Abstract
Circulating tumor cells (CTC) have been identified in several human malignancies, including malignant melanoma. However, whether melanoma CTC are tumorigenic and cause metastatic progression is currently unknown. Here we isolate for the first time viable tumorigenic melanoma CTC and demonstrate that this cell population is capable of metastasis formation in human-to-mouse xenotransplantation experiments. The presence of CTC among peripheral blood mononuclear cells (PBMC) of murine recipients of subcutaneous (s.c.) human melanoma xenografts could be detected based on mRNA expression for human GAPDH and/or ATP-binding cassette subfamily B member 5 (ABCB5), a marker of malignant melanoma-initiating cells previously shown to be associated with metastatic disease progression in human patients. ABCB5 expression could also be detected in PBMC preparations from human stage IV melanoma patients but not healthy controls. The detection of melanoma CTC in human-to-mouse s.c. tumor xenotransplantation models correlated significantly with pulmonary metastasis formation. Moreover, prospectively isolated CTC from murine recipients of s.c. melanoma xenografts were capable of primary tumor initiation and caused metastasis formation upon xenotransplantation to secondary murine NOD-scid IL2Rγnull recipients. Our results provide initial evidence that melanoma CTC are tumorigenic and demonstrate that CTC are capable of causing metastatic tumor progression. These findings suggest a need for CTC eradication to inhibit metastatic progression and provide a rationale for assessment of therapeutic responses of this tumorigenic cell population to promising emerging melanoma treatment modalities.
Keywords: tumor xenotransplantation, immunocompromised mice, interleukin-2 receptor gamma, circulating tumor cells, metastasis, melanoma
1. Introduction
Human melanoma is the most malignant skin cancer, with increasing incident rates worldwide. Although melanoma can be cured by surgical resection before the cancer has spread, metastatic melanoma is one of the most aggressive and drug-resistant cancers with very poor overall survival [1; 2; 3]. A more detailed understanding of the cellular mechanisms that drive metastatic melanoma progression would improve the development and assessment of more effective new or emerging treatment modalities that target disseminated disease.
In metastatic dissemination, primary tumor cells invade basement membranes, intravasate into blood or lymphatic vessels, reach secondary sites through the circulation, and extravasate and colonize again [4]. In recent years, circulating tumor cells (CTC) have been actively investigated as potential prognostic and therapeutic biomarkers [5]. In melanoma patients, CTC were first detected based on expression of tyrosinase RNA in the peripheral circulation [6]. Melanoma CTC have been found to correlate significantly with tumor stage and patient survival [7], and can serve as a biomarker for predicting antitumor responses in patients undergoing systemic therapies [8].
Different strategies have been employed to detect and characterize melanoma CTC, and several genes have been investigated as melanoma CTC biomarkers, including genes involved in melanin biosynthesis or genes encoding melanoma-associated antigens [9]. However, isolation of viable CTC, a prerequisite for functional studies in tumor initiation and metastasis of this cell population, has not been achieved to date. While observations of prognostic relevance of melanoma CTC have suggested that this malignant subpopulation might indeed be tumorigenic and responsible for the development of distant metastases, this hypothesis has not been experimentally proven to date.
Melanoma-initiating cells [10; 11] are minority subpopulations in which clinical virulence resides as a consequence of unlimited self-renewal capacity, resulting in inexorable tumor progression [1; 10; 11]. Our laboratory recently showed human malignant melanoma initiating cells (MMIC) to express the targetable biomarker [10; 12; 13; 14] and multidrug resistance mediator [14; 15], ATP-binding cassette subfamily B member 5 (ABCB5) [10]. In this study, proof of principle of immune-mediated MMIC destruction and consequent inhibition of tumor growth was demonstrated [10]. More recently, we have shown that MMIC employ mechanisms to thwart endogenous anti-tumor immunity [16], which could account for the observed relative enrichment of MMIC in patient lymph node (LN) metastases compared to visceral metastases [10]. Whether ABCB5 might also be expressed by melanoma CTC hypothesized to exert important roles in metastatic progression, has not been evaluated to date.
Here we provide initial evidence for the existence of circulating melanoma cells that are capable of causing tumor initiation and metastatic disease progression, considerably strengthening the rationale for ongoing clinical evaluations of melanoma CTC as a biomarker for disease progression, prognosis and outcome. Furthermore, we find that CTC can be comprised of heterogeneous cancer populations, including ABCB5-positive subsets previously associated with clinical metastatic melanoma progression. This additional result provides a rationale to examine in future studies whether specific CTC subsets might contribute differentially to tumor initiation and metastatic disease progression, of relevance to the selection of appropriate CTC markers for diagnostic, prognostic or therapeutic purposes.
2. Materials and methods
Melanoma specimens
Clinical melanoma cells were derived from surgical specimens according to an Institutional Review Board–approved research protocol (University of Würzburg, Germany). Metastatic human C8161 melanoma cells [17] were provided by Dr. Mary Hendrix (Children’s Memorial Research Center, Chicago, IL) and metastatic human LOX and FEMX1 melanoma cells [18] were provided by Dr. Udo Schumacher (University Hospital Hamburg-Eppendorf, Hamburg, Germany). Melanoma cell lines were cultured as described [15]. Fluorescent protein-expressing cells were generated by stable transfection of C8161, LOX, or clinical melanoma cells with expression vectors encoding red fluorescent protein (RFP) or green fluorescent protein (GFP) genes (BD Bioscience, Franklin Lakes, NJ) as described [10]. Individual clones were generated for RFP-labeled LOX or clinical melanoma cells. Fluorescence-transfected C8161 cells were purified for high RFP or GFP expression using a Cytomation MoFlo sorter (Dako,Carpinteria, CA).
Animals
Athymic nude mice were purchased from Harlan Laboratories (Indianapolis, IN). Nonobese diabetic-severe combined immune deficiency (NOD-scid) mice and NOD-scid IL2Rγnull mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under defined conditions in accordance with the institutional guidelines of Children’s Hospital Boston and Harvard Medical School. Experiments were performed according to experimental protocols approved by Children’s Hospital Boston.
Human-to-mouse xenotransplantation studies
Human melanoma cells (C8161, LOX, FEMX1 or clinical specimen-derived cells) were injected subcutaneously (s.c.) into the right flank of recipient NOD-scid or nude mice (1×106 in 100 μL of PBS per inoculum). Tumor formation was assayed weekly up to 11 weeks and the development of pulmonary metastases was determined by examining hematoxylin-eosin (H&E)-stained paraffin-embedded tissue sections prepared by serial sectioning of the entire lungs of all primary tumor-bearing mice.
Antibodies
The murine IgG1κ anti-ABCB5 monoclonal antibody (mAb) 3C2-1D12 [10; 15; 16; 19] was used in the herein reported studies. Isotype control mAb and allophycocyanin (APC)-conjugated murine anti-human HLA-ABC IgG1 Ab were purchased from BD Bioscience. APC-conjugated isotype murine IgG1 mAb was purchased from Miltenyi Biotec (Auburn, CA). APC-conjugated donkey anti-mouse IgG was purchased from eBioscience (San Diego, CA).
Isolation of circulating melanoma cells
NOD-scid IL2Rγnull mice were inoculated s.c. with RFP-transfected human melanoma cells (2×103 to 2×104 cells/inoculum). Mice were euthanized 5 to 7 weeks upon xenotransplantation and whole blood specimens were collected through heart acupuncture. PBMC were isolated as described [20] and stained with APC-conjugated anti-human HLA-ABC Ab or APC-conjugated isotype control Ab. To isolate CTC, RFP-positive and/or human HLA-ABC antigen-positive cells were purified from cell suspensions using a MoFlo high-speed sorter and collected in growth factor-reduced matrigel (BD Biosciences). Cultured RFP-transfected human melanoma cells or PBMC from tumor-free NOD-scid IL2Rγnull mice were used as positive or negative controls, respectively. Matrigel preparations containing sorted melanoma CTC were injected s.c. into the right flank of secondary NOD-scid IL2Rγnull mice (first-generation CTC xenotransplantation). Tumor formation was monitored weekly for 7 weeks and subsequently, second-generation CTC were isolated as above and xenografted s.c. to tertiary NOD-scid IL2Rγnull mice. Primary tumors and lungs from xenografted mice were collected and the expression of RFP was determined following counterstaining with DAPI by fluorescence microscopy as described [10].
Flow cyotometry
ABCB5 protein expression in human melanoma cultures, primary tumors, distant metastases and CTC was determined by flow cytometry as described [10; 15; 16; 19]. First, NOD-scid IL2Rγnull mice were inoculated s.c. with C8161/RFP or C8161/GFP cells (2×104 cells/inoculum). Subsequently, primary tumors, lungs, and enlarged axillary lymph nodes were harvested and dissociated as described [10]. Cell suspensions were incubated with anti-ABCB5 mAb or isotype control mAb followed by counterstaining with APC-conjugated secondary antibody, and dual- or triple-color flow cytometry was subsequently performed at the FL1 (GFP) or FL2 (RFP) spectra, and the FL4 (APC) spectrum as described [10]. Single-cell suspensions from tumor-free mice were used as negative controls to exclude autofluorescent cells. Melanoma cells were identified as cells positive for RFP or GFP. Statistical differences in the percentages of ABCB5-positive melanoma cells were determined using ANOVA and Bonferroni’s Multiple Comparison Test, with P<0.05 considered statistically significant.
RNA extraction, reverse transcription, and real-time quantitative PCR
Murine blood specimens were collected by cardiac puncture and erythrocytes were lysed using ACK lysis buffer (Invitrogen). Human blood specimens were obtained from metastatic melanoma patients or healthy controls in compliance with institutional IRB regulations and PBMC were isolated as described [21]. Total RNA was extracted and transcribed into complementary DNA (cDNA), and real-time quantitative PCR was performed as described [10]. Human GAPDH expression in PBMC samples of murine melanoma xenograft recipients was determined using a TaqMan assay (Hs99999905_ml, Applied Biosystems) and human ABCB5 expression was assayed using SYBR Green chemistry, with the use of 5’-ATGATGTGACTGATGAAGAGATGGAGAGA-3’ and 5’-CTCTGTTTCTGCCCTCCACTCATTTGAGC-3’ as forward and reverse primers, respectively. A Ct value of 33.5 was set as the threshold for ABCB5 positivity, as determined by using PBMC from tumor-free NOD-scid mice as negative controls. For ABCB5 detection in human metastatic melanoma patient blood specimens, a TaqMan assay (Hs00698751_m1, Applied Biosystems) was performed, with use of a ΔCt value<15 (compared to b-actin expression) as a threshold for significant ABCB5 mRNA detection, as determined by using PBMC from healthy donors, previously found negative for ABCB5 expression [19], as negative controls. Statistically significant associations between GAPDH and/or ABCB5 detection and the presence of melanoma metastases were determined using the Fisher’s Exact Test and/or Relative Risk analyses, with P<0.05 considered significant.
3. Results
Isolation of tumorigenic circulating melanoma cells
In order to demonstrate tumorigenic potential of human melanoma CTC, we developed a novel metastatic melanoma xenotransplantation model whereby fluorescent transgene-expressing metastatic human melanoma cells are xenografted s.c. to immunodeficient mice and, upon primary tumor formation and systemic spreading, human melanoma cells are isolated from the murine circulation for use in further tumorigenicity experiments (Fig.1A). In order to maximize tumor take resulting from xenotransplantation of relatively low CTC numbers in this model, we grafted human melanoma CTC isolated from murine PBMC into severely immune-compromised NOD-scid IL2Rγnull mice, using matrigel [11; 22]. To maximize the efficiency of melanoma CTC detection for flow cytometric cell sorting, melanoma cells were transfected with the fluorescent transgene RFP, and CTC that expressed the RFP marker, or the human MHC Class-I antigen HLA-ABC, or both markers, were flow cytometrically sorted from the murine circulation (Fig.1B). These detection criteria were posited to maximize human CTC detection, because of the possibility of gradual loss of RFP transgene expression during prolonged in vivo tumorigenic growth, and because of the possibility that human melanoma cells might not invariably express human MHC Class I antigens [16]. Subcutaneous xenotransplantation of human melanoma cells (2×103-2×104 cells/inoculum) resulted in consistent primary tumor formation in NOD-scid IL2Rγnull primary recipient mice (n=18/18; Table 1). CTC isolated from the circulation of n=11 primary tumor-bearing mice (first-generation CTC) resulted in tumor formation in 7 of 11 secondary recipients upon s.c xenotransplantation of purified CTC ranging in numbers from 81 to 4.2×103, including 4 of 4 murine recipients of clinical specimen-derived melanoma CTC (Fig.1C, Table 1). Further re-isolated CTC from secondary xenograft recipient (second-generation CTC) performed for C8161 melanomas were likewise capable of tumor initiation in 2 of 2 tertiary xenograft recipients (Fig.1C, Table 1). Purified CTC were not only capable of primary tumor initiation (Fig.1C,D), but also of metastasis formation upon s.c. xenotransplantation (Fig.1D). These results demonstrate a capacity of circulating human melanoma cells for in vivo initiation of tumorigenic growth and metastatic progression.
Figure 1. Isolation of tumorigenic circulating melanoma cells.
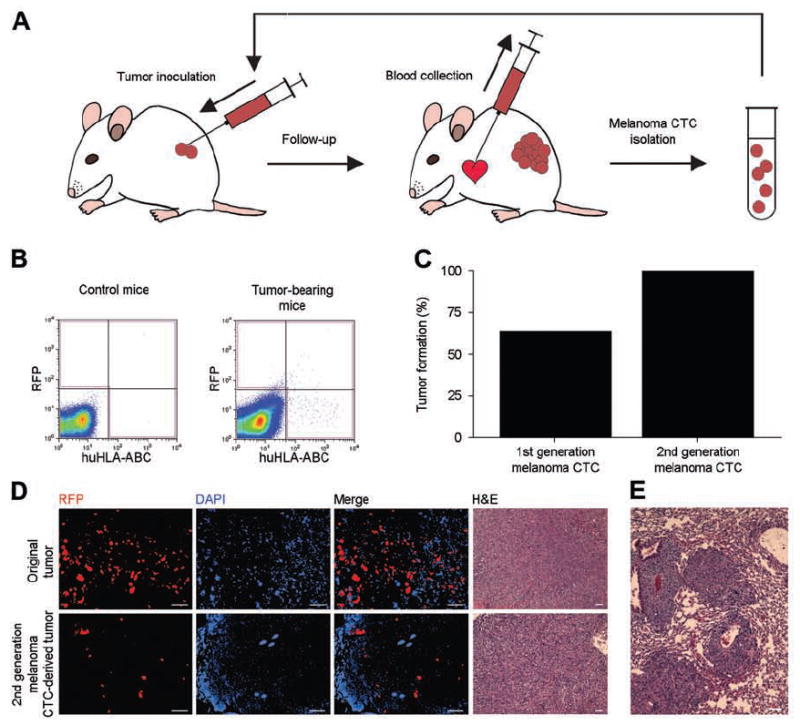
A. Diagram depicting the procedure for CTC isolation and serial xenotransplantation. B. Representative flow cytometry results for RFP and human HLA-ABC antigen detection in blood specimens from tumor-free control mice (left) and mice xenografted with RFP-transfected human melanoma cells (right). The gate used for CTC sorting is shown in red. C. Tumor initiation capacity of CTC in NOD-scid IL2Rγnull mice. D. Top: Representative fluorescent and light microscopy of a tumor derived from RFP-transfected melanoma cells. Bottom: Representative fluorescent and light microscopy of a tumor derived from second-generation CTC. Scale bar, 100 μm. E. Representative image of a pulmonary metastasis derived from second-generation melanoma CTC. Scale bar, 100 μm.
Table 1.
Tumorigenicity of melanoma CTC
| ABCB5 positivity (%) | Original s.c. inoculum-derived tumors/ xenografted mice | First-generation melanoma CTC-derived tumors / xenografted mice | Second-generation melanoma CTC-derived tumors / xenografted mice | |
|---|---|---|---|---|
| Clinical specimen | 1.1 | 4/4 (2×103)* | 4/4 (81 - 3.9×103)* | - |
| C8161 | 1.4 | 9/9 (2×104)* | 2/2 (2.1×103)* | 2/2 (1.4×103 – 2×103)* |
| LOX | 1.8 | 5/5 (2×103)* | 1/5 (2.8×102- 4.2×103)* | - |
| Aggregate tumor formation | 18/18 | 7/11 | 2/2 | |
Numbers in parentheses indicate the number of cells inoculated per mouse
Melanoma CTC contain ABCB5-positive subpopulations associated with metastatic disease progression in human patients
We and others have demonstrated that the chemoresistance mediator ABCB5 [14; 15] marks immunoevasive [16] subpopulations of MMIC that correlate with metastatic malignant melanoma progression in human patients [10; 12; 13] and experimental xenotransplantation models [22]. We therefore examined whether ABCB5 might also be expressed by melanoma CTC found capable of tumor initiation and metastasis formation in this study. Similar to results of previous expression analyses in cultured melanoma cells and patient-derived primary tumor and metastatic specimens, ABCB5 was expressed on melanoma subpopulations ranging from 1.0% to 1.8% in clinical specimen-derived melanoma cells and established C8161, FEMX1 and LOX melanoma cultures (Fig.2A). Further investigation of ABCB5-positive MMIC frequencies at each step of tumorigenesis and metastatic progression revealed similarly low percentages of ABCB5-positive cells among melanoma cells derived from primary tumors and axillary lymph node (LN) metastases in the C8161 xenotransplantation model. Interestingly, ABCB5-positive melanoma cell frequency was significantly increased among melanoma CTC (33.4±7.4%) compared to the frequencies detected among melanoma cells in xenograft inocula, resultant primary tumors, LN metastases or pulmonary metastases (P<0.001, P<0.001, P<0.05 and P<0.001, respectively) (Fig.2B). Thus, circulating melanoma cells represent heterogeneous cell populations that include ABCB5-positive subsets previously associated with clinical metastatic progression [10].
Figure 2. Circulating melanoma cells contain ABCB5-positive tumor subpopulations.

A. Flow cytometry plots depicting ABCB5 expression in melanoma cell inocula for a clinical specimen and C8161, FEMX1 and LOX melanoma cells. The lower panels show isotype control-staining. B. Flow cytometrically-determined percentages of ABCB5-positive melanoma cells in cell inocula and resultant primary tumors, lymph node metastases, melanoma CTC, and pulmonary metastases. Means±SE of n=3-11 samples/group are illustrated.
Melanoma CTC detection correlates with metastatic progression
Based on our demonstration that melanoma CTC can cause tumor formation and metastatic progression in human-to-mouse xenotransplantation experiments, we examined whether detection of melanoma CTC might correlate significantly with metastatic tumor progression. Human melanoma cells (C8161, LOX, FEMX1 or clinical specimen-derived cells) were injected s.c. into the right flank of recipient NOD-scid or nude mice (1×106 in 100 μL of PBS per inoculum), and the presence or absence of CTC and pulmonary metastases was assayed in n=32 xenograft recipients with established primary tumors. The presence of CTC among PBMC of murine recipients of s.c. human melanoma xenografts was established based on mRNA detection for human GAPDH and/or human ABCB5, a marker of drug resistant and immunoevasive MMIC [1; 2; 10; 15; 16]. Absence of CTC among PBMC preparations was defined as dual negativity for human GAPDH and ABCB5 expression. The presence or absence of pulmonary macro- or micrometastases was determined by macroscopic tissue examination and microscopic examination of serial tissue sections of the entire lungs of all primary tumor-bearing mice.
Among 27 CTC-positive primary tumor-bearing mice, 25 (92.6%) had pulmonary metastases, whereas only one of five (20%) of CTC-negative primary tumor-bearing mice showed evidence for pulmonary metastases (Fig.3A, B), demonstrating a significant association between CTC detection and the presence of pulmonary metastases (P<0.01). Mice with metastatic disease hereby exhibited human GAPDH positivity in 92% of cases, whereas mice without metastases were human GAPDH-positive in only 33% of cases (P<0.05) (Fig.3C). Regarding human ABCB5 detection, only mice with metastatic disease showed ABCB5 positivity (19% of metastatic cases), whereas none of the mice without metastases exhibited ABCB5 positivity (P<0.05) (Fig.3D), indicating that ABCB5 might represent a novel relatively specific, albeit relatively insensitive molecular marker for the detection of CTC that correlate with metastasis formation. In support of this possibility, only PBMC specimens derived from n=9 stage IV metastatic melanoma patients showed ABCB5 positivity in 3 of 9 cases, whereas none of the PBMC specimens derived from n=5 healthy human controls were found to express ABCB5 (P<0.05), consistent with previously determined ABCB5 mRNA negativity of human PBMC [19].
Figure 3. Melanoma CTC detection correlates with metastatic progression.

A. Correlation between pulmonary metastases in primary tumor-bearing mice and CTC detection. B. Representative morphology of a primary tumor (left) and pulmonary metastases (right) following xenotransplantation of patient-derived melanoma cells. Scale bar, 100 μm. C. Detection of human GAPDH, and D. human ABCB5 expression in PBMC derived from primary tumor-bearing mice with or without the presence of pulmonary metastases. E. ABCB5 gene expression in PBMC derived from metastatic melanoma patients or healthy humans.
4. Discussion
CTC are thought to be important mediators of tumor dissemination and metastatic disease progression in human patients. They represent unique malignant subpopulations that emigrate from the cellular microenvironment and extracellular matrix support of the primary tumor site, survive in a fluid dynamic environment in the context of immunocompetent PBMC, and possess the capacity to eventually home to and establish tumorigenic growth in secondary sites of metastasis. Not all CTC might necessarily be alike; some might readily adapt to a new microenvironment and reinitiate cellular proliferation causing metastasis, while others might remain dormant for prolonged time periods or ultimately die [23; 24]. Moreover, while some CTC might drive metastatic dissemination of the primary tumor, others have been shown to contribute to primary tumor growth through self-seeding [25].
CTC have previously been detected and phenotypically characterized in human malignant melanoma, using melanoma-specific or melanoma-associated molecular markers [9], and CTC detection has been shown to correlate with clinical tumor progression [26]. However, the existence of tumorigenic melanoma CTC capable of driving metastatic progression, a concept that underlies the posited prognostic value of CTC detection in this malignancy, has not been directly demonstrated prior to this study. Therefore, our finding that melanoma CTC are capable of tumor initiation and metastatic progression significantly strengthens the rationale for further evaluating CTC as a biomarker for melanoma progression, prognosis and outcome, and as potential therapeutic targets in this malignancy. Additionally, the prospective isolation of viable melanoma CTC promises to open new avenues for the functional study and further biological characterization of these cell populations, and to potentially lead to improved methods for evaluating therapeutic responses of melanoma CTC.
A second significant insight of our study is the finding that melanoma CTC represent phenotypically heterogeneous cell populations. This is indicated by our result of heterogeneous expression of the MMIC marker ABCB5 and MHC class I antigens on specific subsets of CTC, similar to previous findings in human primary tumor- or metastasis-derived melanoma cells [10; 16]. Intriguingly, ABCB5-positive tumor cells were >20-fold enriched among melanoma CTC compared to either primary tumors or pulmonary metastases. These results provide a possible explanation for the previously established role of ABCB5 as a marker of metastatic progression in human melanoma patients [10; 12]. Moreover, because ABCB5-positive MMIC and related melanoma initiating cell populations [11] often do not express melanoma-associated antigens such as MART-1 or tyrosinase [11; 16], our results suggest that caution should be applied when selecting melanoma CTC markers for diagnostic, prognostic or therapeutic purposes, as not all disease-relevant CTC populations might be measured or targeted when only select tumor antigens are considered. In addition, our results provide a rationale to examine in future studies whether distinct subpopulations of melanoma CTC might differ in their capacity for tumorigenic growth and metastatic progression.
Lastly, our results suggest that ABCB5 might represent a useful novel molecular marker for the detection and monitoring of tumorigenic melanoma CTC populations associated with metastatic disease, because ABCB5 detection in CTC was a highly specific predictor for the presence of metastatic disease in the here-examined experimental metastatic melanoma model. Moreover, our results revealed selective ABCB5 expression in PBMC of a subset of metastatic human melanoma patients but not in PBMC derived from healthy human controls as previously described [19], underlining the melanoma-specific expression pattern of this MMIC-associated gene. These findings resemble recent observations by others of melanoma-specific ABCB5 expression in a subset of patient sentinel lymph node biopsy specimens, but absent expression in all of a series of non-melanoma case-derived control lymphatic tissues [13].
In summary, our results show that melanoma CTC contain tumorigenic cancer cells and demonstrate that CTC are capable of causing metastatic tumor progression. These findings suggest a need for CTC eradication to inhibit metastatic progression and provide a rationale for assessment of therapeutic responses of this cell population to promising emerging melanoma treatment modalities.
Acknowledgments
This work was supported by by the NIH/NCI (grants 1RO1CA113796 and 1R01CA138231 to M.H.F. and grant 2P50CA093683 (Specialized Program of Research Excellence in Skin Cancer) to M.H.F and G.F.M.
Abbreviations
- CTC
Circulating tumor cells
- MMIC
malignant melanoma initiating cells
- PBMC
peripheral blood mononuclear cells
- ABCB5
ATP-binding cassette subfamily B member 5
- s.c.
subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J, Frank MH. Tumor initiation in human malignant melanoma and potential cancer therapies. Anticancer Agents Med Chem. 2010;10:131–6. doi: 10.2174/187152010790909254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 6.Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–9. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 7.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–13. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi K, O’Day SJ, Boasberg P, Atkins MB, Wang HJ, Gonzalez R, Lewis K, Thompson JA, Anderson CM, Lutzky J, Amatruda TT, Hersh E, Richards J, Weber JS, Hoon DS. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16:2402–8. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medic S, Pearce RL, Heenan PJ, Ziman M. Molecular markers of circulating melanoma cells. Pigment Cell Res. 2007;20:80–91. doi: 10.1111/j.1600-0749.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010 April 14;:1–5. doi: 10.1016/j.jss.2010.03.043. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Gazzaniga P, Cigna E, Panasiti V, Devirgiliis V, Bottoni U, Vincenzi B, Nicolazzo C, Petracca A, Gradilone A. CD133 and ABCB5 as stem cell markers on sentinel lymph node from melanoma patients. Eur J Surg Oncol. 2010 doi: 10.1016/j.ejso.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Cheung ST, Cheung PF, Cheng CK, Wong NC, Fan ST. Granulin-Epithelin Precursor and ATP-Dependent Binding Cassette (ABC)B5 Regulate Liver Cancer Cell Chemoresistance. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 16.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seftor RE, Seftor EA, Stetler-Stevenson WG, Hendrix MJ. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993;53:3411–5. [PubMed] [Google Scholar]
- 18.Thies A, Mauer S, Fodstad O, Schumacher U. Clinically proven markers of metastasis predict metastatic spread of human melanoma cells engrafted in scid mice. Br J Cancer. 2007;96:609–16. doi: 10.1038/sj.bjc.6603594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 20.Izawa A, Schatton T, Frank NY, Ueno T, Yamaura K, Pendse SS, Margaryan A, Grimm M, Gasser M, Waaga-Gasser AM, Sayegh MH, Frank MH. A novel in vivo regulatory role of P-glycoprotein in alloimmunity. Biochem Biophys Res Commun. 2010;394:646–52. doi: 10.1016/j.bbrc.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank MH, Denton MD, Alexander SI, Khoury SJ, Sayegh MH, Briscoe DM. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J Immunol. 2001;166:2451–9. doi: 10.4049/jimmunol.166.4.2451. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga-Kalabis M, Martinez G, Nguyen TK, Kim D, Santiago-Walker A, Roesch A, Herlyn M. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010 doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 24.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 25.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samija I, Lukac J, Maric-Brozic J, Buljan M, Alajbeg I, Kovacevic D, Situm M, Kusic Z. Prognostic value of microphthalmia-associated transcription factor and tyrosinase as markers for circulating tumor cells detection in patients with melanoma. Melanoma Res. 2010 doi: 10.1097/CMR.0b013e32833906b6. [DOI] [PubMed] [Google Scholar]


