Summary
Virus-cell membrane fusion requires a critical transition from positive to negative membrane curvature. St. Vincent et al., in PNAS (St Vincent, et al., 2010), designed a class of antivirals that targets this transition. These Rigid Amphipathic Fusion Inhibitors are active against an array of enveloped viruses.
Comment
Viruses are either membrane-enveloped or not. Although viral membranes originate from cellular membranes, they can be biochemically and physically distinct. Their relative lipid, cholesterol and protein content can be different from a healthy cell’s membrane, and the proper balance between these components are critical for maintaining the virus’ size and shape, and ultimately its ability to fuse with cellular membranes. While the specific mechanics of fusion may differ between classes of viral fusion proteins, some general features are common to this process (Harrison, 2008). Viral membranes have a high positive curvature relative to the host cell membrane. After attachment/binding of the viral particle and triggering of the fusion protein, which requires specific interactions between viral envelope glycoproteins and cognate receptors, virus-cell fusion proceeds through a hemifusion intermediate comprising of a lipid stalk where the outer leaflet of the viral membrane phospholipid bilayer is mixed with its counterpart on the host cell membrane (Chernomordik, et al., 2006) (Figure 1). This step of lipid mixing between viral and cellular membranes is characterized by a transition from positive to negative curvature. The unfavourable energetics of this transition is overcome by the energy released from receptor-induced conformational changes in the viral envelope glycoproteins and cellular membrane destabilization mediated by the viral fusion proteins (Harrison, 2008). Complete fusion (bilayer lipid mixing) and pore formation/enlargement ultimately allows the virus to deliver its content inside the target cell, but this final step requires overcoming another energetic hurdle (Chernomordik, et al., 2006).
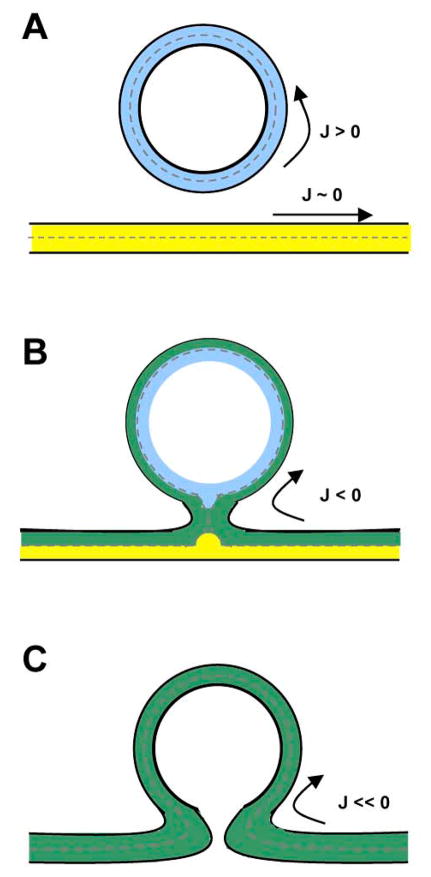
Figure 1. Schematic cross section view of viral-cell membrane fusion.
(A) The viral membrane (in blue) has a strong positive curvature (J > 0) and the cellular membrane (in yellow) looks virtually flat in comparison (J ~ 0). After juxtaposition of membranes and triggering of the fusion proteins (not depicted) (B) the outer leaflets of both membranes start fusing and a stalk, with a highly negative curvature (J ≪ 0) is formed. This step called hemifusion allows for lipid mixing that involves only the outer leaflets of the viral and cellular membrane bilayer (in green). (C) The final step is the pore formation and enlargement, which will ultimately lead to complete bilayer and content mixing (delivery of viral content in the cell’s cytoplasm).
Based on the biophysics of virus-cell membrane fusion, St. Vincent et al. designed and evaluated a class of potentially broad-spectrum antivirals, termed rigid amphipathic fusion inhibitors (RAFIs) (St Vincent, et al., 2010), that can interfere with the positive to negative curvature transitions central to virus-cell fusion (Figure 1). It is known that “inverted-cone” shaped molecules (e.g. lysophospholipids), inducing a positive curvature in membranes, are able to inhibit viral- and cell-cell fusion. However, these lipid-based molecules are often cytotoxic or quickly degraded. Nevertheless, agents that promote the positive curvature of a viral membrane (Figure 2) or otherwise increase the energy barrier required for positive to negative curvature transitions remain appealing as a broad-spectrum anti-viral concept. The study by St. Vincent et al. pursues this concept. The synthetic RAFIs have an “inverted cone” shape with the polar hydrophilic end larger in diameter than the hydrophobic end that inserts into the membrane (Figure 2). Active RAFIs specifically inhibit viral fusion with a mean IC50 of ~50 nM, without overt cytotoxicity (selectivity index ≫100 for most of the active ones). Importantly, RAFIs do not act as detergents: treated viruses (HSV-1) remain intact and are still able to attach to target cells via their cognate receptors. However, content- and lipid-mixing experiments showed that RAFI-treated viruses do not even progress to the hemifusion stage (Figure 1). The RAFIs specifically target membranes and are active against different unrelated enveloped viruses (HSV-1, HSV-2, HCV, VSV and Sindbis virus) albeit with different IC50s, but not non-enveloped viruses (Adenovirus and Poliovirus). Finally, RAFIs disfavor the formation of negatively curved hexagonal phase of dielaidoylphosphatidylethanolamine (DEPE) structures. In toto, the evidence is suggestive that RAFIs inhibit enveloped viral entry by acting on viral membranes and inhibiting, at least in part, the positive to negative curvature transition that is critical for productive membrane fusion (Figure 1).
Figure 2. Schematic cross section view of a viral envelope.
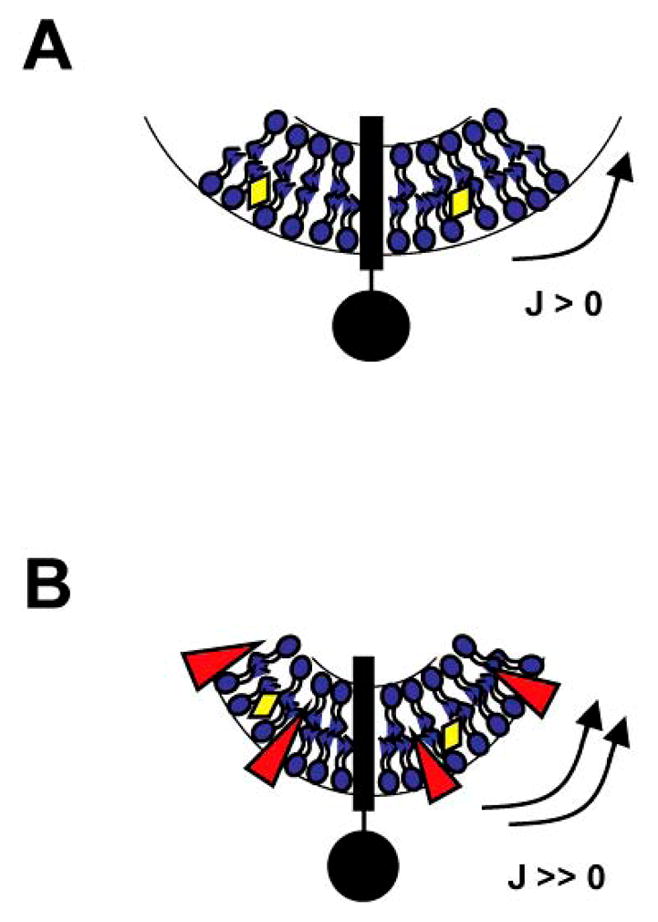
(A) The viral membrane, composed of different lipids (in blue), proteins (e.g. transmembrane envelope glycoprotein in black) and cholesterol (in yellow), has a strong positive curvature (J > 0). (B) The addition of « inverted-cone » shaped RAFIs (in red) increases the curvature (J ≫ 0) of the viral membrane by specifically inserting in the outer leaflet. RAFI-treated viruses are no longer able to fuse with cellular membranes because the energy required to form a stalk (Fig. 1B) is now too high.
The provocative studies by St. Vincent et al. bring to mind the recent study by Wolf et al. describing a series of chemically unrelated compounds (represented by LJ001) with remarkably similar properties (Wolf, et al., 2010). It remains to be determined if LJ001 also acts in a mechanistically similar fashion. However, a few differences can be noted. As opposed to the simple benzene group in the hydrophobic tail of LJ001, the hydrophobic group, perylene, present in effective RAFIs has a structure closely related to hypocrellin A, a well-known photosensitizer with antiviral and antineoplastic activities belonging to the family of quinones (Krishnamoorthy, et al., 2005). Hydrophobic photosensitizers (such as hypocrellin A) insert into membranes and upon photoactivation (activation by light) produce reactive oxygen species (ROS) that may be damaging to membrane components. It may be interesting to determine if this additional mechanism contributes to RAFIs’ antiviral activity. It seems unlikely that just the lipid-binding activity alone can account for the nanomolar activity of RAFIs given the vast excess of cellular membrane components in mixed virus-cell cultures. For that matter, it is curious that RAFIs were not tested for their ability to inhibit cell-cell fusion as even lysophospholipids have been shown to inhibit syncytia formation (Chernomordik, et al., 1995). Finally, as in the Wolf et al. study, whether the concept of a broad-spectrum therapeutic that targets the viral lipid membrane can be translated into in vivo efficacy remains to be determined.
The impressive antiviral activity of these RAFIs broadens the chemotypes that may be developed as potential broad-spectrum antivirals. Broad-spectrum antivirals that target viral membranes have been reported although mechanistic studies have not been as exhaustive. Docosanol (Abreva), a long chain saturated alcohol, seems to target viral membranes and inhibits a variety of enveloped viruses (Katz, et al., 1994; Marcelletti, et al., 1996) although it is unclear why the compound would still be active when pre-incubated with cells alone. Nevertheless, docosanol has been developed as a topical microbicide for treating herpes cold sores. Cosalane also shows activity against some enveloped viruses (e.g. HIV and HSV) although it seems to have unfavourable bioavailability and pharmacokinetic properties that may limit its in vivo utility (Zhan, et al., 2010). More recently, Bavituximab, a monoclonal antibody against phosphatidylserine, has shown in vitro and in vivo activity against multiple viruses (Soares, et al., 2008) although it is not yet clear how generalizable this strategy is: what is the spectrum of viruses that externalize phosphatidylserine? Nevertheless, our increasing understanding of the virus-cell fusion process suggests that physical and physiological differences that exist between viral and cellular membranes may be exploited for the development of antiviral therapeutics. A particularly appealing notion that underlies this antiviral concept is that viral membrane targeting agents would necessarily limit the development of resistance, as it is not even conceptually clear how such resistance may develop. Thus, targeting viral membranes represents an exciting new paradigm to explore regarding the development of broad-spectrum antivirals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References (10 max…)
- Chernomordik L, Leikina E, Cho MS, Zimmerberg J. Journal of virology. 1995;69:3049–3058. doi: 10.1128/jvi.69.5.3049-3058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Zimmerberg J, Kozlov MM. The Journal of cell biology. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC. Nature structural & molecular biology. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DH, Marcelletti JF, Pope LE, Khalil MH, Katz LR, McFadden R. Annals of the New York Academy of Sciences. 1994;724:472–488. doi: 10.1111/j.1749-6632.1994.tb38949.x. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Webb SP, Nguyen T, Chowdhury PK, Halder M, Wills NJ, Carpenter S, Kraus GA, Gordon MS, Petrich JW. Photochemistry and photobiology. 2005;81:924–933. doi: 10.1562/2004-11-23-RA-378. [DOI] [PubMed] [Google Scholar]
- Marcelletti JF, Lusso P, Katz DH. AIDS research and human retroviruses. 1996;12:71–74. doi: 10.1089/aid.1996.12.71. [DOI] [PubMed] [Google Scholar]
- Soares MM, King SW, Thorpe PE. Nature medicine. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, et al. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MC, Freiberg AN, Zhang T, Akyol-Ataman Z, Grock A, Hong PW, Li J, Watson NF, Fang AQ, Aguilar HC, et al. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P, Li Z, Liu X. Mini reviews in medicinal chemistry. 2010;10:966–976. doi: 10.2174/138955710792007222. [DOI] [PubMed] [Google Scholar]