Abstract
AIM: To compare the outcomes of endoscopic resection with transanal excision in patients with early rectal cancer.
METHODS: Thirty-two patients with early rectal cancer were treated by transanal excision or endoscopic resection between May 1999 and December 2007. The patients were regularly re-examined by means of colonoscopy and abdominal computed tomography after resection of the early rectal cancer. Complications, length of hospital-stay, disease recurrence and follow up outcomes were assessed.
RESULTS: Sixteen patients were treated by endoscopic resection and 16 patients were treated by transanal excision. No significant differences were present in the baseline characteristics. The rate of complete resection in the endoscopic resection group was 93.8%, compared to 87.5% in the transanal excision group (P = 0.544). The mean length of hospital-stay in the endoscopic resection group was 2.7 ± 1.1 d, compared to 8.9 ± 2.7 d in the transanal excision group (P = 0.001). The median follow up was 15.0 mo (range 6-99). During the follow up period, there was no case of recurrent disease in either group.
CONCLUSION: Endoscopic resection was a safe and effective method for the treatment of early rectal cancers and its outcomes were comparable to those of transanal excision procedures.
Keywords: Endoscopic resection, Rectal cancer, Transanal excision
INTRODUCTION
Rectal cancer is one of the commonest gastrointestinal cancers worldwide[1]. Low anterior resection and abdomino-perineal resection with total mesorectal excision are the standard treatment methods used for patients with low rectal cancer. However, rectal resection requires surgical intervention with considerable morbidity[2].
Low rectal cancer presents a challenge to surgeons with regard to local disease control and sphincter preservation[3-8]. With conventional abdomino-perineal resection, an acceptable local control rate can be achieved; however, the permanent stoma is associated with an increased risk of sexual and/or urinary dysfunction[9].
Endoscopic resection and transanal excision are regarded as alternative procedures to radical surgery in patients with early rectal cancer[2]. However, until now, no comparisons between transanal excision and endoscopic resection in patients with early rectal cancer have been made.
The aim of the present study was to compare complete resection and recurrence of early rectal cancer after transanal excision to endoscopic resection, and to investigate the safety and efficacy of transanal excision compared to endoscopic resection for early rectal cancer.
MATERIALS AND METHODS
Patients
Between May 1999 and December 2007, 32 patients were selected for the study. Candidates for transanal excision were chosen according to the following criteria: the mobility, size (< 3.5 cm), and accessibility (usually within 10 cm of the anal verge) of the tumor. Criteria for endoscopic resection of early rectal cancer at our institution included the following: (1) well or moderately differentiated adenocarcinoma on the forceps biopsy; (2) the mucosal or minute submucosal (sm1 < 1000 μm) type; (3) no lymphatic or vascular invasion. Whether these criteria were satisfied or not was not known before endoscopic resection. The decision to treat patients with endoscopic resection was therefore based on our own close observation and confirmation of the lesion.
After the transanal excision or endoscopic resection procedures, the patients were regularly re-examined by means of colonoscopy and/or abdominal computed tomography.
Methods
A data collection sheet was designed to obtain the relevant clinical information including baseline characteristics, tumor size, pathology of the tumor specimen, resection method used, margin involvement of specimens and any complications; this information was retrospectively reviewed. The recurrence of early rectal cancer and other associated factors were also analyzed. The study was approved by the Institutional Review Board of our institute.
Endoscopic resection: Endoscopic resection was performed after close observation and confirmation of the lesion. In cases of semi-pedunculated or pedunculated types, the mass was resected by polypectomy method with snaring. If the mass was a flat or excavated type, submucosal hypertonic saline mixed with epinephrine (1:10 000) was injected to make a mucosal bleb. The lesion was incised and dissected if larger than 3 cm (Endoscopic submucosal dissection, ESD) or snared and cut out if smaller than 3 cm (Endoscopic mucosal resection, EMR). The resected specimens were washed in normal saline, fixed in 8% formaldehyde solution, and embedded in paraffin. Complete resection was defined as free of marginal invasion by cancer cells.
Transanal excision: Transanal endoscopic microsurgery (TEM) as the treatment option seems to be superior to transanal excision in someways. However, we had no TEM instruments at our institution. Therefore, transanal excision as the treatment option was always chosen. The procedure was performed under local anesthetic. Prone jack-knife or lithotomy position were the preferred positions. A Parks three-bladed anal retractor was inserted through the anus. After confirmation of the tumor, full-thickness excision with Metzenbaum scissors and electrocautery was performed. The defect was repaired with synthetic absorbable sutures.
Statistical analysis
All analyses were performed using the statistical package for the social sciences program (SPSS, version 14.0, Chicago, IL, USA). The differences between the two groups were compared using the t-test or χ2 test. A P < 0.05 was considered statistically significant.
RESULTS
Complete resection rate
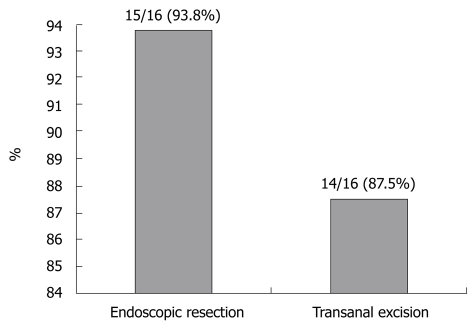
Thirty-two patients were included in the study. One was found to have positive resection margins on the endoscopic resection specimen, and two were found to have positive resection margins on the transanal excision specimen. Therefore, the number of complete resections carried out on the 16 endoscopic resection patients was 15 (93.8%) and the number of complete resections carried out on the 16 transanal excision patients was 14 (87.5%). No significant difference was found between the two groups with regard to complete resection (P = 0.544, Figure 1). The three patients with positive resection margins were excluded from further analysis.
Figure 1.
Complete resection rates for early rectal cancer. No significant difference was found between the two groups (P = 0.544).
The endoscopic resection methods used were ESD in 1, EMR in 9, and polypectomy in 5 patients.
Tumor and patient characteristics
There were 7 males and 7 females in the transanal excision group. The mean age was 57.0 ± 12.7 years in the transanal excision group. There were 7 males and 8 females in the endoscopic resection group. The mean age was 59.8 ± 8.9 years in the endoscopic resection group. No significant difference was found between the two groups with regard to age and gender (P = 0.419 and P = 0.858, respectively).
In the transanal excision group, the mean tumor size was 2.0 ± 1.0 cm and the mean tumor location from the anal verge was 5.2 ± 2.2 cm. In the endoscopic resection group, the mean tumor size was 1.8 ± 1.0 cm and the mean tumor location from the anal verge was 9.6 ± 6.5 cm. No significant difference with regard to tumor size and location was observed in either of the two groups.
The histological diagnosis of the tumors in the transanal excision group was that of well differentiated adenocarcinoma in 13 and moderately differentiated adenocarcinoma in 1 patient. All tumors in the transanal excision group were confined to the rectal mucosa. The histological diagnosis of the tumors in the endoscopic resection group was that of well differentiated adenocarcinoma in all 15 patients. The tumor invasion depth in the endoscopic resection group was mucosa in 14 and sm1 (< 1000 μm) in 1 patient (Table 1).
Table 1.
Characteristics of patient and rectal cancer
| Transanal excision group (n = 14) | Endoscopic resection group (n = 15) | P value | |
| Patient characteristics | |||
| Age (yr) | 57.0 ± 12.7 | 59.4 ± 8.9 | 0.419 |
| Sex (male/female) | 7/7 | 7/8 | 0.858 |
| Rectal tumor characteristics | |||
| Location from AV (cm) | 5.2 ± 2.2 | 9.6 ± 6.5 | 0.188 |
| Size (cm) | 2.0 ± 1.0 | 1.8 ± 1.0 | 0.728 |
| Tumor depth | |||
| Mucosa | 14 | 14 | |
| Submucosa | 0 | 1 | |
| Tumor histology | |||
| Well differentiation | 13 | 15 | |
| Moderate | 1 | 0 |
AV: Anal verge.
Clinical outcomes
The median follow up period was 21.5 mo (6-99 mo) for the transanal excision group and 12.0 mo (6-70 mo) for the endoscopic resection group. These differences were not significant.
There was one episode of delayed bleeding after the endoscopic resection which was managed successfully by endoscopic hemoclipping. This episode of delayed bleeding did not need a transfusion and the patient was hospitalized and treated for 2 d. There were no other serious complications in the two groups.
The mean hospital-stay was 8.9 ± 2.7 d for the patients in the transanal excision group and 2.7 ± 1.1 d for the patients in the endoscopic resection group. The patients in the endoscopic resection group had a shorter hospital-stay duration compared to those in the transanal excision group (P = 0.001, Table 2).
Table 2.
Follow-up and results after transanal excision and endoscopic resection of rectal cancer
| Transanal excision group (n = 14) | Endoscopic resection group (n =15) | P value | |
| Median follow up period (mo) | 21.5 | 12.0 | 0.605 |
| Mean hospital-stay (d) | 8.9 ± 2.7 | 2.7 ± 1.1 | 0.001 |
| Severe complications | |||
| Severe bleeding | 0 | 1 | |
| Recurrence | 0 | 0 |
During a median follow-up period of 21.5 mo, all 14 patients in the transanal local excision group were free of disease recurrence. In addition, during a median follow-up period 12.0 mo, all 15 patients in the endoscopic resection group were free of disease recurrence (Table 2).
DISCUSSION
Colorectal cancer is the second most common cause of cancer death in the Western world. More than 35 000 new rectal cancers are diagnosed every year in the USA, and of these, 25% are stage I disease. Fewer than half of these cases are lesions confined to the mucosa and submucosa[10]. Increasing concerns regarding the burden of rectal cancer have led to growing efforts to achieve early endoscopic detection and treatment of cancer in the rectal mucosa. Secondary prevention of rectal cancer depends on simultaneous detection of early rectal cancers and their premalignant precursors. Early rectal cancers have a better prognosis than advanced rectal cancer.
Local excision of early rectal cancer has potential benefits for patients in terms of sphincter preservation, with low mortality and fast recovery. There are several methods of local excision; transanal excision, transanal endoscopic microsurgery (TEM), and endsocopic resection[11].
Low associated morbidity and semicolon mortality makes the treatment of early rectal cancer by transanal excision an appealing alternative to radical resection[11]. The Association of Coloproctology of Great Britain and Ireland recommends that transanal excision to cure early rectal cancer should be restricted to pT1 cancers with well or moderate differentiation and < 3 cm in diameter[12].
Transanal excision was the most commonly performed procedure for local excision of rectal masses. TEM has long been utilized in Europe but has been adopted much more slowly in the United States[13]. Recent resurgence in local excision of rectal masses has stimulated renewed interest in the procedure. TEM has been advocated by some as a superior technique to transanal excision, offering lower recurrence rates without increases in morbidity[14-16].
On the other hand, the treatment of early rectal cancer by means of endoscopic resection might well be a safe and effective alternative. Endoscopic resection can be used as curative treatment in selected patients with early rectal cancer. It has been accepted not only in Japan but also in Western countries. However, prospective studies are still needed to compare the outcomes of endoscopic resection techniques with laparoscopic surgery for patients with early rectal cancer[17-19]. The criteria for endoscopic resection of early rectal cancer are controversial, but generally include the following: (1) well or moderately differentiated adenocarcinoma; (2) the mucosal or minute submucosal (sm1 < 1000 μm) type; (3) no lymphatic or vascular invasion[20].
No large studies have compared the effectiveness of endoscopic resection with transanal excision.
In this study, the number of complete resections in the 16 endoscopic cases was 15 (93.8%) and in the 16 transanal local excisions it was 14 (87.5%). Moore et al[21] reported a 78% rate of complete resection after transanal excision. Bergmann et al[22] reported a 97% rate of complete resection after endoscopic resection. In our study, no significant difference was found for complete excision between the two groups, consistent with the reports of the previous two studies.
The mean hospital-stay was 8.9 ± 2.7 d for the patients in the transanal excision group and 2.7 ± 1.1 d in the endoscopic resection group. The patients in the endoscopic resection group had a shorter hospital stay, compared to the transanal excision group (P = 0.001). This might be explained by the fact that transanal excision required full-thickness excision, and needed longer observation times.
There were no significant differences between the two study groups with regard to rectal cancer size, location from the anal verge and histological differentiation. All of the patients in both groups were free of recurrence during the follow-up period. For early rectal cancer, transanal excision has a 0%-32% recurrence rate[23-25], whereas TEM has yielded recurrence rates of 5 to 15 percent[26-28]. Few studies have compared TEM to transanal excision for early rectal cancer. The University of Minnesota reported a retrospective analysis of their experience with transanal excision and TEM. Recurrence rates for TEM were lower when compared with transanal excision (9% vs 33%, P < 0.001)[29]. Sengupta et al[6] performed a meta-analysis that demonstrated recurrence rates from 4.2% to 25% for lesions excised by TEM. For pT1 lesions, recurrence rates have been reported as ranging between 0%-12.5%[30].
In this study, the comparison between transanal local excision and endoscopic resection in the patients with early rectal cancer of equal grade showed that, in the selected patients, the two procedures were equally safe and effective with regard to treatment, outcome and disease recurrence.
The results of this study suggest that endoscopic resection can be considered as a treatment option for patients with early rectal cancer. Radical surgery, transanal excision and the associated complications might thus be avoided in high risk groups such as the elderly and those with significant co-morbidity.
The limitations of this study include the following; (1) the number of patients that underwent endoscopic resection or transanal excision was small, (2) the follow period was short (3) and the study design was retrospective and non-randomized. However, to the best of our knowledge, this is the first report to compare endoscopic resection and transanal excision as treatments for early rectal cancers.
In conclusion, endoscopic resection was safe and effective for the treatment of early rectal cancers; the outcomes were comparable to patients undergoing a transanal excision. In addition, the endoscopic resection had the advantage of a shorter hospital recovery.
COMMENTS
Background
Screening colonoscopy enables us to detect early colorectal cancer, which is demanding relative non-invasive treatment strategy. This study compared the results of two non-invasive methods for early rectal cancer; transanal excision and colonoscopic resection.
Research frontiers
Transanal endoscopic microsurgery (TEM) have been gaining the attention for the treatment option of early rectal cancer.
Innovations and breakthroughs
This is the first study which compared the results of the transanal resection with that of the endoscopic resection. Endoscopic resection is safe and effective for the treatment of early rectal cancer and comparable to the outcomes of the transanal resection.
Applications
These results mean that the two non-invasive methods might be the options for the early rectal cancer in clinical practice. The feasibility and accessibility should be evaluated according to the site within the rectum. The comparative study are needed between TEM and endoscopic resection.
Peer review
This is an interesting paper focused on effectiveness of endoscopic resection for early rectal cancer comparing to transanal resection. Although this paper has limitations due to retrospective, non-randomized and small numbers based design, it will contribute to readers in the field of therapeutic endoscopy. For more benefit, a number of points clarifying and certain statements require further justification.
Footnotes
Peer reviewer: Omar Javed Shah, Professor, Head, Department of Surgical Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India
S- Editor Zhang HN L- Editor Herholdt AV E- Editor Ma WH
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Koninger J, Muller-Stich BP, Autschbach F, Kienle P, Weitz J, Buchler MW, Gutt CN. Endoscopic posterior mesorectal resection as an option to combine local treatment of early stage rectal cancer with partial mesorectal lymphadenectomy. Langenbecks Arch Surg. 2007;392:567–71. doi: 10.1007/s00423-007-0211-4. [DOI] [PubMed] [Google Scholar]
- 3.Stahl TJ, Murray JJ, Coller JA, Schoetz DJ Jr, Roberts PL, Veidenheimer MC. Sphincter-saving alternatives in the management of adenocarcinoma involving the distal rectum. 5-year follow-up results in 40 patients. Arch Surg. 1993;128:545–549; discussion 549-550. doi: 10.1001/archsurg.1993.01420170079011. [DOI] [PubMed] [Google Scholar]
- 4.Steele GD Jr, Herndon JE, Bleday R, Russell A, Benson A 3rd, Hussain M, Burgess A, Tepper JE, Mayer RJ. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433–441. doi: 10.1007/s10434-999-0433-5. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. doi: 10.1097/00000658-200003000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345–1361. doi: 10.1007/BF02234796. [DOI] [PubMed] [Google Scholar]
- 7.Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, Garcia-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071; discussion 1071-1074. doi: 10.1007/BF02236551. [DOI] [PubMed] [Google Scholar]
- 8.Bleday R. Local excision of rectal cancer. World J Surg. 1997;21:706–714. doi: 10.1007/s002689900295. [DOI] [PubMed] [Google Scholar]
- 9.Min BS, Kim NK, Ko YT, Lee KY, Baek SH, Cho CH, Sohn SK. Long-term oncologic results of patients with distal rectal cancer treated by local excision with or without adjuvant treatment. Int J Colorectal Dis. 2007;22:1325–1330. doi: 10.1007/s00384-007-0339-2. [DOI] [PubMed] [Google Scholar]
- 10.Blair S, Ellenhorn JD. Transanal excision for low rectal cancers is curative in early-stage disease with favorable histology. Am Surg. 2000;66:817–820. [PubMed] [Google Scholar]
- 11.Whitehouse PA, Armitage JN, Tilney HS, Simson JN. Transanal endoscopic microsurgery: local recurrence rate following resection of rectal cancer. Colorectal Dis. 2008;10:187–193. doi: 10.1111/j.1463-1318.2007.01291.x. [DOI] [PubMed] [Google Scholar]
- 12.The Association of Coloproctology of Great Britain and Ireland. Guidelines for the Management of Colorectal, 2001:30–31. [Google Scholar]
- 13.Cataldo PA. Transanal endoscopic microsurgery. Surg Clin North Am. 2006;86:915–925. doi: 10.1016/j.suc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Middleton PF, Sutherland LM, Maddern GJ. Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum. 2005;48:270–284. doi: 10.1007/s10350-004-0804-8. [DOI] [PubMed] [Google Scholar]
- 15.Guerrieri M, Baldarelli M, Morino M, Trompetto M, Da Rold A, Selmi I, Allaix ME, Lezoche G, Lezoche E. Transanal endoscopic microsurgery in rectal adenomas: experience of six Italian centres. Dig Liver Dis. 2006;38:202–207. doi: 10.1016/j.dld.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Dixon MR, Finne CO, Madoff RD. Transanal endoscopic microsurgery improves outcomes in local treatment of early rectal cancer. Dis Colon Rectum. 2006;49:715–716. [Google Scholar]
- 17.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 18.Hurlstone DP, Cross SS, Brown S, Sanders DS, Lobo AJ. A prospective evaluation of high-magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc. 2004;59:642–650. doi: 10.1016/s0016-5107(04)00156-7. [DOI] [PubMed] [Google Scholar]
- 19.Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 20.Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, Mori M, Itoh H. Endoscopic submucosal dissection for rectal tumors. Endoscopy. 2007;39:423–427. doi: 10.1055/s-2007-966237. [DOI] [PubMed] [Google Scholar]
- 21.Moore JS, Cataldo PA, Osler T, Hyman NH. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum. 2008;51:1026–1030; discussion 1030-1031. doi: 10.1007/s10350-008-9337-x. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann U, Beger HG. Endoscopic mucosal resection for advanced non-polypoid colorectal adenoma and early stage carcinoma. Surg Endosc. 2003;17:475–479. doi: 10.1007/s00464-002-8931-6. [DOI] [PubMed] [Google Scholar]
- 23.Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12:3186–3195. doi: 10.3748/wjg.v12.i20.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentrem DJ, Okabe S, Wong WD, Guillem JG, Weiser MR, Temple LK, Ben-Porat LS, Minsky BD, Cohen AM, Paty PB. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg. 2005;242:472–477; discussion 477-479. doi: 10.1097/01.sla.0000183355.94322.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bretagnol F, Rullier E, George B, Warren BF, Mortensen NJ. Local therapy for rectal cancer: still controversial? Dis Colon Rectum. 2007;50:523–533. doi: 10.1007/s10350-006-0819-4. [DOI] [PubMed] [Google Scholar]
- 26.Maslekar S, Pillinger SH, Monson JR. Transanal endoscopic microsurgery for carcinoma of the rectum. Surg Endosc. 2007;21:97–102. doi: 10.1007/s00464-005-0832-z. [DOI] [PubMed] [Google Scholar]
- 27.Winde G, Nottberg H, Keller R, Schmid KW, Bunte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39:969–976. doi: 10.1007/BF02054683. [DOI] [PubMed] [Google Scholar]
- 28.Stipa F, Burza A, Lucandri G, Ferri M, Pigazzi A, Ziparo V, Casula G, Stipa S. Outcomes for early rectal cancer managed with transanal endoscopic microsurgery: a 5-year follow-up study. Surg Endosc. 2006;20:541–545. doi: 10.1007/s00464-005-0408-y. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud N, Madoff R, Rothenberger D, Finne C. Transanal endoscopic microsurgery reduces the incidence of positive margins compared with transanal excision for rectal tumors. Dis Colon Rectum. 2001;44:A30. [Google Scholar]
- 30.Saclarides TJ. TEM/local excision: indications, techniques, outcomes, and the future. J Surg Oncol. 2007;96:644–650. doi: 10.1002/jso.20922. [DOI] [PubMed] [Google Scholar]