chaudhry, Kevin Costa, Martin Schwarz (Cardiovascular Research Center, Mount Sinai Heart, New York) and Prof. Dr. Ihor Lemischka, (Black Family Stem Cell Institute, Mount Sinai School of Medicine, New York) for comments and reviewing the article. This study was supported in part by R01 HL-078691, HL-071763, HL-080498, and HL-083156 grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, and by a grant from the Leducq Trans-Atlantic Network grant (to R. J. H.). I.K. was supported by the Louis B. Mayer Foundation. Confocal laser scanning microscopy performed at the MSSM-Microscopy Shared Resource Facility was supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504) and an NIH shared instrumentation grant (1 S10 RR0 9145-01).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Aasen T. Raya A. Barrero M.J., et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008;26:1276–1282. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77, 88: Screening for fetal chromosomal abnormalities. Obstet. Gynecol. 2007;109:217–228. doi: 10.1097/00006250-200701000-00054. [DOI] [PubMed] [Google Scholar]
- Aoi T. Yae K. Nakagawa M., et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Baker M. Embryonic-like stem cells from a single human hair. Nat. Rep. Stem Cells. 2008. Published online: dol:10.1038/stemcells.2008.142.
- Brambrink T. Foreman R. Welstead G.G., et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B.W. Markoulaki S. Hanna J., et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl. Acad. Sci. USA. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P. Bartsch G. Siddiqui M.M., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Ebert A.D. Yu J. Rose F.F., et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D. Birkhoff G. Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat. Rev. Mol. Cell Biol. 2008;9:505–516. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauza O. Amniotic fluid and placental stem cells. Best Practice Res. Clin. Obstet. Gynecol. 2004;18:877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gosden CM. Amniotic fluid cell types and culture. Br. Med. Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- Hipp J. Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- In't Anker P.S. Scherjon S.A. Kleijburg-van der Keur C., et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K. Norrby K. Paca A., et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B. Zaehres H. Wu G., et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Li C. Zhou J. Shi G., et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum. Mol. Genet. 2009;18:4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- Lowry W.E. Richter L. Yachechko R., et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N. Hochedlinger K. Induced pluripotency of mouse and human somatic cells. Cold Spring Harbor Symp. Quant. Biol. 2008;73:157–162. doi: 10.1101/sqb.2008.73.017. [DOI] [PubMed] [Google Scholar]
- Mali P. Ye Z. Hommond H.H., et al. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- Meissner A. Wernig M. Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Nakagawa M. Koyanagi M. Tanabe K., et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Park I.H. Lerou P.H. Zhao R., et al. Generation of human-induced pluripotent stem cells. Nat. Protoc. 2008;3:1180–1186. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- Polgar K. Sipka S. Abel G., et al. Neutral-red uptake by amniotic-fluid macrophages in neural-tube defects: a rapid test. N. Engl. J. Med. 1984;310:1463–1464. doi: 10.1056/nejm198405313102217. [DOI] [PubMed] [Google Scholar]
- Polgar K. Adany R. Abel G., et al. Characterization of rapidly adhering amniotic fluid cells by combined immunofluorescence and phagocytosis assays. Am. J. Hum. Genet. 1989;45:786–792. [PMC free article] [PubMed] [Google Scholar]
- Priest R.E. Marimuthu K.M. Priest J.H. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab. Invest. 1978;39:106–109. [PubMed] [Google Scholar]
- Prusa A.R. Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med. Sci. Monit. 2002;8:RA253–RA257. [PubMed] [Google Scholar]
- Prusa A.R. Marton E. Rosner M., et al. OCT4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Hu Y. Epidermal neural crest stem cells (EPI-NCSC) and pluripotency. Stem Cell Rev. 2008;4:256–260. doi: 10.1007/s12015-008-9042-0. [DOI] [PubMed] [Google Scholar]
- Siegel N. Rosner M. Hanneder M., et al. Human amniotic fluid stem cells: a new perspective. Amino Acids. 2008;35:291–293. doi: 10.1007/s00726-007-0593-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wernig M. Meissner A. Foreman R., et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Woltjen K. Michael I.P. Mohseni P., et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Ye L. Chang J.C. Lin C., et al. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc. Natl. Acad. Sci. USA. 2009;106:9826–9830. doi: 10.1073/pnas.0904689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Vodyanik M.A. Smuga-Otto K., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]