Abstract
Intracranial atherosclerotic disease (ICAD) contributes to a significant number of ischemic strokes. There is debate in the recent literature concerning the impact of the location of stenosis in ICAD on outcome. Some reports have suggested that disease processes and outcomes vary by vessel location, potentially altering the natural history and indications for intervention. Here we have performed a comprehensive, critical review of the natural history of ICAD by vessel in an attempt to assess the differences in disease specific to each of the vascular territories. Our assessment concludes that only minor differences exist between patients with different vessels affected in vessel-specific ICAD. We have found that middle cerebral artery disease confers a lower mortality than vessel-specific ICAD in other intracranial vessels, asymptomatic disease follows a more benign course than symptomatic disease, and that plaque progression or the detection of microemboli on transcranial Doppler may predict poor outcome. Given the expanding indications for treatment of ICAD and rapidly developing endovascular techniques to confront this disease, a thorough understanding of the natural history of ICAD aids the interventional neuroradiologist in determining when to treat and how to predict outcome in this patient population.
Keywords: Intracranial atherosclerosis, Natural history, Stenosis
INTRODUCTION
Patients with stroke or transient ischaemic attack (TIA) are found to have intracranial atherosclerotic disease (ICAD) in at least 9% of cases[1]. Depending on the populations studied, and particularly in Asian populations, ICAD may account for up to 29% of all ischemic events[2-4]. Besides race and ethnicity, medical risk factors associated with ICAD include insulin-dependent diabetes mellitus, hypercholesterolemia, cigarette smoking and hypertension[1,5,6]. Despite advances in endovascular and pharmaceutical technology, the prognosis for patients with ICAD remains poor. We have previously characterized the natural history of ICAD on a vessel-by-vessel basis[7] in an effort to identify the potentially variable risk represented by stenosis of the different intracranial vessels. Care should be taken when interpreting the finding of single stenosed intracranial vessels, since intracranial atherosclerosis is likely to be a diffuse process affecting multiple locations[8]. For this reason, atherosclerotic stenosis identified in any one vessel also confers an increased risk of subsequent stroke in other vascular distributions. The location of stenosis has no clear effect on stenosis progression and stroke[7].
Though most recent reports on ICAD aggregate stenoses at all locations in the intracranial arteries in their outcome assessment, some investigators have identified pathogenic differences that may contribute to the observed survival discrepancies identified in the literature. Bang et al[9], investigated the mechanisms underlying stroke in patients with atherosclerotic lesions in the internal carotid artery and proximal atherosclerotic lesions (MCA), finding that biomarkers of inflammation were more elevated in those with carotid stenosis (P < 0.01). These authors concluded that atherosclerotic intracranial carotid stenosis may be prone to instability with plaque rupture and subsequent ischemic stroke while atherosclerotic stenosis of the MCA appears more stable and less prone to plaque rupture.
Because of apparent differences in morbidity and mortality based upon location in the intracranial circulation, we critically review the natural history of ICAD using a vessel-by-vessel approach, and discuss the possible common features and management.
EPIDEMIOLOGY AND PATHOGENESIS OF ICAD
The proportion of patients with atherosclerotic stenoses in the major vessels varies widely in the medical literature. Mazighi et al[10], reported on a series of patients with stenosis > 30%: the ICA was affected in 16.3%, MCA in 18.3%, basilar artery (BA) in 15.9% and vertebral artery (VA) in 7.6%. In a prospective series of 267 patients with intracranial large vessel occlusion, the MCA was affected in 38%, ICA in 6%, ACA in 1.3%, and posterior circulation arteries in 7%[11]. However, the Groupe d’Etude des Stenoses Inta-Cranieenes Atheromateuses symptomatiques (GESICA) was a prospective series including 102 patients with symptomatic intracranial stenosis and showed relatively equal distribution of disease affecting VA, BA, MCA and ICA[12].
ICAD occurs more commonly in patients of Asian, African or Hispanic origin than in Caucasians. Studies in Chinese, Thai, Korean, Japanese and Singaporean patients with stroke have demonstrated rates of ICAD between 30%-50%[13]. By contrast, approximately 8 to 9% of strokes are attributable to ICAD in Caucasians, while African- and Hispanic-Americans were shown to have a relative risk of 5 to 6 for ICAD-related stroke compared to Caucasians[14]. The development of this racial difference has been hypothesized to be related to the emergence of a stroke-suppressor genotype among Europeans that has primarily affected intracranial arteries[15].
The prevalence and true impact of ICAD is likely underestimated because most patients are evaluated using primarily cross-sectional imaging studies such as computed tomography[16] or magnetic resonance imaging[17] rather than catheter angiography which can most accurately diagnose and characterized intracranial stenoses. In particular, recent studies suggest that even stenoses < 50% can potentially be associated with ischemic stroke and may be significant[10]. Nevertheless, the Warfarin-Aspirin Symptomatic Intracranial Diseases (WASID) Trial showed that intracranial stenosis 70%-99% were associated with the greatest risk of stroke[3], and an autopsy study of 339 consecutive patients who died of stroke found in intracranial stenoses in 43% of cases, not all of which were symptomatic[10].
Controversy remains about the prognostic significance of ICAD, either discovered prior to a stroke or secondarily identified post-stroke or TIA. Since the risk factors for ICAD are similar to those for arterial disease in other parts of the body (diabetes, hypertension, cigarette smoking, and hypercholesterolemia[1,18,19]), it is not surprising that patients with ICAD have increased rates of vascular occlusive disease in other vascular territories.
ICAD is part of a generalized vasculopathy due to genetic and environmental factors that puts these patients at a significantly higher risk for a range of vascular events. ICAD has been associated with high levels of circulating pro-inflammatory cytokines and inhibitors of fibrinolysis[20]. In particular, increased levels of C-reactive protein and PAI-1 predicted progression to symptomatic ICAD. High levels of lipoprotein-(a) and diabetes have been found to predict higher levels of ICAD and may be useful markers of risk for this disease[21].
INTERNAL CAROTID ARTERY ICAD
A number of retrospective studies have investigated annual mortality and ipsilateral stroke rates following diagnosis of intracranial ICA atherosclerotic disease in the 1980s[22-25]. The reported rates of annual mortality ranged from 7.8% to 17.2%, with recurrent ipsilateral stroke occurring at a rate of 3.1 to 8.1%. These reports have limitations because the patient populations investigated were heterogeneous with baseline stenosis ranging from 20%-70%, longitudinal evaluation varied from 25.5-50 mo, and the series were small, with fewer than 100 symptomatic and asymptomatic patients. The primary outcomes are difficult to compare because there is no common objective measure of stroke severity. In aggregate, these case series can only be considered Level 2b evidence by the Oxford Centre for Evidence Based Medicine scheme[26].
A number of important hypotheses emerged from this early work on carotid ICAD. Craig et al[22] found that recurrent cerebral ischemic events were more frequent in patients with symptomatic rather than asymptomatic ICA stenosis. Wechsler et al[25] showed that impaired flow on angiography in symptomatic patients due to hemodynamically significant carotid siphon stenosis contributed to TIA symptomatology, while strokes primarily resulted from distal embolization from the stenosis. Even though ICAD occurs in the condition of diffuse vascular disease, strokes subsequent to the diagnosis of ICAD occurred primarily in the same vascular territory supplied by the area of lesion identified[23,24]. In a prospective series of patients with suspected stroke, multivariate analysis demonstrated that the location of stenosis is associated with clinical outcome: ICAD at the internal carotid terminus was significantly associated with poor outcome (modified Rankin scale score 3-6) over a 23-mo follow-up[11].
MIDDLE CEREBRAL ARTERY ICAD
The early, retrospective cohorts investigating the natural history of MCA ICAD show a 12.5%-24% risk of recurrent stroke during 6.5 years follow-up[27,28]. The annual stroke rate ranged from 2.8%-3.7%, and in some cases occurred very quickly, prior to initiation of medical therapy[27]. More recently, a number of prospective series have investigated the same population. Sub-group analysis of the extracranial-intracranial bypass study population[29] reported an annual stroke rate of 5%, with 25% of 138 patients experiencing stroke during 55.8 mo follow-up. The patients in this group had > 70% stenosis at the beginning of the study, and may represent a higher risk group than the earlier cohorts including patients with < 50% stenosis. Arenillas et al[20] showed that stenosis progression, as measured by transcranial Doppler (TCD), independently predicted stroke recurrence in symptomatic patients with MCA ICAD. Subsequently, Gao et al[30] showed that the presence of microembolic signals (MES) on TCD ultrasonography in symptomatic patients with known MCA stenoses predicted recurrent ipsilateral stroke. The annual ipsilateral stroke rate was 7.8% and annual mortality was 7.0%[30]. The results of these two studies suggest that symptomatic MCA ICAD is a condition with significant risk of death or recurrent stroke, which is primarily embolic in origin. Furthermore, regular evaluation using TCD, including evaluation of MES, may be valuable in identifying high-risk patients with MCA stenosis.
Stroke prevention in symptomatic ICAD patients remains a subject of discussion and on-going research. Generally, systemic anti-coagulation is no longer primary therapy based on WASID and Warfarin-Aspirin Rcurrent Stroke Study (WARSS)[3,31]. The Aspirin or anticoagulants in stenosis of the middle cerebral artery (MCA) (AVASIS) trial, which was intended to clarify the findings of WASID concerning efficacy of aspirin vs warfarin was halted due to slow enrollment. Neither group reached the primary endpoint in a final analysis of the collected data[32]. In the United States, an update of the American Heart Association/American Stroke Association summarizes existing data on stroke prevention in patients with prior stroke or TIA[33].
VERTEBROBASILAR ICAD
The second most common location for ICAD is the vertebrobasilar arteries[2]. ICAD at this location confers a substantial risk of subsequent ischemic events. In GESICA, BA atherosclerosis conferred the highest risk for recurrent stroke over an average 23-mo follow up[12]. In a prospective series of patients presenting with suspected stroke, basilar and internal carotid terminus occlusions independently predicted poorer outcome on multivariate analysis (relative risk for a good outcome was 0.4 for basilar occlusion and 0.47 for ICA occlusion)[11]. Data from the WASID study group demonstrated that BA stenosis conferred a higher risk of subsequent events than VA stenosis[34]. The posterior circulation has also been found to have significantly higher rates of complications after angioplasty and stenting than ICAD affecting the anterior circulation[35].
Most of the data regarding the natural history of vertebrobasilar ICAD comes from retrospective cohorts. In four retrospective series published over the last 20 years, the rates of annual stroke mortality ranged from 1.1%-14.3%[34,36-39]. The annual vertebrobasilar-territory stroke rate and overall annual stroke rate ranged from 0%-8.7% and 3%-14.3%, respectively. Kaplan-Meier analysis revealed that the majority of symptomatic patients experienced a stroke and/or death within 5 years of initial presentation. The best data specifically about posterior circulation stroke likely comes from the New England Stroke Registry[40]. In this study of 407 registry patients, 59% had posterior circulation strokes without TIA, 24% presented with TIAs then strokes, and only 16% had TIAs, 14% of which were due to intra-arterial lesions[40].
Voetsch et al[41] performed a subgroup analysis of patients with basilar stenosis artery in the New England Stroke Registry. Mortality was lower than expected (2.6%) and 62% had minor or no permanent deficits at follow-up over an 8 years period. Longer-term follow up from this cohort would be welcomed, but has not been reported to date. It has been proposed that, as for ICAD in other regions, the risk of recurrent ischemia is highest in the first 2 years following a stroke[36]. Indeed, Thijs et al[42] showed that the risk of recurrent stroke and death on treatment can be quite high. In a series of 52 patients with TIA or stroke due to ICAD, 56% had recurrent events during treatment with antithrombotic or anti-platelet medications. 52% of these events occurred within 36 d (median) and 15% were major strokes or death. However, an analysis of the WASID cohort failed to reveal any difference in patients who were on or off (i.e. had failed) antithrombotic therapy at the time of the initial stroke[43].
MEDICAL AND INTERVENTIONAL MANAGEMENT OF ICAD
Optimal management of ICAD continues to evolve but currently focuses on the use of anti-aggregating (anti-platelet) medications as first line therapy and revascularization procedures (usually endovascular) for refractory cases. Recent medical management, which evolved from the treatment of systemic atherosclerosis has included combinations of aspirin, dipyridamole, statins, ticlopidine, clopidogrel, warfarin and angiotensin-converting enzyme inhibitors[2,42]. Since the publication of the WASID and the WARSS trial, the benefit of warfarin over aspirin has been questioned[3,31]. For example, WASID was stopped prematurely by its safety monitoring committee due to a significantly increased risk of hemorrhage in the warfarin group. At a mean follow-up of 1.8 years, there was no difference in the primary endpoint of ischemic stroke, brain hemorrhage or death from other vascular causes, but the rates of each endpoint individually were significantly lower in the aspirin group. Although some commentators have voiced concerns about the study design and applicability of outcome and complication measures[44], use of warfarin to treat this group of high-risk patients based on the foregoing data requires caution.
For patients who develop recurrent symptoms on antithrombotic therapy, the risk of recurrent stroke or TIA is believed to be extremely high and revascularization is often considered (Figure 1). The efficacy of angioplasty[45] or stent-supported angioplasty (PTAS) for ICAD remains controversial. Marks et al[46], reported clinical outcomes in their series of 120 patients with 124 symptomatic intracranial stenoses using PTA alone. Including periprocedural strokes and deaths, the annual stroke rate in the territory of treatment was 3.2% and 4.4% overall[46]. However, PTA alone is not always successful. In Marks’ series, 12.9% of patients required immediate stenting when angioplasty failed[46].
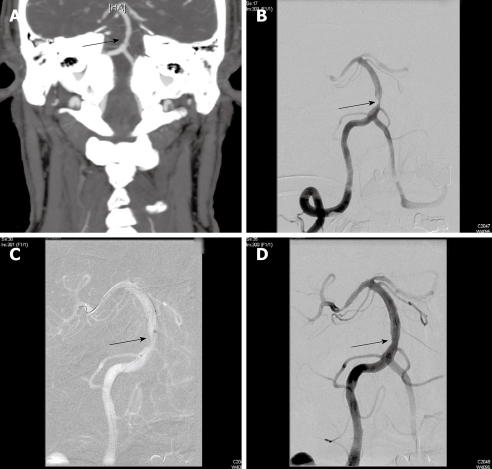
Figure 1.
Seventy-nine years old man with hypertension and hyperlipidemia developed episodic dizziness, visual distortion, dysarthria, and somnolence refractory to anti-platelet therapy using aspirin and dipyridamole. A: Computed tomography of the brain with contrast, CTA protocol and coronal reconstructions, shows severe focal stenosis of the basilar artery (arrow); B: Catheter arteriography of the right vertebral artery during arterial phase in frontal projection confirms 80% stenosis the proximal basilar artery (arrow); C: Fluororadiography during endovascular revascularization using stent angioplasty shows placement of a 3.0 mm × 15 mm Wingspan® self-expanding nitinol stent (arrow) symmetrically across the stenosis after angioplasty using a 2.5 mm × 9 mm Gateway® angioplasty balloon catheter; D: Catheter arteriography of the right vertebral artery during the arterial phase in frontal projection at the conclusion of the procedure shows residual 40% stenosis of the basilar artery after angioplasty and stent placement (arrow). Out-patient follow-up with non-invasive imaging using transcranial Doppler ultrasonography shows stable normal velocities in the treated artery. The patient remains stable on aspirin and clopidogrel.
There are also numerous series reviewing single and multi-center experience with intracranial stent angioplasty. SSYLVIA (Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries) was a prospective multi-center non-randomized feasibility trial to evaluate the Neurolink® intracranial stent (Guidant, Indianapolis, IN). Sixty one patients underwent treatment with 95% technical success, 6.6% stroke and 0% mortality. Unfortunately, the incidence of stroke increased to 7.3% between 30 d and 1 year with a 35% rate of restenosis in the treated arteries[47]. In 2005, Henkes et al[48] reported on the use of a novel self-expanding stent called Wingspan® (Boston Scientific, Fremont, CA). Forty five patients underwent treatment for ICAD > 50% stenosis with 98% technical success, composite 30-d ipsilateral or death of 4.5%, and 6-mo all cause stroke rate of 9.5%. Based on these data, the FDA granted Boston Scientific a humanitarian device exemption to treat symptomatic patients with ICAD stenosis > 50% and refractory to medical therapy. Fiorella et al[49], reported on 78 patients with 82 intracranial stenoses > 50% who were treated with Wingspan®. With a technical success rate of nearly 99%, there were 6.1% major peri-procedural complications or deaths with good target artery revascularization but a 32% rate of in-stent restenosis[50]. The SAMMPRIS (Stenting vs aggressive medical management for preventing recurrent stroke in intracranial stenosis) is an National Institute of Health-funded randomized trial comparing best medical therapy with stent-angioplasty using the Wingspan device plus best medical therapy and is now enrolling in the United States[51].
Across centers in the Americas and Europe, the results of PTA/PTAS have not been uniform. Recent non-randomized, retrospective studies have found no significant difference in vascular ischemic endpoints between patients with ICAD treated with medical therapy or PTA/PTAS[52]. In a systematic review of PTAS for ICAD, Gröschel et al[35], concluded that the use of PTA/PTAS outside the context of randomized, controlled trials cannot be recommended due to the widely variable complication rates of PTA/PTAS, the natural history of the ICAD, and lack of clear effect on patient outcome. A comprehensive review of this area is beyond the scope of this review, and is covered in detail elsewhere[17,53].
CONCLUSION
Symptomatic ICAD is associated with a significant risk of stroke despite medical therapy. Vessel-specific patterns of outcome have emerged in the old and recent literature on ICAD. Studies examining the ICA terminus have shown that stenosis in this region predicts poor outcome, arguing for intervention in some cases. Stenosis specific to the MCA distribution confers a lower rate of morbidity and mortality than other vessels, suggesting that medical management may play a larger role in this vessel. The presence of ICAD in the vertebrobasilar system confers the highest rate of stroke, but interventional neuroradiologists also report the highest rate of complications following angioplasty and stenting in these vessels. Within the posterior circulation, the ICAD of the BA has the highest rate of mortality and morbidity.
Examination of the natural history of ICAD evidences the new potential role for endovascular revascularization of the most severe intracranial stenoses. Percutaneous transluminal angioplasty and stent-supported angioplasty are now feasible with modern microcatheter technologies. Studies designed to compare complication rates of medical and interventional therapies are now underway. Restenosis with stent-supported angioplasty may be less frequent than after angioplasty alone at early follow-up evaluations. However, the long-term patency of stented cerebral arteries and rates of recurrent stroke remain unclear. For these reasons, a multi-disciplinary approach to patients with symptomatic ICAD is important to achieve optimal outcomes across the spectrum of cerebrovascular occlusive diseases.
Footnotes
Peer reviewers: Ender Uysal, MD, Sisli Etfal Training and Research Hospital. Clinic of Radiology, Sisli Etfal Eğitim ve Araştırma Hastanesi Radyoloji Kliniği, Etfal sok. Sisli, Istanbul 34377, Turkey; Hadi Rokni Yazdi, MD, Associate Professor, Department of radiology, Central Radiology, Imam Khomeini Hospital, Tehran University of Medical Sciences, Keshavarz Blvd, Tehran, 1419733141, Iran
S- Editor Cheng JX L- Editor Lalor PF E- Editor Zheng XM
References
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, Pessin MS, Weichel E, Sila CA, Furlan AJ. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 3.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 4.Rundek T, Elkind MS, Chen X, Boden-Albala B, Paik MC, Sacco RL. Increased early stroke recurrence among patients with extracranial and intracranial atherosclerosis: the Northern Manhattan Stroke Study. Neurology. 1998;50 Suppl 4:A75. [Google Scholar]
- 5.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 6.Ingall TJ, Homer D, Baker HL Jr, Kottke BA, O'Fallon WM, Whisnant JP. Predictors of intracranial carotid artery atherosclerosis. Duration of cigarette smoking and hypertension are more powerful than serum lipid levels. Arch Neurol. 1991;48:687–691. doi: 10.1001/archneur.1991.00530190033011. [DOI] [PubMed] [Google Scholar]
- 7.Komotar RJ, Wilson DA, Mocco J, Jones JE, Connolly ES Jr, Lavine SD, Meyers PM. Natural history of intracranial atherosclerosis: a critical review. Neurosurgery. 2006;58:595–601; discussion 595-601. doi: 10.1227/01.NEU.0000204102.88016.33. [DOI] [PubMed] [Google Scholar]
- 8.Arenillas JF, Alvarez-Sabín J. Basic mechanisms in intracranial large-artery atherosclerosis: advances and challenges. Cerebrovasc Dis. 2005;20 Suppl 2:75–83. doi: 10.1159/000089359. [DOI] [PubMed] [Google Scholar]
- 9.Bang OY, Lee PH, Yoon SR, Lee MA, Joo IS, Huh K. Inflammatory markers, rather than conventional risk factors, are different between carotid and MCA atherosclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1128–1134. doi: 10.1136/jnnp.2004.054403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, Gonzalez G, Schaefer PW, Dillon WP, Koroshetz WJ, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, Woimant F. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187–1191. doi: 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- 13.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 14.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 15.Mak W, Cheng TS, Chan KH, Cheung RT, Ho SL. A possible explanation for the racial difference in distribution of large-arterial cerebrovascular disease: ancestral European settlers evolved genetic resistance to atherosclerosis, but confined to the intracranial arteries. Med Hypotheses. 2005;65:637–648. doi: 10.1016/j.mehy.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 16.McTaggart RA, Jayaraman MV, Haas RA, Feldmann E. Intracranial atherosclerotic disease: epidemiology, imaging and treatment. Med Health R I. 2009;92:412–414. [PubMed] [Google Scholar]
- 17.Leung TW, Kwon SU, Wong KS. Management of patients with symptomatic intracranial atherosclerosis. Int J Stroke. 2006;1:20–25. doi: 10.1111/j.1747-4949.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 18.Segura T, Serena J, Castellanos M, Teruel J, Vilar C, Dávalos A. Embolism in acute middle cerebral artery stenosis. Neurology. 2001;56:497–501. doi: 10.1212/wnl.56.4.497. [DOI] [PubMed] [Google Scholar]
- 19.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–1980. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 20.Arenillas JF, Alvarez-Sabín J, Molina CA, Chacón P, Fernández-Cadenas I, Ribó M, Delgado P, Rubiera M, Penalba A, Rovira A, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 21.Arenillas JF, Molina CA, Chacón P, Rovira A, Montaner J, Coscojuela P, Sánchez E, Quintana M, Alvarez-Sabín J. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004;63:27–32. doi: 10.1212/01.wnl.0000132637.30287.b4. [DOI] [PubMed] [Google Scholar]
- 22.Craig DR, Meguro K, Watridge C, Robertson JT, Barnett HJ, Fox AJ. Intracranial internal carotid artery stenosis. Stroke. 1982;13:825–828. doi: 10.1161/01.str.13.6.825. [DOI] [PubMed] [Google Scholar]
- 23.Marzewski DJ, Furlan AJ, St Louis P, Little JR, Modic MT, Williams G. Intracranial internal carotid artery stenosis: longterm prognosis. Stroke. 1982;13:821–824. doi: 10.1161/01.str.13.6.821. [DOI] [PubMed] [Google Scholar]
- 24.Bogousslavsky J. Prognosis of carotid siphon stenosis. Stroke. 1987;18:537. [PubMed] [Google Scholar]
- 25.Wechsler LR, Kistler JP, Davis KR, Kaminski MJ. The prognosis of carotid siphon stenosis. Stroke. 1986;17:714–718. doi: 10.1161/01.str.17.4.714. [DOI] [PubMed] [Google Scholar]
- 26.Heneghan C. EBM resources on the new CEBM website. Evid Based Med. 2009;14:67. doi: 10.1136/ebm.14.3.67. [DOI] [PubMed] [Google Scholar]
- 27.Feldmeyer JJ, Merendaz C, Regli F. [Symptomatic stenoses of the middle cerebral artery] Rev Neurol (Paris) 1983;139:725–736. [PubMed] [Google Scholar]
- 28.Hinton RC, Mohr JP, Ackerman RH, Adair LB, Fisher CM. Symptomatic middle cerebral artery stenosis. Ann Neurol. 1979;5:152–157. doi: 10.1002/ana.410050208. [DOI] [PubMed] [Google Scholar]
- 29.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 30.Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. 2004;35:2832–2836. doi: 10.1161/01.STR.0000147035.31297.b6. [DOI] [PubMed] [Google Scholar]
- 31.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP Jr, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 32.Martí-Fàbregas J, Cocho D, Martí-Vilalta JL, Gich I, Belvís R, Bravo Y, Millán M, Castellanos M, Rodríguez-Campello A, Egido J, et al. Aspirin or anticoagulants in stenosis of the middle cerebral artery: A randomized trial. Cerebrovasc Dis. 2006;22:162–169. doi: 10.1159/000093450. [DOI] [PubMed] [Google Scholar]
- 33.Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–1652. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke. 1998;29:1389–1392. doi: 10.1161/01.str.29.7.1389. [DOI] [PubMed] [Google Scholar]
- 35.Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40:e340–e347. doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 36.Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke. 1986;17:938–942. doi: 10.1161/01.str.17.5.938. [DOI] [PubMed] [Google Scholar]
- 37.Pessin MS, Kwan ES, DeWitt LD, Hedges TR 3rd, Gale D, Caplan LR. Posterior cerebral artery stenosis. Ann Neurol. 1987;21:85–89. doi: 10.1002/ana.410210115. [DOI] [PubMed] [Google Scholar]
- 38.Pessin MS, Gorelick PB, Kwan ES, Caplan LR. Basilar artery stenosis: middle and distal segments. Neurology. 1987;37:1742–1746. doi: 10.1212/wnl.37.11.1742. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, Zaidat OO, Suarez JI, Wityk RJ. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52:1033–1039; discussion 1039-1040. [PubMed] [Google Scholar]
- 40.Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, Teal P, Dashe JF, Chaves CJ, Breen JC, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 41.Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2004;61:496–504. doi: 10.1001/archneur.61.4.496. [DOI] [PubMed] [Google Scholar]
- 42.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]
- 43.Turan TN, Maidan L, Cotsonis G, Lynn MJ, Romano JG, Levine SR, Chimowitz MI. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–509. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koroshetz WJ. Warfarin, aspirin, and intracranial vascular disease. N Engl J Med. 2005;352:1368–1370. doi: 10.1056/NEJMe058022. [DOI] [PubMed] [Google Scholar]
- 45.Kassab MY, Gupta R, Majid A, Farooq MU, Giles BP, Johnson MD, Graybeal DF, Rappard G. Extent of intra-arterial calcification on head CT is predictive of the degree of intracranial atherosclerosis on digital subtraction angiography. Cerebrovasc Dis. 2009;28:45–48. doi: 10.1159/000219296. [DOI] [PubMed] [Google Scholar]
- 46.Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, Do HM. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016–1020. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 47.Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 48.Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan) Neuroradiology. 2005;47:222–228. doi: 10.1007/s00234-005-1351-2. [DOI] [PubMed] [Google Scholar]
- 49.Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Hanel RA, Woo H, Rasmussen PA, Hopkins LN, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–887. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 50.Albuquerque FC, Levy EI, Turk AS, Niemann DB, Aagaard-Kienitz B, Pride GL Jr, Purdy PD, Welch BG, Woo HH, Rasmussen PA, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23–27; discussion 27-28. doi: 10.1227/01.NEU.0000335067.53190.A2. [DOI] [PubMed] [Google Scholar]
- 51.SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) 2008. Available from: http://www.strokecenter.org/trials/TrialDetail.aspx?tid=819. [Google Scholar]
- 52.Samaniego EA, Hetzel S, Thirunarayanan S, Aagaard-Kienitz B, Turk AS, Levine R. Outcome of symptomatic intracranial atherosclerotic disease. Stroke. 2009;40:2983–2987. doi: 10.1161/STROKEAHA.109.549972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyers PM, Schumacher HC, Tanji K, Higashida RT, Caplan LR. Use of stents to treat intracranial cerebrovascular disease. Annu Rev Med. 2007;58:107–122. doi: 10.1146/annurev.med.58.121205.100631. [DOI] [PubMed] [Google Scholar]