Abstract
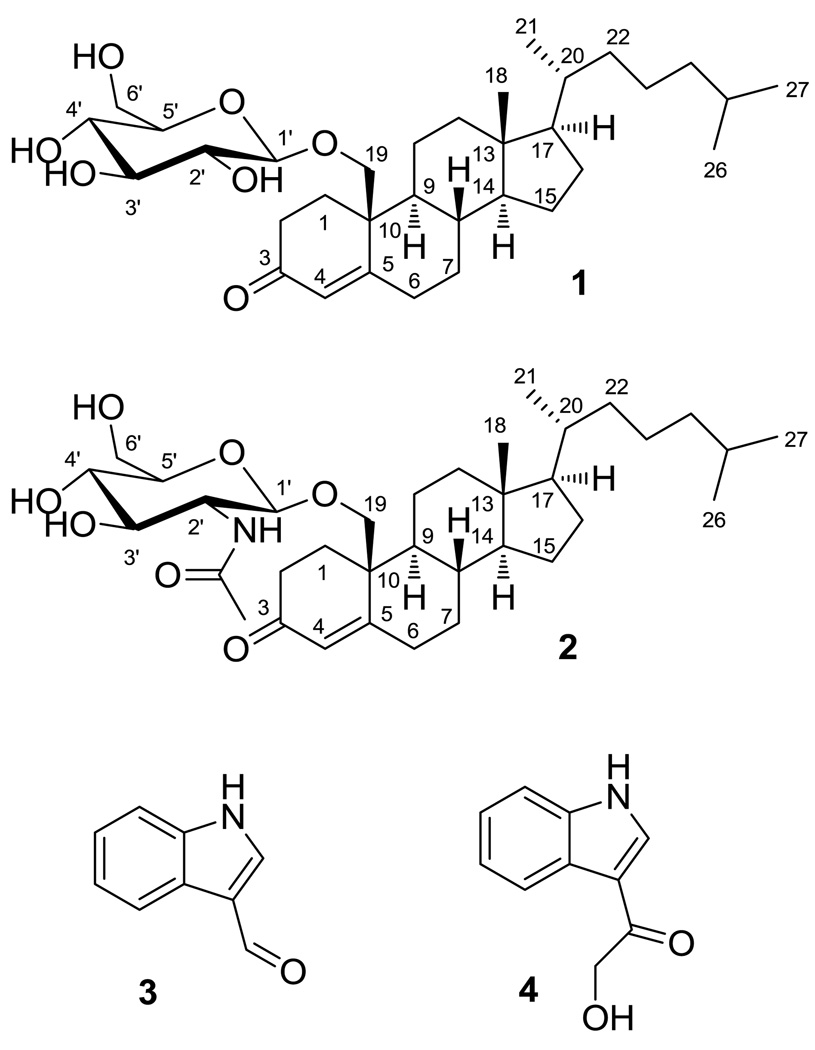
Bioactivity-guided fractionation of the extract from a Fijian red alga Peyssonnelia sp. led to the isolation of two novel sterol glycosides 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) and 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2), and two known alkaloids indole-3-carboxaldehyde (3) and 3-(hydroxyacetyl)indole (4). Their structures were characterized by 1D and 2D NMR and mass spectral analysis. The sterol glycosides inhibited cancer cell growth with mean IC50 values (for 11 human cancer cell lines) of 1.63 and 1.41 µM for 1 and 2, respectively. The most sensitive cancer cell lines were MDA-MB-468 (breast) and A549 (lung), with IC50s in of 0.71–0.97 µM for 1 and 2. Modification of the sterol glycoside structures revealed that the α,β-unsaturated ketone at C-3 and oxygenation at C-19 of 1 and 2 are crucial for anticancer activity, whereas the glucosidic group was not essential but contributed to enhanced activity against the most sensitive cell lines.
Keywords: Sterol glycoside, Peyssonnelia, cytotoxicity, anticancer
1. Introduction
Red algae (Rhodophycota) have been a rich source for a large variety of novel bioactive natural products, including many terpenes and halogenated polyphenols.1 Our efforts to discover new bioactive secondary metabolites from Fijian marine organisms have led us to collect over 300 red algal samples, representing more than 50 species found in the tropical South Pacific. Initial screening of fractionated extracts from Peyssonnelia sp. showed good anti-proliferation activity against human colon (HCT-116) and breast (MDA-MB-468) cancer cell lines, as well as moderate antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA).
Red algae of the genus Peyssonnelia are among those crustose algae that have pantropic distributions within coral reef habitats. Peyssonnelia spp. are typically found encrusting various hard substrates under ledges and in caves to deeper than 20 m.2,3 Some Peyssonnelia have been found as deep as 274 m, making them the deepest known photosynthetic organisms on earth.4 Given the difficulty of collection, there are few reports of chemical studies of Peyssonnelia spp. and their secondary metabolites remain largely unknown, although eight compounds have been reported representing terpene hydroquinone and fatty acid-derived structural classes.5–7 The Peyssonnelia species examined in this study was collected in 2006 from 30 m deep reef overhangs near Tuvuca Island, Fiji. Bioassay-guided fractionation of its methanol extract yielded two new sterol glycosides, 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) and 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2), and two known alkaloids, indole-3-carboxaldehyde (3) and 3-(hydroxyacetyl)indole (4). Synthetic modification of the sterol glycosides and anticancer testing of commercially available derivatives enabled examination of structure-activity relationships.
2. Results and discussion
2.1 Novel natural products
Freshly collected Peyssonelia sp. was frozen until processed for extraction in the lab. Frozen tissue was ground and repeatedly extracted with methanol. The crude organic extract was subjected to cytotoxicity-guided fractionation using cell lines HCT-116 and MDA-MB-468, yielding compounds 1–4.
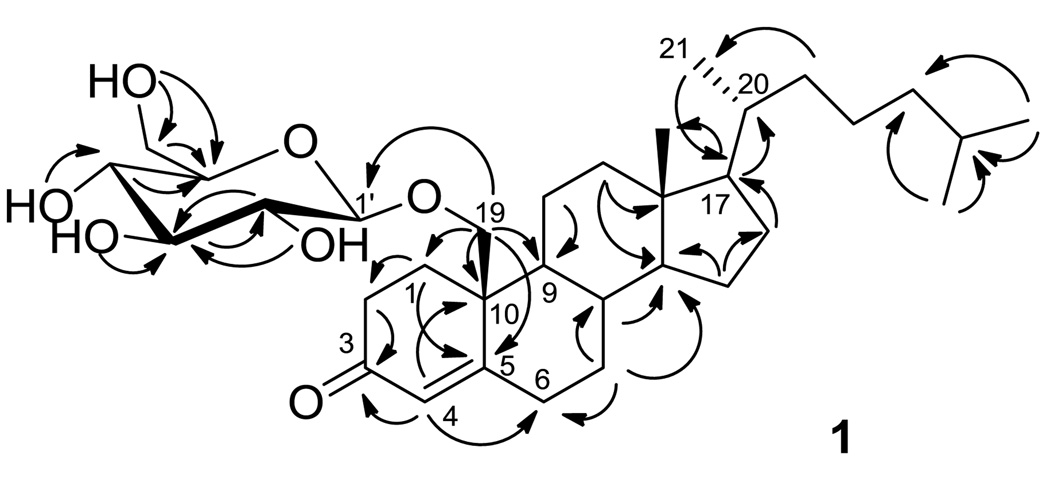
The positive ESI-MS of 1 exhibited an [M + H]+ peak at m/z 563. The molecular formula of C33H54O7 was established on the basis of its HR-ESI-MS [M + Na]+ ion at m/z 585.3689, suggesting seven degrees of unsaturation. Inspection of 1H, 13C, HSQC, and COSY NMR spectra for 1 revealed four hydroxy signals at δ 6.43–7.44, one anomeric proton at δ 4.96, and six carbinol methine/methylene protons signals at δ 4.00–4.60, in good agreement with the literature for a β-d-glucopyranoside moiety (Table 1).8,9 In the 13C NMR spectrum, a carbonyl signal at δ 200.1 (C-3), a downfield shifted quaternary carbon at δ 167.7 (C-5), and an olefinic methine at δ 126.8 (C-4) suggested an α,β-unsaturated ketone. Considering the degrees of unsaturation and the remaining 27 carbons bearing three downfield shifted tertiary carbons at δ 56.9, 56.6, and 55.0 that resembled the resonances of C-17, C-14, and C-9 of cholesterol, a steroid structure with tetracyclic cyclopentanoperhydrophenanthrene nucleus was suggested (Figure 1).10 The α,β-unsaturated ketone was assigned at C-3 based on HMBC correlations from δ 6.02 (H-4) to C-3, δ 34.0 (C-6), and δ 43.3 (C-10). Inspection of COSY and HMBC spectroscopic data concluded a 1,5-dimethylhexanyl (C-20 – C-27) function, with C-17 connected to C-20 based on HMBC correlations from δ 1.06 (H-17) to δ 36.3 (C-20), and from δ 0.95 (H2-21) to δ 56.9 (C-17) (Figure 2). HMBC correlations from oxygenated methylene protons at δ 3.96 and 4.70 (H2-19) to δ 34.7 (C-1), δ 167.7 (C-5), δ 55.0 (C-9) and C-10 concluded the aglycone structure of 19-hydroxy-cholest-4-en-3-one, a known aglycone consistent with previous reports.11,12 The glucosyl moiety was linked through C-19 based on HMBC correlations from H2-19 to the anomeric carbon δ 105.6 (C-1’).
Table 1.
NMR spectroscopic data (500 M Hz, pyridine-d5) for 1 and 2a
| 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) | 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2) | |||
|---|---|---|---|---|
| # | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 1 | 34.7 | 2.47 m, 1.72m | 34.3 | 2.48 m, 2.13m |
| 2 | 36.1 | 3.50 m, 2.47m | 35.7 | 3.00 td (15.5, 4.5), 2.42m |
| 3 | 200.1 | 199.5 | ||
| 4 | 126.8 | 6.08 s | 126.3 | 6.02 s |
| 5 | 167.7 | 168.1 | ||
| 6 | 34.0 | 2.58 td (14.0, 4.5), 2.15 dt (14.0, 2.0) | 34.2 | 2.36 m, 1.60 m |
| 7 | 33.0 | 1.63 m, 0.91 m | 32.8 | 1.64 m, 0.87 m |
| 8 | 36.5 | 1.40 m | 36.3 | 1.35 m |
| 9 | 55.0 | 0.92 m | 54.9 | 0.86 m |
| 10 | 43.3 | 43.1 | ||
| 11 | 22.2 | 1.55 m, 1.48 m | 22.3 | 1.50 m, 1.46 m |
| 12 | 28.8 | 1.82 m, 1.2 4m | 28.8 | 1.79 m, 1.22 m |
| 13 | 43.0 | 43.1 | ||
| 14 | 56.6 | 0.84 m | 56.6 | 1.01 m |
| 15 | 24.5 | 1.55 m, 1.39 m | 24.5 | 1.46 m, 1.38 m |
| 16 | 40.7 | 1.94 dt (7.5, 2.0), 1.18m | 40.7 | 1.94 d (12.5), 1.04 d (11.5) |
| 17 | 56.9 | 1.06 m | 56.9 | 1.01 m |
| 18 | 12.7 | 0.62 s | 12.7 | 0.66 s |
| 19 | 74.1 | 3.96 d (9.5), 4.70 d (9.5) | 72.8 | 4.52 d (9.5), 3.84 d (9.5) |
| 20 | 36.3 | 1.52 m | 36.4 | 1.59 m |
| 21 | 19.2 | 0.95 d (6.5) | 19.2 | 0.93 d (6.5) |
| 22 | 36.8 | 1.39 m, 1.02 m | 36.8 | 1.35 m, 1.01 m |
| 23 | 24.5 | 1.18 m, 0.92 m | 24.5 | 1.13 m, 1.01 m |
| 24 | 40.1 | 1.19 m, 1.19 m | 40.1 | 1.13 m, 1.13 m |
| 25 | 28.6 | 1.52 m | 28.6 | 1.49 m |
| 26 | 23.3 | 0.91 d (6.5) | 23.3 | 0.88 d (6.5) |
| 27 | 23.1 | 0.91 d (6.5) | 23.1 | 0.88 d (6.5) |
| Glc 1’ | 105.6 | 4.96 d (8.0) | 102.9 | 5.16 d (7.0) |
| 2’ | 75.2 | 4.00 m | 57.9 | 4.41 m |
| 3’ | 79.4 | 4.22 m | 76.4 | 4.42 m |
| 4’ | 72.0 | 4.20 m | 72.9 | 4.14 m |
| 5’ | 79.1 | 4.03 m | 79.2 | 4.00 m |
| 6’ | 63.2 | 4.60 m, 4.42 m | 63.2 | 4.57 dd (11.5, 5.0), 4.35 dd (11.5, 6.0) |
| OH-2’ | 7.44 d (5.0) | |||
| OH-3’ | 7.29 br s | 7.23 d (3.5) | ||
| OH-4’ | 7.17 d (3.5) | 7.33 d (4.5) | ||
| OH-6’ | 6.43 t (6.0) | 6.45 t (6.0) | ||
| NH | 8.96 d (7.5) | |||
| OAc | 170.7 | |||
| 24.0 | 2.13 s | |||
br = broad; s = singlet; d = doublet; dd = doublet of doublets; m = multiplet.
Figure 1.
Novel natural products 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) and 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2), and two known alkaloids, indole-3-carboxaldehyde (3) and 3-(hydroxyacetyl)indole (4) from Peyssonnelia sp.
Figure 2.
Key HMBC correlations of 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1).
ROESY cross peaks connecting H-19a and H-6a, H-6a and H-7a, H-7a and H-8, H-8 and CH3-18, CH3-18 and H-20 indicated the β configurations of H-19, H-8, Me-13, and H-19. Large coupling constants (7.5–9.5 Hz) between adjacent protons from H-1’ to H-5’ of the sugar moiety were observed in CDCl3 (Supporting Information), indicating a β-glucopyranoside structure. Thus, the structure 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) of 1 was established as shown in Figure 1, with H-9 and H-14 oriented alpha as in cholesterol.
The positive ESI-MS of 2 exhibited a [M + H]+ peak at m/z 604. The HR-ESI-MS molecular ion at m/z 602.3766 [M - H]− was appropriate for a molecular formula of C35H57NO7 and suggested eight degrees of unsaturation. The UV spectrum of 2 showed the same maximal absorption wavelength at 242 nm as 1, indicating a similar chromophore. In comparing the 1H, 13C, HSQC, HMBC, and COSY NMR spectroscopic data with those of 1, identical signals associated with the α,β-unsaturated ketone, tetracyclic steroid, and 1,5-dimethylhexanyl functions revealed a same 19-hydroxy-cholest-4-en-3-one aglycone structure for 2. The anomeric proton at δ 5.16 together with six proton signals at δ 4.00–4.57 suggested a glycosyl moiety similar to 1. Among these carbinol methine and methylene protons, the signal at δ 4.41 (H-2’) correlated with an upfield-shifted carbon signal at δ 57.9 (C-2’) in the HSQC spectrum, indicating that this glycosyl moiety was an aminoglucoside.13,14 In addition, an acetyl function [1H (δ 2.13) and 13C (δ 170.7 and 24.0)] was identified. HMBC correlations from H-2’ and NH (δ 8.96) to the carbonyl carbon at δ 170.7 indicated an N-acetylglucosamine structure.15,16 The linkage between the glycoside and aglycone was confirmed by HMBC correlations from both oxygenated methylene protons at δ 4.52 and 3.84 (H2-19) to δ 102.9 (C-1’) and from δ 5.16 (H-1’) to δ 72.8 (C-19). Thus the structure of 2 was established as 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one.
Indole-3-carboxaldehyde (3) and 3-(hydroxyacetyl)indole (4) were isolated and their structures were identified by 1H NMR and mass spectroscopic analysis in comparison with the literature.17,18
2.2 Structural modification of sterol glycosides
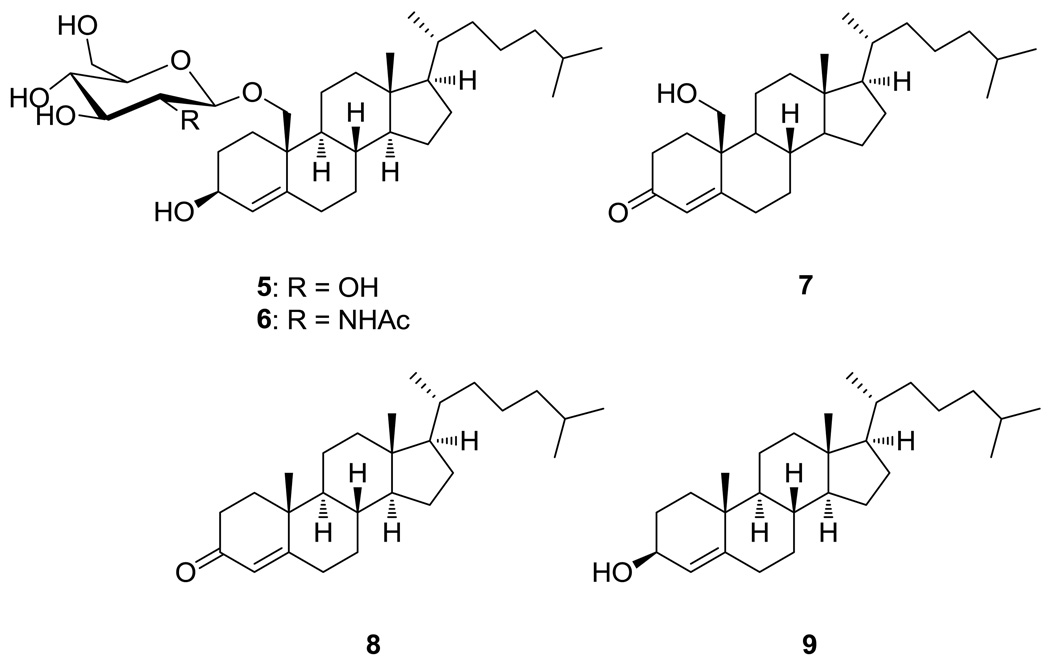
Steroids contribute to a wide range of therapeutic applications in humans, such as cardiotonic, contraceptive and anti-inflammatory agents.19 Some synthetic steroids have been reported for their anticancer potential.20,21 Cholestene derivatives, however, were recently reported to exhibit weak to no cytotoxic activity.22,23 In order to uncover structure-activity relationships of these novel natural products, we carried out structural modifications by semi-synthesis. To obtain the cholest-4-en-3-ol analogs of 1–2, the Luche reduction was used to reduce the α,β-unsaturated ketone of C-3 in 1 and 2.24 With sodium borohydride as reducing agent, this highly stereoselective synthesis was achieved in combination with CeCl3 in MeOH, yielding 19-O-β-d-glucopyranosyl-cholest-4-en-3β,19-diol (5) from 1, and 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-cholest-4-en-3β,19-diol (6) from 2, each in 21–23 % yield. To acquire the cholest-4-en-3-one aglycone from 2, a mild condition of refluxing with pyridium p-toluenesulfonic acid (PPTS) in benzene was used, and 19-hydroxy-cholest-4-en-3-one (7), was acquired in 56 % yield.25 Commercially available 4-cholesten-3-one (8) was reduced to 4-cholesten-3β-ol (9) in 86% yield by Luche reduction (Figure 3).
Figure 3.
19-O-β-d-glucopyranosyl-cholest-4-en-3β,19-diol (5), 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-cholest-4-en-3β,19-diol (6), and 19-hydroxy-cholest-4-en-3-one (7) synthesized by modification of 1–2; commercially available 4-cholesten-3-one (8) and its reduction product 4-cholesten-3β-ol (9).
2.3 Pharmacological evaluation
Both sterol glycosides 1 and 2 displayed moderate activity toward all human cancer cell lines tested with IC50s of 0.86–3.44 and 0.71–2.44 µM, respectively (Table 2). More specifically, 1 and 2 displayed good cytotoxicity toward human breast cancer MDA-MB-468 with IC50 = 0.71 and 0.86 µM, respectively. Human lung cancer cell line A549, which is often more resistant to cytotoxins than other commonly used cells,26 was inhibited by 1 and 2 with IC50s of 0.93 and 0.97 µM, respectively.
Table 2.
Cytotoxicities of Peyssonnelia sp. natural products and derivatives
| IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|
| Cell lines | 1 | 2 | 5 | 6 | 7 | 8 | 9 |
| DU4775 | 1.29 | 2.44 | 6.09 | 6.12 | 1.00 | 6.74 | >25.0 |
| MDA-MB-468 | 0.86 | 0.71 | 6.69 | 7.13 | 3.99 | 6.09 | >25.0 |
| PC-3 | 3.44 | 2.40 | >25.0 | 9.61 | 1.96 | >25.0 | >25.0 |
| LNCaP-FGC | 3.40 | 1.48 | 24.2 | 21.39 | 15.63 | 5.96 | >25.0 |
| HCT116 | 3.13 | 2.23 | >25.0 | >25.0 | 20.95 | 9.58 | >25.0 |
| MDA-MB-231 | 1.27 | 1.01 | 16.94 | 10.20 | 22.33 | 13.51 | >25.0 |
| Du145 | 1.31 | 1.35 | >25.0 | >25.0 | 5.05 | 20.30 | >25.0 |
| BT-549 | 1.26 | 1.24 | 11.87 | 10.06 | - | - | - |
| A549 | 0.93 | 0.97 | >25.0 | >25.0 | - | - | - |
| A2780/DDP-S | 1.39 | 1.35 | >25.0 | 8.98 | - | - | - |
| CCRF-CEM | 1.97 | 1.45 | 9.82 | 10.73 | - | - | - |
| Meana | 1.63 | 1.41 | 16.71 | 11.98 | 7.09 | 12.45 | >25.0 |
| Cell line selectivity (IC50 max/IC50 min) | 4.00 | 3.43 | 4.10 | 4.08 | 22.33 | 4.11 | 1.00 |
Mean IC50 of all cancer cell lines tested (see the Experimental Section for details) - Cell line not tested
Reduced derivatives 5 and 6 exhibited anticancer IC50s from 6.09 to >25.0 µM and from 6.12 to >25.0 µM, respectively, against 11 human cancer cell lines (Table 2). Thus, the 3-keto sterol glycosides were 2.5–27 times more cytotoxic than their 3β-hydroxy counterparts, indicating the importance of oxidation state at C-3. The importance of the glucopyranosyl moiety towards anticancer activity was less pronounced. Relative to 1–2, their aglycone (i.e., 19-hydroxy-cholest-4-en-3-one (7)) exhibited slightly improved cytotoxicity toward human breast cancer cell line DU4775 and human prostate cancer cell line PC-3 with IC50s of 1.00 and 1.96 µM, respectively, but higher IC50 values for the other tested cell lines. Removal of the 19-hydroxy group to 4-cholesten-3-one (8) resulted in only moderate further declines in cytotoxicity, but as expected reduction to the 3β-hydroxy form of the aglycone (i.e., 4-cholesten-3β-ol (9)) led to complete loss of activity (Table 2).
Sterol glycosides 1 and 2 were further tested for their effects on cell cycle arrest and apoptosis against the A2780/DDP-S ovarian cancer cell line. Neither compound demonstrated any significant perturbation of the cell cycle nor induction of PARP cleavage at 10 µM for 23 h. A longer incubation time of 48 h also did not affect a change in cell cycle or apoptosis (data not shown).
Sterol glycosides 1 and 2 exhibited moderate antibacterial activity toward methicillin-resistant Staphylococcus aureus with MICs of 6.3 and 3.1 µg/mL, respectively, and vancomycin-resistant Enterococcus faecium, IC50 = 6.3 and 12.5 µg/mL, respectively. Weak or no potency was detected in the assays against amphotericin-resistant Candida albicans (Table 3), Mycobacterium tuberculosis (>40 µM, data not shown), and the malaria parasite Plasmodium falciparum (>40 µM, data not shown).
Table 3.
Antibacterial and antifungal activities of 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1) and 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2).
| antibacterial activity (µg/mL) | antifungal activity (µg/mL)a | ||
|---|---|---|---|
| cmpd. | MRSA MIC | VREF MIC | MIC |
| 1 | 6.3 | 6.3 | 25 |
| 2 | 3.1 | 12.5 | 25 |
Using amphotericin-resistant Candida albicans. MRSA=methicillin-resistant Staphylococcus aureus; VREF=vancomycin-resistant Enterococcus faecium.
3. Experimental
3.1 General experimental procedures
Optical rotations were acquired on a Jasco P-1010 spectropolarimeter. UV spectra were determined in MeOH with a Spectronic 21D spectrophotometer. NMR spectra were measured on a Bruker DRX-500 instrument, using a 5 mm broadband or inverse detection probe for 1H, 13C, 1H-1H COSY, HSQC, HMBC, and ROESY experiments. LC-MS analyses were conducted using a Waters 2695 HPLC with Waters spectrometer with 2996 diode-array UV detection and Micromass ZQ 200 mass spectrometer with electrospray ionization in both positive and negative mode. LC-MS chromatography was achieved with an Xterra NS-C-18 3.5 µm column measuring 2.1 × 15 mm and gradient mobile phases of aqueous methanol with 0.1% acetic acid. High-resolution mass spectra were measured using electrospray ionization with an Applied Biosystems QSTAR-XL hybrid quadrupole-time-of-flight tandem mass spectrometer and Analyst QS software. Semipreparative HPLC was performed using a Waters 2690 pump and 996 diode-array UV detector, controlled by Waters Millenium software. Compound purification by HPLC was performed on Agilent Zorbax SB-C-18 (5 µm, 9.4 × 250 mm) and Phenomenex Develosil C30 RPAQUEOUS (5 µm, 4.6 × 250 mm) columns. All commercial chemicals were reagent grade. Optima grade (Fisher Scientific Co.) solvents were used for HPLC and LC-MS. NMR solvents were purchased from Cambridge Isotope Laboratories.
3.2 Algal material
Peyssonnelia sp. (Rhodophycota) was collected from a coral reef at 30 m depth near Tuvuca Island (S17° 39’28”, W178° 50’36”), Fiji. Fresh tissue samples were frozen aboard the Vanuabalavu Fisheries vessel (TUI-NI-WASABULA) at −20 °C. All samples were transferred to a −80 °C freezer at University of the South Pacific until further processed for extraction in the laboratory. Multiple voucher samples were preserved in 10% aqueous formalin and are stored at the University of the South Pacific in Suva, Fiji and at Georgia Institute of Technology, Atlanta, GA USA with voucher identification ICBG-G-0349.
3.3 Extraction and isolation
Frozen Peyssonnelia sp. (227.0 g wet weight) was extracted with MeOH (500 mL) five times. The extracts were combined and reduced in vacuo to afford 4.23 g crude extract. The extracts were fractionated by HP20ss gel column chromatography and eluted sequentially with 1:1 MeOH/H2O, 4:1 MeOH/H2O, 100% MeOH, and 100% acetone (150 mL each) to give 4 fractions (F1–F4). Fraction F3 (363 mg) was separated by C18 reversed-phase HPLC using an isocratic system of 95% MeOH and 5% H2O to afford 1 (8.0 mg) and 2 (7.6 mg); F2 (130 mg) was separated by C18 reversed-phase HPLC using a isocratic system of 1:1 MeOH/H2O to afford 3 (1.0 mg) and 4 (0.8 mg). Pure compounds (10 µg/mL) were analyzed by LC-MS to determine λmax and molecular mass, and quantified by 1H NMR spectroscopy using 2,5-dimethylfuran as internal standard.27
3.3.1. 19-O-β-d-glucopyranosyl-19-hydroxy-cholest-4-en-3-one (1)
White amorphous solid (0.0035 % wet mass); [α]23D +19.4 (c 0.038, MeOH); UV (MeOH) λmax (log ε) 242 (3.77) nm; 1H NMR (pyridine-d5, 500 MHz) and 13C NMR (pyridine-d5, 125 MHz) data, Table 1; 2D NMR data, Supporting Information; HRESIMS [M+Na]+ m/z 585.3689 (calcd for C33H54O7Na, 585.3767).
3.3.2. 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-19-hydroxy-cholest-4-en-3-one (2)
White needles obtained from MeOH (0.0033 % wet mass); [α]23D +11.4 (c 0.038, MeOH); UV (MeOH) λmax (log ε) 242 (3.88) nm; 1H NMR (pyridine-d5, 500 MHz) and 13C NMR (pyridine-d5, 125 MHz) data, Table 1; 2D NMR data, Supporting Information; HRESIMS [M+Na]+ m/z 626.4068 (calcd for C35H57NO7Na, 626.4033).
3.4 Reduction of 1 and 2
NaBH4 (0.0060 mmol) and CeCl3·7H2O (0.0060 mmol) were dissolved in 1.0 mL MeOH, and 1 (2.0 mg) was added. The reaction was stirred at room temperature for 3 h until no starting material was detected by TLC. Aqueous HCl was added to the reaction mixture, and then extracted with EtOAc (4 × 2 mL).19 The EtOAc layers were combined and dried in vacuo, and the products were purified by HPLC using an isocratic system of 9:1 MeOH/H2O to afford 5 (0.47 mg, yield 23%). The same method was applied for 2 to afford 6 (0.43 mg, yield 21%).
3.4.1. 19-O-β-d-glucopyranosyl-cholest-4-en-3β,19-diol (5)
White amorphous solid; 1H NMR (pyridine-d5, 500 MHz) δ 5.91 (br s, H-4), 4.90 (d, J=8.0, H-1’), 4.54 (m, H-19a), 4.52 (m, H-3), 3.85 (m, H-19b), 2.30 (m, H-2a); HRESIMS [M+Na]+ m/z 587.3895 (calcd for C33H56O7Na, 587.3923).
3.4.2. 19-O-β-d-N-acetyl-2-aminoglucopyranosyl-cholest-4-en-3β,19-diol (6)
White needles obtained from MeOH; 1H NMR (pyridine-d5, 500 MHz) δ 5.89 (br s, H-4), 5.02 (d, J=8.5, H-1’), 4.59 (d, J=9.5 H-19a), 4.48 (m, H-3), 3.71 (d, J=9.5, H-19b), 2.19 (m, H-2a); HRESIMS [M+Na]+ m/z 628.4214 (calcd for C35H59NO7Na, 628.4189).
3.5 Aglycone hydrolysis
Pyridium p-toluenesulfonic acid (PPTS, 0.1 eq) and 2 (1.6 mg) were dissolved in 2.0 mL benzene. The reaction was refluxed at 90 °C for 3.5 h until no starting material was detected by TLC. Water (10 mL) was added to the reaction mixture which was then extracted with EtOAc (5 × 2 mL). The EtOAc layers were combined and dried in vacuo, and the product was purified by HPLC using a isocratic system of 49:1 MeOH/H2O to afford 7 (0.59 mg).
3.5.1. 19-hydroxy-cholest-4-en-3-one (7)
White wax (0.59 mg); 1H NMR (pyridine-d5, 500 MHz) δ 6.38 (t, J=5.0 Hz, OH), 6.18 (s, H-4), 4.27 (dd, J=10.5, 4.0 Hz, H-19a), 4.13 (dd, J=11.0, 5.5 Hz, H-19b), 3.18 (m, H-2a), 2.57 (m, H-6a), 2.50 (m, H-1a, H-2b), 2.26 (d, J=13.5 Hz, H-6b), 1.98 (d, J=12.5 Hz, H-16a), 1.84 (m, H-12a), 1.77-1.02 (m, 21H), 0.97 (d, J=6.5 Hz, Me-21), 0.91 (d, J=6.5 Hz, Me-26 and 27), 0.70 (s, Me-18); 13C NMR (pyridine-d5, 125 MHz) δ 199.5 (C-3), 168.4 (C-5), 126.5 (C-4), 76.3 (C-19), 56.5 (C-17), 56.3 (C-14), 54.5 (C-9), 44.4 (C-10), 42.7 (C-13), 40.4 (C-16), 39.7 (C-24), 36.5 (C-8), 36.3 and 36.1 (C-20 and 22), 35.9 (C-2), 34.2 (C-2), 33.9 (C-6), 32.6 (C-7), 28.5 (C-25), 28.3 (C-12), 24.3 and 24.2 (C-15 and 23), 22.9 (C-26), 22.7 (C-27), 21.9 (C-11), 18.8 (C-21), 12.3 (C-18); HRESIMS [M+Na]+ m/z 423.3230 (calcd for C27H44O2Na, 423.3239).
3.6 Pharmacological assays
The pharmacological assays were performed as previously reported.28,29 Anticancer assays were conducted using 11 human cancer cell lines including breast (BT-549, DU4475, MDA-MB-468 and MDA-MB-231), colon (HCT-116), lung (A549), prostate (PC-3, LNCaP-FGC and DU145), ovarian (A2780/DDP-S) and leukemia (CCRF-CEM).30 Antibacterial assays were performed using methicillin-resistant Staphylococcus aureus (MRSA, ATCC 33591) and vancomycin-resistant Enterococcus faecium (VREF, ATCC 700221) as test pathogens.28,29 Antifungal assays were performed using amphotericin B-resistant Candida albicans (ATCC 90873).28,29 Antitubercular activity was assessed against Mycobacterium tuberculosis strain H37Rv (ATCC 27294) using the microplate alamar blue assay (MABA).31 Antimalarial activity was determined with a SYBR Green based parasite proliferation assay.28,29
Cell cycle arrest and apoptosis experiments using the A2780/DDP-S ovarian cancer cell line were conducted for 1–2 at 1.0 µM, 3.0 µM and 10 µM, over 24 and 48 h. Using flow cytometry analysis, cell cycle was assessed by propidium iodine staining of DNA and apoptosis was assessed by the presence of cleaved PARP (a marker of caspase activation in apoptotic cells).32
Supplementary Material
Acknowledgement
This research was supported by the U.S. National Institutes of Health’s International Cooperative Biodiversity Groups program (Grant No. U01 TW007401). We thank the Government of Fiji for the permission to perform research in their territorial waters and for permission to export samples. We thank the Qoliqoli Lomaloma (Department of Fisheries and Forests) and the village of Lomaloma on Vanuabalavu Island, Fiji for their support in making this collection expedition possible. We especially thank the Captain and crew of the Tui Ni Wasabula and the faculty and staff of the University of the South Pacific (USP) for facilitating this work. We are also thankful to S. Franzblau and B. Wan for antituberculosis assays; K. L. Roch and J. Prudhomme for antimalarial assays; T. Davenport for antimicrobial assays; C. Redshaw for LC-MS assistance; M. C. Sullards and D. Bostwick for mass spectroscopic analyses; L. Gelbaum for NMR assistance; and A. Bommarius and T. Rogers for use of their spectropolarimeter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
1H and 13C NMR spectra and COSY, HMBC, and NOE data tables for 1–2 can be found in the online version.
References and notes
- 1.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2010;27:165–237. doi: 10.1039/b906091j. [DOI] [PubMed] [Google Scholar]
- 2.Littler DS, Littler MM. South Pacific Reef Plants. Washington, D. C: Offshore Graphics, Inc.; 2003. p. 90. [Google Scholar]
- 3.Littler DS, Littler MM. Caribbean Reef Plants. Washington, D. C: Offshore Graphics, Inc.; 2000. pp. 86–90. [Google Scholar]
- 4.Littler MM, Littler DS. Biologie in unserer Zeit. 1994;24:330–335. [Google Scholar]
- 5.Lane AL, Mular L, Drenkard EJ, Shearer TL, Engel S, Fredericq S, Fairchild CR, Prudhomme J, Le Roch K, Hay ME, Aalbersberg W, Kubanek J. Tetrahedron. 2010;66:455–461. doi: 10.1016/j.tet.2009.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPhail KL, France D, Cornell-Kennon S, Gerwick WH. J. Nat. Prod. 2004;67:1010–1013. doi: 10.1021/np0400252. [DOI] [PubMed] [Google Scholar]
- 7.Talpir R, Rudi A, Kashman Y, Loya Y, Hizi A. Tetrahedron. 1994;50:4179–4184. [Google Scholar]
- 8.Lin A-S, Chang F-R, Wu C-C, Liaw C-C, Wu Y-C. Planta Med. 2005;71:867–870. doi: 10.1055/s-2005-871292. [DOI] [PubMed] [Google Scholar]
- 9.Kodai T, Umebayashi K, Nakatani T, Ishiyama K, Noda N. Chem. Pharm. Bull. 2007;55:1528–1531. doi: 10.1248/cpb.55.1528. [DOI] [PubMed] [Google Scholar]
- 10.Seo S, Tomita Y, Tori K, Yoshimura Y. J. Am. Chem. Soc. 1978;100:3331–3339. [Google Scholar]
- 11.Ino S, Oinuma H, Yamakado R, Morisaki M, Furukawa Y, Hata T. Chem. Pharm. Bull. 1991;39:3335–3337. [Google Scholar]
- 12.Rabinowitz MH, Djerassi C. J. Am. Chem. Soc. 1992;114:304–317. [Google Scholar]
- 13.Yamada-Hada J, Hada N, Aoki K, Yamamoto K, Takeda T. Chem. Pharm. Bull. 2004;52:473–476. doi: 10.1248/cpb.52.473. [DOI] [PubMed] [Google Scholar]
- 14.Boullanger P, Chevalier Y, Croizier M-C, Lafont D, Sancho M-R. Carbohydr. Res. 1995;278:91–101. [Google Scholar]
- 15.Watanabe K, Tanaka R, Sakurai H, Iguchi K, Yamada Y, Hsu C-S, Sakuma C, Kikuchi H, Shibayama H, Kawai T. Chem. Pharm. Bull. 2007;55:780–783. doi: 10.1248/cpb.55.780. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka I, Hada N, Ohtaka H, Sugita M, Takeda T. Chem. Pharm. Bull. 2002;50:600–604. doi: 10.1248/cpb.50.600. [DOI] [PubMed] [Google Scholar]
- 17.Bernart M, Gerwick WH. Phytochemistry. 1990;29:3697–3698. [Google Scholar]
- 18.Tan J, Bednarek P, Liu J, Schneider B, Svatos A, Hahlbrock K. Phytochemistry. 2004;65:691–699. doi: 10.1016/j.phytochem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Robbers JE, Speedie MK, Tyler VE. Pharmacognosy and Pharmacobiotechnology. Maryland: Williams & Wilkins; 1996. p. 108. [Google Scholar]
- 20.Banday AH, Shameem SA, Gupta BD, Kumar HMS. Steroids. 2010;75:801–804. doi: 10.1016/j.steroids.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Banday AH, Mir BP, Lone IH, Kumar HMS. Steroids. 2010;75:805–809. doi: 10.1016/j.steroids.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Lee J-S, Nakazawa T, Ukai K, Mangindaan REP, Wewengkang DS, Rotinsulu H, Kobayashi H, Tsukamoto S, Namikoshi M. Steroids. 2009;74:758–760. doi: 10.1016/j.steroids.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiang C-S, Huang C-G, Feng B, Li J, Gong J-X, Kutan T, Guo Y-W. Steroids. 2010;75:1153–1163. doi: 10.1016/j.steroids.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Bisogno FR, Orden AA, Pranzoni CA, Cifuente DA, Giordano OS, Sanz MK. Steroids. 2007;72:643–652. doi: 10.1016/j.steroids.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Frye LL, Cusack KP, Leonard DA. J. Med. Chem. 1993;36:410–416. doi: 10.1021/jm00055a012. [DOI] [PubMed] [Google Scholar]
- 26.Miao Z-H, Tong L-J, Zhang J-S, Han J-X, Ding J. Int. J. Cancer. 2004;110:627–632. doi: 10.1002/ijc.20026. [DOI] [PubMed] [Google Scholar]
- 27.Gerritz SW, Sefler AM. J. Comb. Chem. 2000;2:39–41. doi: 10.1021/cc990041v. [DOI] [PubMed] [Google Scholar]
- 28.Kubanek J, Prusak AC, Snell TW, Giese RA, Hardcastle KI, Fairchild CR, Aalbersberg W, Raventos-Suarez C, Hay ME. Org. Lett. 2005;23:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane AL, Stout EP, Lin A-S, Prudhomme J, Le Roch K, Fairchild CR, Franzblau SG, Hay ME, Aalbersberg W, Kubanek J. J. Org. Chem. 2009;74:2736–2742. doi: 10.1021/jo900008w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee FYF, Borzilleri R, Fairchild CR, Kim SH, Long BH, Raventos-Suarez C, Vite GD, Rose WC, Kramer RA. Clin. Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 31.Falzari K, Zhu Z, Pan D, Liu H, Hongmanee P, Franzblau SG. Antimicrob. Agents Chemother. 2005;49:1447–1454. doi: 10.1128/AAC.49.4.1447-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litzenburger BC, Kim H-J, Kuiatse I, Carboni JM, Attar RM, Gottardis MM, Fairchild CR, Lee AV. Clin. Cancer Res. 2009;15:226–237. doi: 10.1158/1078-0432.CCR-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.