Abstract
Age-related declines in immune function have an impact on both primary and memory responses. In this study, we have examined the ability of naive CD4 T cells from young and aged T cell receptor transgenic mice to establish functional memory. We found that memory cells generated from young CD4 T cells responded well to antigen, even a year after generation, whereas memory cells derived from CD4 T cells from aged mice responded poorly both ex vivo and in vivo. Memory cells generated from aged naive cells proliferate less, produce reduced levels of cytokines, and exhibit reduced cognate helper function, compared with memory cells generated by using young naive cells. These results indicate that it is the age of the naive T cell when it first encounters antigen, rather than the age when it reencounters antigen, that is critical for good memory CD4 T cell function.
Because immune system function declines with age, the elderly are more susceptible to infection and are, therefore, prime candidates for vaccination. However, the ability of the immune system to respond vigorously to vaccination is also impaired with age (1–4). Although it is generally accepted that immunological memory generated early in life is maintained well into old age (reviewed in refs. 5 and 6), few studies have specifically examined memory CD4 T cell function generated during old age.
The model that we have used to study memory CD4 T cells involves the transfer of in vitro generated, polarized T cell receptor (TCR) transgenic (Tg) effectors into syngeneic nontransgenic hosts that have been thymectomized, lethally irradiated, and bone-marrow-reconstituted (ATxBM) (7). These hosts lack endogenous T cells and provide an empty space for repopulation with the transferred effectors. We have used this model to compare memory cell generation from young and aged TCR Tg mice. We and others have demonstrated that naive CD4 T cell function decreases dramatically with age (8–13). Our published studies have shown that naive CD4 T cells from aged TCR Tg mice produce less IL-2, expand less, and differentiate less on initial antigen stimulation than CD4 T cells from young individuals (9, 10). These aged effectors, generated without the addition of exogenous IL-2, produce low levels of cytokines and expand poorly. We have also shown that this defect in effector generation by aged CD4 T cells can be corrected in vitro by the addition of exogenous IL-2 (9). On the addition of IL-2, aged naive Tg CD4 T cells expand well in response to antigen, and these effectors express a highly activated phenotype. Aged naive CD4 T cells can also generate highly activated polarized T helper (Th) 1 and Th2 effectors in the presence of exogenous IL-2. These polarized aged effectors secrete high levels of the appropriate cytokines, similar to effectors generated from young mice.
In this current study, we examined the function of memory CD4 T cells generated from naive cells from young and aged mice. We show that memory cells generated from naive TCR Tg CD4 T cells from young mice function well early on and, importantly, retain their ability to respond well to antigen, even a year after adoptive transfer. Although primary effectors from aged naive cells, generated in the presence of exogenous IL-2 and polarizing cytokines, expand considerably and produce high levels of cytokines, they generate memory cells that function poorly. Our results indicate that even when vigorous primary responses are induced, they do not always lead to good CD4 T cell memory. Most importantly, it is the age of the naive CD4 T cell when it first encounters antigen that is a critical factor in robust memory-cell function.
Materials and Methods
Animals. AND TCR Tg mice, which express a Vβ3/Vα11 TCR Tg recognizing the 88–104 fragment of pigeon cytochrome c (PCCF) (14), were housed under specific pathogen-free conditions in positive-pressure ventilated racks at the Trudeau Institute until their use at 2–4 months (young) and 14–16 months (aged). B10.BR mice, purchased from The Jackson Laboratory, were thymectomized at 4–6 weeks of age, lethally irradiated [950 rads (1 rad = 0.01 Gy)], and reconstituted with T-depleted syngeneic bone marrow to generate ATxBM hosts. Experimental procedures were approved by Trudeau Institute Institutional Animal Care and Use Committee.
Cell Culture and Effector Generation. Lymphocytes were harvested from the spleens and peripheral lymph nodes of young or aged TCR Tg mice. Enrichment by negative selection and culturing of naive Tg CD4 T cell populations has been described (9). For some experiments, Tg CD4 T cells were purified by using a fluorescence-activated cell sorter (FACSVantage SE, Becton Dickinson) on Vα11/Vβ3/CD4+ populations. CD4 T cell effector populations were generated by using DCEK-ICAM, a fibroblast cell line, as an antigen-presenting cell (APC). TCR Tg CD4 cells (2 × 105 per ml) were cultured for 4 days with 5 mM PCCF and DCEK-ICAM cells (2:1 T/APC) in the presence of polarizing cytokines: IL-2 (80 units/ml), IL-12 (2 ng/ml), and anti-IL-4 (11B11, 10 mg/ml) for Th1; IL-2 (80 units/ml), IL-4 (15 ng/ml), and anti-IFN-γ (XMG1.2, 10 mg/ml) for Th2. Recombinant murine cytokines used for effector generation (IL-2, IL-4, and IFN-γ) were obtained from culture supernatant of X63.Ag8-653 cells transfected with cDNA for the respective cytokines (15). Recombinant murine IL-12 was a generous gift of S. Wolf (Genetics Institute, Cambridge, MA).
Before adoptive transfer, effector populations were examined for cell-surface phenotype by FACS analysis as described below. Effectors were also washed thoroughly and 1 × 105 cells were restimulated with Ag/APC. Culture supernatants were then collected after 24 h and assayed for cytokines as described below. To generate young and aged memory CD4 T cells, 107 4-day effectors were transferred i.v. into ATxBM hosts and then recovered 3 weeks to 12 months later.
Ex Vivo Memory Cell Function. CD4 memory T cells were recovered from spleens and lymph nodes of the adoptive hosts and were enriched as described (9). The donor memory cell recovery (Vβ3+ CD4+) from each individual host was determined by FACS analysis as described below. Memory cells were then pooled from three to four individual memory mice and cultured (2 × 105 per ml) with Ag/APC with or without IL-2 for 1–4 days to generate memory effectors. Culture supernatants were collected at indicated times and assayed for cytokines as described below. Fold expansion was determined by dividing the number of cells recovered after culture by the original input number. In some experiments CD4 memory-cell populations were labeled with the dye carboxy-fluorescein succinimidyl ester (CFSE; Molecular Probes) as described (9) before culture with Ag/APC.
In Vivo Memory Cell Function. Three to 6 weeks after transfer of effector populations, adoptive hosts were immunized with 200 μg of 4-hydroxy-3-nitrophenyl acetyl (NP)-conjugated PCC (NP-pigeon cytochrome c) or PBS in alum i.p. (two to three mice per group). To examine the expansion of Tg CD4 memory T cells, mice were given BrdUrd (1 mg/ml) in drinking water for 3 days beginning on day 0. On day 3, splenocytes were harvested, surface-stained for Vβ3 and CD4 expression, stained for BrdUrd incorporation as described (16), and subjected to FACS analysis. The percent of Vβ3/CD4 cells staining positive for BrdUrd was determined by subtracting the staining in the Vβ3/CD4 population of PBS-immunized hosts from those in NP-PCC-immunized hosts.
To examine CD4 T cell helper function, splenocytes were harvested on day 6 after immunization. NP-specific B cells were identified by staining with NP conjugated to the fluorochrome APC (NP-APC). The CD38 and peanut agglutinin (PNA) phenotype of the NP+ population was examined by FACS analysis. Additionally, serum was collected and NP-specific IgG1 and IgG2a were determined by ELISA. NP-PCC and NP-APC were prepared as described (17).
Immunofluorescent Staining. Staining was done at 4°C in PBS with 1% BSA and 0.1% NaN3. The following antibodies were used (purchased from BD Pharmingen): CyC-anti-CD4 (clone RM4-5), PE anti-CD44 (clone IM7), PE anti-CD62L (clone Mel 14), PE anti-CD25 (clone PC61), biotin, PE anti-Vβ3 (clone KJ25), FITC anti-Vα11 (clone RR8.1), and PE anti-CD38 (clone 90). FITC-PNA was purchased from Sigma. NP-APC was prepared as described (17). Isotype control antibodies and streptavidin-APC were purchased from BD Pharmingen. Flow cytometry was carried out with a FACSCalibur flow cytometer (Becton Dickinson), and data were analyzed with CELLQUEST software (BD Biosciences).
Cytokine Detection. CD4 T cell populations were stimulated with Ag/APC at a 2:1 TCR Tg CD4/APC ratio. Culture supernatants collected after 24 h of culture were assayed for the presence of IL-2 (units per milliliter) in a bioassay with NK-3 cells and for IL-4 (picograms per milliliter), IL-5 (units per milliliter), and IFN-γ (nanograms per milliliter) by ELISA.
Results
Memory mice were generated by reconstituting young ATxBM hosts with in vitro generated Th1 and Th2 effector populations, which were routinely assessed for appropriate cell-surface phenotype and cytokine production before adoptive transfer. By generating effector populations in vitro from naive T cells, we can control stimulation conditions, thus ensuring equivalent effector generation from young and aged CD4 T cell populations. After a period (from 4 weeks to 12 months), memory-cell recovery and ex vivo and in vivo function of memory CD4 T cells was assessed.
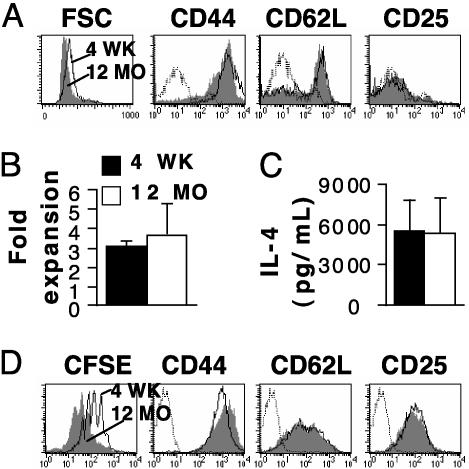
Memory Cells Generated from Young Naive Cells. Th2 effector populations were generated by using purified TCR Tg CD4 cells from young mice and then adoptively transferred to ATxBM hosts. Memory cells generated in this manner exhibited a small resting, typical memory phenotype (CD44hiCD62LhiCD25neg), even 12 months after transfer (Fig. 1A), consistent with our published observations (7). At this time (12 months after transfer), these memory cells derived from 2- to 4-month-old mice are equivalent in age to naive cells in 14-month-old Tg mice, which, as we have shown, display significant age-related functional defects (9). No significant difference occurred in the number of memory cells recovered at 4 weeks (3.5 ± 1.8 × 105 per host) and 12 months (2.9 ± 1.7 × 105 per host) after transfer.
Fig. 1.
Effect of aging on memory cells generated from young naive CD4 T cells. Th2 effectors generated by using naive cells from young mice (2–4 months old) were adoptively transferred to young ATxBM hosts. At 4 weeks and 12 months after transfer, phenotype and function of the resulting memory cell populations were examined. (A) Phenotype of resting Th2 memory cells. FACS histograms show cell-surface phenotype of 4-week (open) and 12-month (shaded) Vβ3+CD4+-gated Th2 memory cells generated with naive cells from young mice. FSC, forward scatter. (B) Four-week (filled) and 12-month (open) Th2 memory cells (1 × 105 Tg CD4 per sample) were restimulated for 3 days with Ag/APC and fold expansion was determined. The mean ± SE for three separate experiments is shown. (C) Four-week and 12-month Th2 memory cells (1 ×105 Tg CD4 per sample) were restimulated for 24 h with Ag/APC. Supernatants were collected and assayed for IL-4. The mean ± SE for three separate experiments is shown. (D) FACS histograms showing CFSE profiles and cell-surface phenotype of 4-week (open) and 12-month (shaded) Vβ3+CD4+-gated Th2 memory cells on day 3 after stimulation with Ag/APC. For all FACS histograms, dotted lines represent isotype controls, and the data shown represent three experiments.
In addition, 4 weeks and 12 months after transfer, populations underwent equivalent expansion (Fig. 1B) and produced similar levels of IL-4 (Fig. 1C) on ex vivo restimulation of equal numbers of memory cells. Expansion of these memory cells was also examined by labeling with the fluorescent dye CFSE before ex vivo stimulation with Ag/APC. The CFSE profiles, and CD44, CD62L, and CD25 expression, of these memory cells on day 3 after stimulation are shown in Fig. 1D. Both memory-cell populations expanded well, as shown by the CFSE profiles, and exhibited a typical effector phenotype (CD44hiCD62LlowerCD25hi). In fact, the 12-month-posttransfer memory cells exhibited even better expansion (more cell divisions, as shown by CFSE staining) than the 4-week posttransfer memory cells. These results show that even 12 months after transfer, memory cells generated from naive CD4 T cells from young mice retain their ability to respond well to antigen.
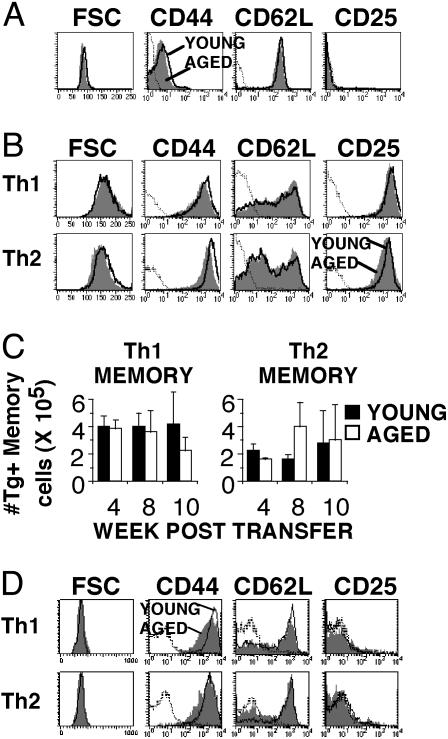
Memory Cells Generated from Aged Naive Cells. Because we determined that memory cells generated from young mice functioned well, even a year after initial generation, we next examined the effect of the age of naive CD4 T cells on memory generation and function. TCR Tg CD4 cells from young and aged mice express a naive phenotype (Fig. 2A), thus ensuring that effectors are generated with equivalent naive populations. CD4 T cells from young (2- to 4-month-old) and aged (14- to 16-month-old) TCR Tg mice were stimulated in vitro (in the presence of exogenous IL-2) to generate polarized effector populations. These effectors had similar phenotypes before adoptive transfer (Fig. 2B), consisting of large lymphoblasts with an activated phenotype (CD44hi CD62Llower CD25hi), although the Th2 effectors down-regulated CD62L to a greater extent than the Th1 effectors.
Fig. 2.
Phenotype of memory cells generated from young and aged naive CD4 T cells. Th1 and Th2 effectors were generated by using naive cells from young (2- to 4-month-old) and aged (14- to 16-month-old) Tg mice and transferred to young ATxBM hosts. Memory-cell recovery and phenotype was then assessed. (A) FACS histograms showing the cell-surface phenotype of naive Vβ3+CD4+ lymphocytes from young (open) and aged (shaded) TCR Tg mice used to generate effector populations. (B) FACS histograms, gated on Vβ3+CD4+ lymphocytes, showing cell-surface phenotype of young (open) and aged (shaded) Th1 and Th2 effector populations before adoptive transfer. (C) At each time point after transfer into ATxBM hosts, memory cells generated from young (filled) and aged (open) effectors were recovered from spleen and lymph nodes. The percent of Tg CD4 memory cells was determined by FACS analysis and gating on Vβ3+CD4+ cells, and was used to calculate the total Tg CD4 memory cell numbers. Results are shown as mean ± SE from two to four experiments. (D) FACS histograms show the cell-surface phenotype of Vβ3+CD4+-gated memory cells generated from young (open) and aged (shaded) naive cells. For all FACS data, dotted lines represent isotype controls, and the data shown represent four separate experiments. FSC, forward scatter.
Effector populations were transferred i.v. to ATxBM hosts and allowed to return to a resting state. No age-related difference occurred in recovery of memory cells at any of the time points examined (Fig. 2C). Both young and aged Th1 and Th2 effectors repopulated the hosts similarly, constituting from 1% to 3% of the total lymphocytes by 10 weeks after transfer. Memory CD4 T cells generated from naive cells from both young and aged mice exhibited a typical memory phenotype (Fig. 2D) (18–20). They were small resting cells based on forward scatter histograms with no CD25 expression. Additionally, both Th1 and Th2 memory cells up-regulated CD62L expression when compared with the pretransfer phenotype shown in Fig. 2B. These results indicate that the transferred young and aged CD4 T cell effector populations returned to a resting state after adoptive transfer.
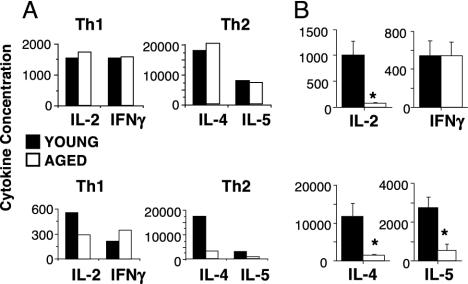
Cytokine Production by Memory Cells from Young and Aged Naive Cells. One of the hallmark features of memory CD4 T cells is rapid production of high quantities of polarized cytokines on restimulation with Ag/APC. Fig. 3A shows cytokine production by effector populations generated from young and aged naive cells and by the memory cells generated from these effectors. Primary Th1 and Th2 effector populations generated from aged naive cells produced high levels of polarized cytokines similar to effectors generated from young cells. As we have shown (9), Th1 effectors from both young and aged naive cells secreted IL-2 and IFN-γ and Th2 effectors secreted IL-4 and IL-5 (Fig. 3A, top graphs), with no age-related differences. When these effector populations were used to generate memory cells, the resulting memory-cell populations retained their polarized cytokine production pattern. However, significant age-related decreases in cytokine production became apparent in the memory cells derived from aged effectors (Fig. 3A, bottom graphs). On ex vivo restimulation of equal numbers of memory cells with Ag/APC for 24 h, Th1 memory cells generated from aged naive cells produced 50% less IL-2, whereas no age-related reduction in IFN-γ was observed. Th2 memory cells generated from aged naive cells produced only 20% of the levels of both IL-4 and IL-5 generated by memory cells from young naive cells. Fig. 3B shows a summary of memory-cell cytokine production for all experiments performed. Th1 memory cells exhibited a dramatic age-related decrease in IL-2, whereas, consistently, no decrease in IFN-γ secretion was observed. Th2 memory cells generated from aged naive cells also exhibited a significant decrease in both IL-4 and IL-5 production. In addition, Th1 and Th2 memory cells generated from aged naive cells showed similar reductions in cytokine production at 48 and 72 h after ex vivo stimulation (data not shown). These results demonstrate that even though young and aged effector populations produced similar levels of cytokines before adoptive transfer, age-related reductions in cytokine production become apparent when these effectors develop into resting memory cells.
Fig. 3.
Cytokine production by primary effector and memory populations. (A) Representative experiment showing cytokine production by primary and memory populations. Effectors (1 × 105 Tg CD4 per sample) generated by using young (filled) or aged (open) naive cells were restimulated for 24 h with Ag/APC before transfer (top graphs). Supernatants were collected and assayed for IL-2 (units per milliliter), IL-4 (picograms per milliliter), IL-5 (units per milliliter), and IFN-γ (nanograms per milliliter). Memory cells generated from these effector populations 6 weeks after transfer (1 × 105 Tg CD4 per sample) were recovered and restimulated for 24 h with Ag/APC (bottom graphs). Supernatants were collected and assayed for cytokines. (B) Summary of 24-h cytokine production from all Th1 (top graph) and Th2 (bottom graph) memory cell experiments as described in A; the data shown are mean ± SE for four to eight experiments; *, P < 0.05, statistically significant difference by Student's t test.
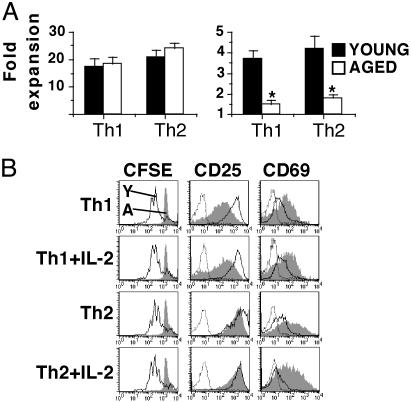
Ex Vivo Expansion of Memory Cells. We have shown that aged naive CD4 T cells do not expand as well as young cells unless exogenous IL-2 is provided (9). Therefore, we compared the expansion of young and aged naive cells during primary effector generation to the expansion of resting memory cells recovered from the adoptive hosts on ex vivo stimulation with Ag/APC. No age-related difference occurred in the expansion of Th1 and Th2 primary effector populations, generated in the presence of exogenous IL-2 (Fig. 4A Left). This result was in dramatic contrast to the memory populations derived from these effectors, which exhibited statistically significant age-related decreases in expansion in both Th1 and Th2 subsets (Fig. 4A Right).
Fig. 4.
Ex vivo expansion of effector and memory cell populations. (A) Expansion of young (filled bars) and aged (open bars) primary effector populations after 4 days of culture (Left) and memory effector populations after 3 days of culture (Right). Primary effector cultures were generated under the appropriate conditions for each effector population; memory cultures were stimulated with Ag/APC alone. *, P < 0.05, statistically significant difference by Student's t test; n = 4. (B) Th2 memory cells generated from young and aged naive cells were recovered 4 weeks after transfer, CFSE-labeled, and cultured with Ag/APC alone or with added IL-2 for 3 days. Histograms are gated on Tg (Vβ3+) CD4 cells and show CFSE profiles and CD25 and CD69 expression of young (open histograms) and aged (shaded histograms) memory cells; dotted lines represent isotype controls. The results are representative of three experiments.
The ex vivo expansion of young and aged memory cells was also examined by using the fluorescent dye CFSE. Memory cells generated by using young and aged naive cells were recovered from the adoptive hosts and were CFSE-labeled. Equal numbers of memory cells were then stimulated with Ag/APC alone or with added IL-2. In all cultures, memory cells generated from young naive cells divided several times during the 3-day culture period, whereas memory cells generated from aged naive cells had not undergone any division even on the addition of exogenous IL-2 (Fig. 4B). This result is in contrast to naive CD4 cells from aged mice, which, as we have shown, proliferated well in the presence of Ag/APC plus exogenous IL-2 (9). The memory cells generated by using aged naive cells did become activated and up-regulated both CD25 and CD69 expression. However, the memory cells generated by using aged naive cells exhibited lower CD25 expression (Th1) and higher CD69 expression (both Th1 and Th2) than young cells did. Furthermore, addition of other exogenous cytokines, such as IL-4, IL-7, or IL-15, also had no enhancing effect on aged memory-cell expansion (data not shown). These ex vivo functional defects predict that the memory cells generated by using naive cells from aged mice will also function poorly in vivo.
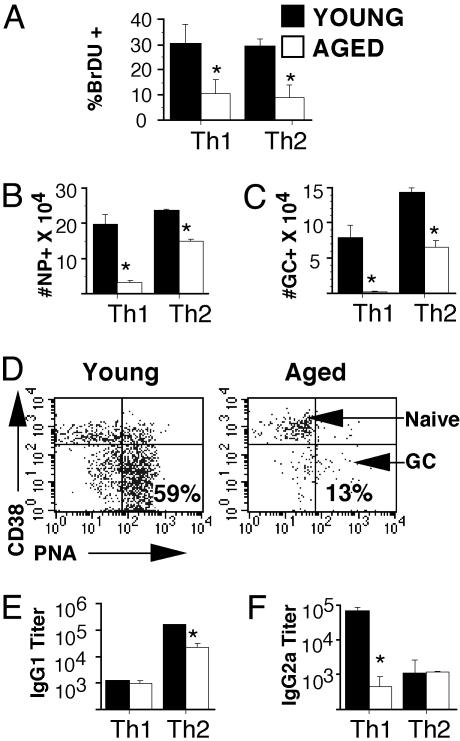
In Vivo Function of Memory Cells. To determine whether this ex vivo proliferative defect of memory cells generated from aged naive cells also occurred in vivo, memory mice were immunized with the hapten NP conjugated to whole PCC protein (NP-PCC) or PBS in alum and BrdUrd (1 mg/ml) was administered in the drinking water (day 0). On day 3, BrdUrd incorporation of young and aged memory cells was determined by FACS analysis. About 30% of Vβ3/CD4 memory cells from young naive cells stained positive for BrdUrd incorporation, whereas only ≈10% of the memory cells from aged naive cells stain positive (Fig. 5A). These results indicate that the ex vivo proliferative defect of the Vβ3/CD4 memory cells from aged naive cells, as shown in Fig. 4, also occurred in vivo after immunization.
Fig. 5.
In vivo function of memory cells. Memory mice generated with young and aged Th1 and Th2 effectors (3 weeks after transfer) were immunized with NP-PCC/alum i.p. and given BrdUrd (BrDU) in drinking water. (A) On day 3, spleens were harvested, and cells were stained for CD4, Vβ3, and BrdUrd. The graph shows the percent of BrdUrd+ staining in CD4/Vβ3-gated populations of Th2 memory cells generated from young (filled bars) or aged (open bars) naive cells. Isotype staining was <5%. (B) On day 6, spleens were harvested and NP-specific B cells were examined. The total number of NP+ B cells generated in memory mice generated by using young (filled bars) or aged (open bars) Th1 and Th2 effectors was determined by staining splenocytes with NP-APC. (C) The total number of germinal center + NP+ cells in Th1 and Th2 memory mice from B was determined by FACS analysis. (D) Representative FACS dot plots showing CD38 and PNA expression on NP + B cells in immunized Th2 memory mice generated by using sort-purified young or aged naive cells. (E) NP-specific serum IgG1 titers on day 14 after immunization of memory mice were determined by ELISA. (F) NP-specific serum IgG2a titers on day 14 after immunization of memory mice were determined by ELISA. For all experiments, data shown are compiled from four separate experiments; *, P < 0.05, statistically significant difference by Student's t test.
B Cell Helper Function of Memory Cells. Other researchers have shown that CD4 T cells from aged mice exhibit greatly decreased B cell helper activity (21–26), resulting in the production of lower titers of antibodies and reduced affinity maturation of these antibodies in the aged. Therefore, we tested the ability of Th1 and Th2 memory cells generated from young and aged naive Tg CD4 T cells to help NP-specific B cell expansion and differentiation. To ensure that no other contaminating T cells were present to account for our observed results, these experiments were also performed with FACS-sorted naive Vαll/Vβ3/CD4+ T cells from young and aged mice, in addition to our standard negative selection of CD4 T cells. The results of experiments using young and aged naive CD4 T cells prepared by both methods are compiled in Fig. 5 B and C. In addition, representative results using sort purified naive T cells is also shown in Fig. 5D.
Memory mice were immunized with NP-PCC/alum 3 weeks after transfer of effector populations generated from young and aged naive cells. The expansion and phenotype of NP-specific B cells, which can be identified with a NP-APC staining reagent, was determined by FACS analysis. On day 6 after immunization, the number of NP-binding cells and the number of germinal center + NP-binding cells in memory mice reconstituted with young Th1 or Th2 effectors was significantly increased compared with mice generated with effectors produced from aged naive cells (Fig. 5 B and C, respectively). This finding is also shown in the representative FACS dot plots shown in Fig. 5D, comparing Th2 memory mice generated with young or aged Th2 effectors. Dramatic differentiation to germinal-center phenotype (PNAhi CD38lo) (27) occurred in memory mice generated with young effectors, whereas little differentiation of NP-specific cells occurred in memory mice generated with aged effectors. In all experiments, the NP-binding population in PBS-immunized controls did not expand (data not shown).
Memory cells generated from young naive cells also promoted the production of high levels of NP-specific IgG. Th2 memory cells from young effectors induced significantly higher levels of IgG1 (Fig. 5D) and Th1 memory cells from young effectors induced significantly higher levels of IgG2a (Fig. 5E) than memory cells generated from aged effectors.
Together, these results indicate that memory cells generated by using naive cells from young mice exhibited good cognate helper function, which lead to the production of high levels of antibody, whereas memory cells generated from aged naive T cells did not. This finding also correlates with our ex vivo results, showing that CD4 memory T cells generated by using naive cells from aged mice exhibited dramatic age-related defects in response to antigen.
Discussion
Because the elderly exhibit a greater susceptibility to infectious diseases such as influenza (28, 29), they are often prime candidates for vaccinations. However, evidence is now increasing that the efficacy of these vaccinations in the elderly is greatly reduced compared with younger populations (30–32). As we have shown in this and other studies (7), memory generated by using naive T cells from young mice responds well, even 1 year after priming. In contrast, memory generated during old age is defective both in vitro and in vivo, suggesting that naive CD4 T cells from aged mice exhibit a heritable defect.
Before adoptive transfer, Th1 and Th2 effector populations generated from young and aged naive cells in the presence of exogenous IL-2 and polarizing cytokines expanded equivalently and produced similarly high levels of cytokines, with no age-related differences. In addition, the cell-surface phenotypes of these young and aged effector populations were very similar, including up-regulation of CD25 expression. By generating effector populations in vitro, we ensured that the primary responses of the young and aged CD4 T cells were similar. Therefore, at the time of transfer, the young and aged in vitro generated effector populations were phenotypically and functionally comparable by several criteria. These effectors were then used to generate the corresponding young and aged memory-cell populations. This approach allowed us to compare similar populations of young and aged antigen-specific cells and to examine subsequent memory-cell generation and function.
We found no age-related defect in the quantitative establishment of memory phenotype CD4 T cells in our adoptive-transfer model. CD4 T cell effector populations generated by using naive cells from young and aged mice reconstitute hosts similarly and generate memory T cell populations with a typical memory-cell phenotype. However, after the young and aged effector populations were adoptively transferred and allowed to return to a resting state, we found that the Th1 and Th2 memory populations generated from aged naive cells exhibited striking defects in proliferation and effector cytokine production. The defect in proliferation in response to antigen occurred both ex vivo and in vivo.
Our results are similar to a recent study by Kapasi et al., which examined the establishment and function of antigen-specific CD4 and CD8 memory T cells in young and aged mice after infection with lymphocytic choriomeningitis virus (33). This study found that generation of CD8 T cell memory is defective in old mice, whereas maintenance of CD8 memory is not affected by aging. This study also examined CD4 memory cells and found that no age-related differences occurred in memory-cell persistence or IFN-γ production. Although these results are limited, they are very similar to our results, where we see no age-related difference in persistence of memory cells (Fig. 2) or IFN-γ production (Fig. 3). The differences that we do observe are in proliferation in response to antigen stimulation, in IL-2 production by Th1 and IL-4 and IL-5 production by Th2 memory cells, and in cognate helper function.
Although our observations are similar to those of Kapasi et al. (33), our model allows more definitive answers. In our model, we induce an equivalent primary response of naive CD4 T cells from young and aged mice. Kapasi et al. (33) show that they begin with more antigen-experienced cells from aged mice compared with mostly naive cells from young mice, and their model does not allow them to compare similar numbers of responding cells. Additionally, in their study, primary responses of young and aged CD4 and CD8 T cells is dramatically different, including reduced expansion and cytokine production in the aged population. Because an age-related reduction occurs in the primary response, it is not surprising that an age-related defect is found in the subsequent memory population. Our model addresses this issue by priming young and aged CD4 T cells in vitro to ensure that we have equivalent primary stimulation. We then show that even when young and aged cells are equally primed, a defect occurs in the secondary response of the memory cells derived from aged naive CD4 T cells.
In addition to expansion and cytokine production, our results show that the cognate helper function of aged memory cells had a profound in vivo age-related defect. This defect in the CD4 memory population generated from aged naive cells resulted in reduced antigen-specific B cell expansion, germinal center development, and IgG production. This age-related reduction in cognate helper function of newly generated memory CD4 T cells could be responsible for the often-observed reduction in antibody titers that occurs in elderly populations on immunization (1–4, 34). If our results were proven applicable to humans, they would suggest that vaccinations should be given before old age to be optimally effective.
The results presented in this study demonstrate two key points. First, the age of the CD4 T cell when it first encounters antigen is a critical factor in determining whether robust memory responses are achieved. Memory cells generated by using naive CD4 T cells from young mice respond well on restimulation with antigen, even when they are chronologically old, whereas those derived from comparably aged naive cells respond poorly. Second, even though naive CD4 cells from aged mice can be induced to become highly activated effectors in the presence of exogenous IL-2, they exhibit age-associated defects when they return to a resting memory state. These age-related defects lead to reduced ex vivo and in vivo function of memory CD4 T cells generated by using aged naive cells. Our results imply that the aged naive cells develop defective responses over time, which are the result of heritable changes, because they are also evident in the resulting memory population. It is also interesting that memory cells generated by using naive cells from young mice do not seem to develop similar defects as they age, implying that intriguing differences exist in the impact of aging on naive and memory CD4 T cells.
Acknowledgments
We thank Drs. Stephen Smiley, Richard Dutton, David Woodland, and Deborah Brown for their critical reading of this manuscript. This work was supported by National Institutes of Health/National Institute on Aging Grants AG01743 (to S.L.S.) and AG21054 (to L.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TCR, T cell receptor; Tg, transgenic; ATxBM, adult thymectomized, lethally irradiated, bone marrow reconstituted; PCC, pigeon cytochrome c; PCCF, 88–104 peptide fragment of PCC; NP, 4-hydroxy-3-nitrophenyl acetyl; CFSE, carboxy-fluroscein succinimidyl ester; FACS, fluorescence-activated cell sorter; APC, antigen-presenting cell; Th, T helper; PNA, peanut agglutinin.
References
- 1.Phair, J., Kauffman, A., Bjornson, A., Adams, L. & Linnemann, C. (1978) J. Lab. Clin. Med. 92, 822-828. [PubMed] [Google Scholar]
- 2.Musher, D. M., Chapman, A. J., Goree, A., Jonsson, S., Briles, D. & Baughn, R. E. (1986) J. Infect. Dis. 154, 245-256. [DOI] [PubMed] [Google Scholar]
- 3.Cook, J. M., Gualde, N., Hessel, L., Mounier, M., Michel, J. P., Denis, F. & Ratinaud, M. H. (1987) Cell. Immunol. 109, 89-96. [DOI] [PubMed] [Google Scholar]
- 4.Burns, E. A., Lum, L. G., L'Hommedieu, G. & Goodwin, J. S. (1993) J. Gerontol. 48, B231-B236. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed, R. & Gray, D. (1996) Science 272, 54-60. [DOI] [PubMed] [Google Scholar]
- 6.Kaech, S. M., Wherry, E. J. & Ahmed, R. (2002) Nat. Rev. Immunol. 2, 251-262. [DOI] [PubMed] [Google Scholar]
- 7.Swain, S. L. (1994) Immunity 1, 543-552. [DOI] [PubMed] [Google Scholar]
- 8.Haynes, L., Eaton, S. M. & Swain, S. L. (2000) Vaccine 18, 1649-1653. [DOI] [PubMed] [Google Scholar]
- 9.Haynes, L., Linton, P.-J., Eaton, S. M., Tonkonogy, S. L. & Swain, S. L. (1999) J. Exp. Med. 190, 1013-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linton, P.-J., Haynes, L., Klinman, N. R. & Swain, S. L. (1996) J. Exp. Med. 184, 1891-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linton, P.-J., Haynes, L., Tsui, L., Zhang, X. & Swain, S. (1997) Immunol. Rev. 160, 9-18. [DOI] [PubMed] [Google Scholar]
- 12.Miller, R. A. (1999) in Fundamental Immunology, ed. Paul, W. E. (Lippincott-Raven Publishers, Philadelphia), pp. 947-966.
- 13.Miller, R. A. (1996) Science 273, 70-74. [DOI] [PubMed] [Google Scholar]
- 14.Kaye, J., Hsu, M.-L., Sauron, M.-E., Jameson, S. C., Gascoigne, N. R. J. & Hedrick, S. M. (1989) Nature 341, 746-749. [DOI] [PubMed] [Google Scholar]
- 15.Karasuyama, H. & Melchers, F. (1988) Eur. J. Immunol. 18, 97-104. [DOI] [PubMed] [Google Scholar]
- 16.Mohri, H., Bonhoeffer, S., Monard, S., Perelson, A. S. & Ho, D. D. (1998) Science 279, 1223-1227. [DOI] [PubMed] [Google Scholar]
- 17.Lalor, P. A., Nossal, G. J., Sanderson, R. D. & McHeyzer-Williams, M. G. (1992) Eur. J. Immunol. 22, 3001-3011. [DOI] [PubMed] [Google Scholar]
- 18.Hengel, R. L., Thaker, V., Pavlick, M. V., Metcalf, J. A., Dennis, G., Jr., Yang, J., Lempicki, R. A., Sereti, I. & Lane, H. C. (2003) J. Immunol. 170, 28-32. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadzadeh, M., Hussain, S. F. & Farber, D. L. (2001) J. Immunol. 166, 926-935. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708-712. [DOI] [PubMed] [Google Scholar]
- 21.Goidl, E. A., Innes, J. B. & Weksler, M. E. (1976) J. Exp. Med. 144, 1037-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti, C. & Cerny, J. (1991) Cell. Immunol. 133, 72-83. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti, C. & Cerny, J. (1992) Cell. Immunol. 144, 332-346. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti, C., Yang, X. & Cerny, J. (1993) J. Immunol. 150, 543-549. [PubMed] [Google Scholar]
- 25.Miller, C. & Kelsoe, G. (1995) J. Immunol. 155, 3377-3384. [PubMed] [Google Scholar]
- 26.Zheng, B., Han, S., Takahashi, Y. & Kelsoe, G. (1997) Immunol. Rev. 160, 63-77. [DOI] [PubMed] [Google Scholar]
- 27.Shinall, S. M., Gonzalez-Fernandez, M., Noelle, R. J. & Waldschmidt, T. J. (2000) J. Immunol. 164, 5729-5738. [DOI] [PubMed] [Google Scholar]
- 28.Couch, R. B., Kasel, J. A., Glezen, W. P., Cate, T. R., Six, H. R., Taber, L. H., Frank, A. L., Greenberg, S. B., Zahradnik, J. M. & Keitel, W. A. (1986) J. Infect. Dis. 153, 431-440. [DOI] [PubMed] [Google Scholar]
- 29.Glezen, W. P., Decker, M., Joseph, S. W. & Mercready, R. G., Jr. (1987) J. Infect. Dis. 155, 1119-1126. [DOI] [PubMed] [Google Scholar]
- 30.Keren, G., Segev, S., Morag, A., Zakay-Rones, Z., Barzilai, A. & Rubinstein, E. (1988) J. Med. Virol. 25, 85-89. [DOI] [PubMed] [Google Scholar]
- 31.McElhaney, J. E., Beattie, B. L., Devine, R., Grynoch, R., Toth, E. L. & Bleackley, R. C. (1990) J. Am. Geriatr. Soc. 38, 652-658. [DOI] [PubMed] [Google Scholar]
- 32.Murasko, D. M., Bernstein, E. D., Gardner, E. M., Gross, P., Munk, G., Dran, S. & Abrutyn, E. (2002) Exp. Gerontol. 37, 427-439. [DOI] [PubMed] [Google Scholar]
- 33.Kapasi, Z. F., Murali-Krishna, K., McRae, M. L. & Ahmed, R. (2002) Eur. J. Immunol. 32, 1567-1573. [DOI] [PubMed] [Google Scholar]
- 34.Burns, E. A., Lum, L. G., Seigneuret, M. C., Giddings, B. R. & Goodwin, J. S. (1990) Mech. Ageing Dev. 53, 229-241. [DOI] [PubMed] [Google Scholar]