Abstract
With the increase in average life expectancy, the rate of occurrence of gastric cancer in elderly patients is also rising. While many clinical trials have been conducted to examine the effect of chemotherapy treatment on gastric cancer, age limits for eligible subjects have prevented the establishment of standards for chemotherapy in elderly patients with gastric cancer. As of March 2009, evidence-based standard chemotherapy regimens were established. In the Western world, debates centered on the ECF (Epirubicin/cisplatin/5-FU) or DCF (Docetaxel/cisplatin/5-FU) regimens based on the phase III randomized controlled trial at the Royal Marsden Hospital (RMH) or the V325 study, respectively. The JCOG9912 and SPIRITS trials emerged from Japan indicating attractive regimens that include S-1 for advanced gastric cancer patients. Using these active anticancer drugs, the trials that studied the efficacy of adjuvant therapies or surgical approaches, such as the Int-116/MAGIC/ACTS-GC trials, have actually succeeded in demonstrating the benefits of adjuvant therapies in gastric cancer patients. For cases of gastric cancer in elderly patients, treatment policies should consider these studies while analyzing not only the therapeutic effects but also drug toxicity, individual general health conditions, and social factors to select treatments that emphasize quality of life.
Keywords: Gastric cancer, Elderly patients, Chemotherapy, Regimen comparison
INTRODUCTION
In 2002, there were an estimated 934 000 new cases of gastric cancer world-wide, accounting for 8.6% of all carcinomas, making gastric cancer the fourth most common cancer following lung cancer, breast cancer, and colorectal cancer. In addition, the mortality rate for gastric cancer is only second to that of lung cancer, with approximately 700 000 fatal cases, indicating a poor prognosis compared to breast cancer or colorectal cancer. With decreases in salt intake due to progress in methods of storing and preserving food, the incidence of gastric cancer is decreasing in many advanced countries, however, due to increases in population and average age, the number of afflicted patients in 2010 is predicted to be approximately 1 100 000 worldwide. Areas with a high incidence rate of gastric cancer include East Asia, Eastern Europe, and South America, while the incidence of gastric cancer is low in Africa, South Asia, North America, Australia and New Zealand[1].
Japan has the highest incidence rate of gastric cancer in the world, despite a decrease since 1990, and gastric cancer remains the most common form of cancer in relation to site of organ[2]. Patients 65 years or older account for two-thirds of all cases, while patients 70 years or older constitute half of all cases; thus the treatment of gastric cancer in elderly patients has become a particularly important issue in countries with a large elderly population.
Regarding chemotherapy for gastric cancer, many clinical trials have been conducted to establish standard treatments. However, many of these clinical trials impose age limits for eligible subjects, targeting subjects up to 75 years of age in most cases, and cases with impaired organ function, which are common among elderly patients, are excluded. Therefore, it is difficult to evaluate the efficacy and safety of chemotherapy treatment for gastric cancer in elderly patients using only the results from these clinical trials. In this manuscript, we first review standard evidence-based chemotherapy for gastric cancer in both the West and Japan, and then we discuss the clinical potential of therapeutic applications and future prospects for elderly gastric cancer patients.
APPROACHES TO CHEMOTHERAPY FOR ELDERLY PATIENTS
It must first be noted that in chemotherapy for elderly patients functional impairments of the liver, kidneys, and lungs, which provide the main metabolic and excretory routes for drugs, can cause increases in blood concentrations that easily result in adverse reactions. Key organ functions deteriorate with age, but the level of deterioration varies greatly among individuals and the indication of chemotherapy cannot be determined on the basis of chronological age alone. If a patient’s performance status is good and their key organ functions are maintained and there are no uncontrollable complications, it is considered possible to perform standard chemotherapy even with elderly patients. However, in some cases, unexpected adverse events occur in elderly patients due to functional deterioration that was not apparent from laboratory test values. Thus, a thorough understanding of adverse events that can be caused by anticancer drugs, together with careful observation and quick responses to adverse reactions, is required in elderly patients.
CHEMOTHERAPY FOR INOPERABLE OR RECURRENT GASTRIC CANCER
In a randomized comparative study of non-resectable or recurrent (far advanced) gastric cancer subjects, which were not thus indicative of surgical treatment, with a performance status (PS) of 0-2 in a symptomatic therapy (best supportive care, BSC) group not receiving anticancer drugs and a chemotherapy group receiving anticancer drugs, survival time was extended in the chemotherapy group, thus confirming the usefulness of chemotherapy in gastric cancer[3-5]. It has therefore been accepted that chemotherapy should be the first option for cases of far advanced gastric cancer with relatively good general health conditions.
The response rate for single agents that have been conventionally used for far advanced gastric cancer, such as 5-fluorouracil (5-FU), cisplatin (CDDP), adriamycin (ADR), epirubicin (EPI), and mitomycin C (MMC), is generally approximately 10% to 20%, and studies of combination therapies have been conducted to obtain higher therapeutic effects[6]. On the other hand, regimens such as 5-FU + ADR + MMC therapy (FAM therapy), 5-FU + ADR + methotrexate therapy (FAMTX therapy), 5-FU + CDDP therapy (CF therapy), 5-FU + leucovorin therapy (FL therapy), EPI + CDDP + 5-FU (ECF therapy), and irinotecan + CDDP therapy have obtained higher response rates of approximately 30% to 50% in phase II studies. Life prolongation and/or quality of life (QOL), however, do not necessarily correlate with these response rates. Any such correlation must be ultimately verified in a phase III comparative study for promising regimens using survival time and QOL as indices.
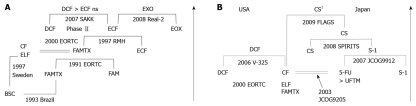
The results of one milestone comparative study conducted by the European Organization for Research and Treatment of Cancer (EORTC) indicated that FAMTX therapy is superior to FAM therapy[7], but in a subsequent randomized controlled trial (RCT) at the Royal Marsden Hospital (RMH), ECF therapy was shown to be further superior to FAMTX therapy in both the response rate and survival time (Figure 1A)[8]. In the ECF regimen, EPI was chosen instead of ADR because of its lower toxicity. Grade 2 alopecia representing pronounced or total reversible hair loss is characterized by Anthracyclins (EPI or ADR)-including regimens (Table 1). Based on these results, in Europe, ECF therapy has been considered the standard treatment. Recently, however, in a two by two design, a comparative study of EPI-included therapy (Real-2) was conducted, using combinations such as EPI + CDDP + capecitabine or 5-FU therapy (ECX or ECF therapy), and EPI + oxaliplatin + capecitabine therapy or 5-FU (EOX or EOF therapy), and the best combination was shown to be capecitabine and oxaliplatin with EPI (EOX therapy), which overcame ECF therapy with a hazard ratio for death of 0.80 (95% CI, 0.66-0.97, P = 0.02)[9] (Figure 1A).
Figure 1.
Phase III trials are the most successful for far advanced gastric cancer in Europe (A) and in both United States and Japan (B). Arrows show chronological direction. BSC: Best supportive care; FAMTX: 5-FU/Adriamycin/Methotrexate; CF: Cisplatin/5-FU; ELF: Etoposide/Leucovorin/5-FU; FAM: 5-FU/Adriamycin/Mitomycin-C; ECF: Epirubicin/Cisplatin/5-FU; EOX: Epirubicin/Oxaliplatin/Capecitabine; DCF: Docetaxel/Cisplatin/5-FU; EORTC: European Organization for Research and Treatment of Cancer; RMH: Royal Marsden Hospital; SAKK: Swiss Group for Clinical Cancer Research; ns: Not significant. UFTM: UFT/Mitomycin-C; CS: S-1/Cisplatin; 1CS regimens in the FLAGS study used 25 mg/m2 of S-1 differently from the SPILITS trial (40 mg/m2 of S-1).
Table 1.
Grade 3/4 side effects in active regimens for advanced gastric cancer
| FAMTX | ECF | EOX | CF | DCF | S1 | CS | |
| Anemia | 10 | 8.0-13.1 | 8.6 | 26 | 18 | 4.0-12.8 | 26 |
| Thrombocytopenia | 8 | 4.0-4.7 | 5.2 | 13 | 8 | 0.0-1.3 | 5 |
| Neutropenia | 58 | 36.0-41.7 | 27.6 | 57 | 82 | 5.6-11.0 | 40 |
| Febrile neutropenia | 20 | 8.0-9.3 | 7.8 | 12 | 29 | 0.0-1.0 | 3 |
| Diarrhea | 7 | 2.6-6.0 | 11.9 | 8 | 19 | 3.0-7.7 | 4 |
| Stomatitis | 1.3 | 2.2 | 27 | 21 | 0.0 | 1 | |
| Hand-foot syndrome | 1 | 3.0-4.3 | 3.1 | 0.0 | 0 | ||
| Nausea and vomitting | 5 | 10.2-17.0 | 11.4 | 17 | 14 | 1.0-5.6 | 11 and 4 |
| Peripheral neuropathy | 0.4 | 4.4 | 3 | 8 | 2.0 | 0 | |
| Lethargy | 16.6 | 24.9 | 14 | 19 | 2.0-5.1 | 4 | |
| Alopecia1 | 42 | 44.2-56.0 | 28.8 | ||||
| Increased creatinine | 3 | 1.0 | 0.0 | 0 | |||
| Ref. | [8] | [8,9] | [9] | [12] | [12] | [16,17] | [16] |
Alopecia is grade 2 according to CTCAE ver2. FAMTX: 5-FU/Adriamycin/Methotrexate; ECF: Epirubicin/cisplatin/5-FU; EOX: Epirubicin/Oxaliplatin/Capecitabine; CF: Cisplatin/5-FU; DCF: Docetaxel/cisplatin/5-FU; CS: S-1/Cisplatin.
On the other hand, in the V325 study in the United States, docetaxel + CDDP + 5-FU therapy (DCF therapy) was shown to produce significantly improved results compared to CF therapy in both response rate and survival time[10], and it is now considered to be one of the standard treatments (Figure 1B). This therapy has a significantly increased toxicity, however, exceeding grade 3 (neutropenia of 82%, diarrhea of 21%, nausea/emesis of 19%, anemia of 18%, lethargy of up to 19%, and thrombocytopenia of 8%, as shown in Table 1).
Using the results of a meta-analysis, Wagner et al[11] reported that combination therapy is more effective than single-agent therapy, and that therapy with a three-drug regimen is more effective than therapy with a two-drug regimen. In addition, the Swiss Group for Cancer Research conducted a phase II trial to compare ECF therapy, docetaxel + CDDP therapy (DC therapy), and DCF therapy. They reported that the response rate was the best for DCF therapy, though there were no differences in survival times, putatively due to a small number tested in the phase II trial[12] (Figure 1A). From the results of these clinical trials, in Europe and the United States, combination therapy using two or three drugs is considered standard treatment for cases with relatively good general health, but neither the Wagner et al[11] nor the Swiss group study discussed therapeutic effects or safety for patients in different age groups, and thus the best Western regimens for elderly patients remain elusive.
Trumper et al[13] analyzed the relationship between age (70 years older and under 70 years old) and therapeutic effects and safety in 1080 cases that had been registered in 3 studies conducted in the United Kingdom. Although some treatment methods included only a small number of subject cases aged 70 years or older, suggesting caution in evaluation, in all treatment methods [including regimens of CDDP (platinum-containing regimen), ECF therapy, 5-FU continuous intravenous infusion (PVI 5-FU) ± MMC therapy, and FAMTX therapy], there were no differences in either the therapeutic effects (response rate and survival time) or toxicity between either age group, and treatments were considered to be useful even for those aged 70 years or older. From this data, age may not be a serious issue to consider in administrating chemotherapy.
In Japan, independent clinical trials have studied treatment methods for far advanced gastric cancer (Figure 1B). In the JCOG 9205 study conducted in the 1990s[14], tegafur-uracil (UFT) + MMC (MC) therapy and CF therapy were evaluated and compared to monotherapy with 5-day continuous intravenous infusion of 5-FU (5-FU ci). There were no significant differences in survival time, which was the primary endpoint, although CF therapy yielded a superior response rate. Therefore, in Japan, 5-FU ci has been considered to be a standard therapy until recently.
S-1, an oral fluorinated pyrimidine drug developed in Japan in the 1990s, has obtained response rates exceeding 40% when used as a single agent, which is comparable to the response rates for conventional combination therapy[15,16], and there were high expectations that it would be a useful treatment method for advanced and recurrent gastric cancer. In the JCOG 9912 study, the noninferiority of single S-1 therapy compared to 5-FU ci and the superiority of irinotecan + CDDP therapy were examined[17]. For overall survival, all other treatment methods yielded better results than 5-FU ci, where noninferiority of single S-1 therapy against 5-FU ci was confirmed statistically, but the superiority of irinotecan + CDDP therapy against 5-FU ci was not demonstrated. Concerning safety, irinotecan + CDDP therapy lead to leukopenia, neutropenia, and loss of appetite exceeding grade 3 at a rate exceeding 30%, while single S-1 monotherapy had a toxicity equivalent to that of single 5-FU therapy and a low incidence rate of adverse events exceeding grade 3 (Table 1)[17].
In the SPIRITS trial, conducted at approximately the same time as the JCOG 9912 study, S-1 + CDDP (CS) therapy showed significantly better results for overall survival and progression-free survival than S-1 monotherapy[18], where the incidence rate of leukopenia (11%), neutropenia (40%), anemia (26%), nausea (11%) and loss of appetite (30%) exceeding grade 3 was higher in CS therapy than in single S-1 monotherapy. Based on the results of these studies, in Japan, CS therapy is currently considered a standard therapy for far advanced gastric cancer.
Subsequently, a comparative study of S-1 monotherapy and S-1 + irinotecan therapy failed to show a significant difference in overall survival[19] and, currently, in a joint study in Japan and South Korea, a comparative study of single S-1 therapy and S-1 + docetaxel therapy is being conducted (Clinical Trials.gov Identifier: NCT00287768).
Regarding the toxicity of S-1 (adverse events exceeding grade 3), the results of a post-marketing survey of 3808 cases showed no differences with the initial survey in the incidence rate of adverse events, which suggests S-1 could be a safe agent for advanced gastric cancer. However, the incidence rate of adverse events is high in cases with reduced creatinine clearance, thus indicating the need for caution when administering drugs in cases with impaired renal function, such as in elderly patients[20].
In Japan, treatments using S-1 are taking a leading role, and when S-1 is combined with CDDP, the median overall survival (MST) exceeds 1 year. Even with S-1 alone, an MST of 11 mo has been obtained. Although a simple comparison is not possible, this exceeds the MST of the three-drug combination therapies used in the Western world. In addition, while dosages of S-1 need to be reduced due to the effects of racial differences in CYP2A6 gene polymorphisms in Europe and the United States[21], a Phase I/II study has obtained an MST of 10.4 mo, and a global Phase III study (FLAGS study) comparing CF therapy with CS therapy revealed that S-1 did not show a significant benefit for survival compared to 5-FU in combination treatments of CDDP, however, many side effect was clearly reduced[22]. Its use is likely considered to be one of the most promising standard treatments around the world.
The proportion of elderly patients in recent phase III clinical trials on far advanced gastric cancer worldwide is shown in Table 2. Only the SPIRITS trial disclosed the actual proportion of elderly patients 70 years old or over (17%) and, interestingly, recent studies have tended to include more elderly patients. These findings may suggest that chemotherapy has been judged as feasible even for elderly patients. It is important to expand the number of treatment options for elderly patients and to provide effective and less toxic treatment. In the SPIRITS trial, the therapeutic effects of CS therapy were better than single S-1 therapy in the subgroup of subjects aged under 60 years (hazard ratio, 0.75; 95% CI, 0.61-0.92), but no difference in effects were seen in the subgroups aged 60-69 years and subjects aged 70 years or older[18]. On the other hand, the most recent study of Arbeitsgemeinschaft Internistische Onkologie (AIO) revealed that oxaliplatin (FLO therapy) in replacement with CDDP (FLP therapy) was significantly effective only in patients older than 65 years, suggesting that oxaliplatin seemed to be very promising in elderly patients with far advanced gastric cancer[18,23].
Table 2.
Phase III milestone trials of chemotherapy for far advanced gastric cancer and information of the elderly patients
| Publication | Trial | Regimens | OS (number) | Selected regimens | Median | Age range | Proportion of elderly patients (70 years old or over) | Ref. | Country |
| 1991 | EORTC | 5-FU/Adriamycin/MMC (FAM) | 4 (79) | FAMTX | 58 | 23-69 | No information | [7] | EORTC |
| 5-FU/Adriamycin/Methotrexate (FAMTX) | 6 (81) | 57 | 28-77 | ||||||
| 1993 | Murad et al | FAMTX | 9 (30) | FAMTX | 58 | 26-72 | No information | [3] | Brazil |
| Best supportive care (BSC) | 3 (10) | 57 | 30-71 | ||||||
| 1995 | Pyrhönen et al | 5-FU/Epirubicin/Methotrexate (FEMTX) | 12.3 (21) | FEMTX | 58 | 42-75 | No information | [5] | Finland |
| BSC | 3.1 (20) | 58 | 42-71 | ||||||
| 1997 | Glimelius et al | 5-FU/Leucovorin/Etoposide (FLE) | 8 (31) | ELF | 64 | 45-75 | No information | [4] | Sweden |
| BSC | 5 (31) | 63 | 40-74 | ||||||
| 1997 | Webb et al | EPI/CDDP/5-FU (ECF) | 8.9 (111) | ECF | 59 | 35-79 | No information | [8] | UK |
| FAMTX | 5.7 (108) | 60 | 29-78 | ||||||
| 2000 | Vanhoefer et al | FAMTX | 6.7 (133) | Similar efficacy | 58 | 30-74 | No information | [44] | EORTC |
| ELF | 7.2 (132) | 59 | 25-74 | ||||||
| 5-FU/CDDP (FUP) | 7.2 (134) | 57 | 24-74 | ||||||
| 2003 | JCOG9205 | 5-FU | 7.1 (105) | 5-FU | 63 | 27-75 | No information | [14] | Japan |
| 5-FU/CDDP (CF) | 7.3 (105) | 63 | 19-75 | ||||||
| UFT/MMC | 6 (70) | 60.5 | 30-75 | ||||||
| 2006 | V325 | Docetaxel/CDDP/5-FU (DCF) | 9.2 (227) | DCF | 55 | 26-79 | 24%-25%1 | [10] | USA |
| CDDP/5-FU (CF) | 8.6 (230) | 55 | 25-76 | ||||||
| 2007 | JCOG9912 | S1 | 11.4 (234) | S1 | 64 | 39-75 | No information | [17] | Japan |
| 5-FU | 10.8 (234) | 63 | 24-75 | ||||||
| CPT11/Docetaxel | 12.3 (236) | 63 | 32-75 | ||||||
| 2008 | SPIRITS | S1 | 11 (150) | S1/CDDP | 62 | 28-74 | 17% | [18] | Japan |
| S1/CDDP (CS) | 13 (148) | 62 | 33-74 | ||||||
| 2008 | REAL-2 | EPI/CDDP/capecitabine (ECX) | 9.9 (241) | EOX | 64 | 25-82 | No information | [9] | UK |
| ECF | 9.9 (249) | 65 | 22-83 | ||||||
| EPI/oxaliplatin/capecitabine (EOX) | 11.2 (239) | 62 | 25-80 | ||||||
| EPI/oxaliplatin/5-FU (EOF) | 9.3 (235) | 61 | 33-78 | ||||||
| 2008 | Al-Batran et al | 5-FU/LV/Oxaliplatin (FLO) | 10.7 (112) | FLO | 64 | 33-86 | 41%-45%2 | [23] | AIO |
| 5-FU/LV/CDDP (FLP) | 8.8 (108) | 64 | 27-85 |
This is proportion of patients with 65 years old or over;
This is proportion of patients over 65 years old.
ADJUVANT CHEMOTHERAPY FOR ADVANCED GASTRIC CANCER
When evaluating the indications for postsurgical adjuvant chemotherapy in gastric cancer cases, the standard options for resection, upon which the chemotherapy is based, are important. One problem is the range of lymph node dissection in Europe and the United States is different from that in East Asian countries, such as Japan. While D2 surgery is a standard procedure in East Asia, D2 surgery is not performed as standard in Europe or the United States. However, even in Japan where D2 surgery is performed as a standard procedure with curative intent, the 5-year survival rate is 69% in Stage II, 50% in Stage IIIA, and 28% in Stage IIIB[24], which are not satisfactory outcomes for curative gastric cancer. Surgery alone does not improve survival rates, and therefore it is believed that some level of adjuvant therapy is required. There are many reports from around the world regarding postoperative adjuvant chemotherapy for gastric cancer in which meta-analysis has indicated extended survival times[25-30], but none of these evaluations used a particular regimen, and it had been concluded that examinations based on large-scale comparative studies are required.
In an INT-116 study conducted in the United States, postoperative adjuvant radiation chemotherapy (5-FU + leucovorin + radiation) lead to significant improvements in overall survival time and recurrence-free survival time and significant reductions in the local recurrence rate, compared with cases undergoing surgery only[31]. On the other hand, in a Magic trial conducted mainly in the United Kingdom, preoperative and postoperative chemotherapy (perioperative chemotherapy) with ECF therapy, which is even effective for far advanced gastric cancer, significantly extended the overall survival time and recurrence-free survival time[32]. Based on these results, postoperative radiation chemotherapy or perioperative chemotherapy with ECF therapy is a standard adjuvant therapy in the Western world.
However, most of the cases registered in the INT-116 study were cases that had undergone D0 or D1 surgery, with only 10% of the cases having undergone D2 surgery. The cases registered in the Magic trial, however, also included cases of lower esophagus cancer, cases that could not undergo surgery, cases that ultimately underwent a noncurative resection, and cases of D1 surgery. In addition, the rate of completion of postoperative chemotherapy was low and the efficacy of postoperative adjuvant chemotherapy remained unclear. It was therefore determined that these results could not be applied to Japan, where D2 surgery is a standard procedure, and it was deemed necessary to examine effective adjuvant chemotherapy treatments through comparative studies using a control group of patients who underwent surgery only[33]. In the ACTS-GC, which was started in 2001, the usefulness of postoperative adjuvant S-1 therapy was examined, and interim analysis results indicated that overall survival in the surgery + S1 group was significantly better than in the surgery alone group[34]. Based on these results, postoperative adjuvant chemotherapy with the administration of S-1 alone is considered to be a standard option in Japan. In addition, in the AGTS-GC approximately one-fourth of the registered cases were elderly subjects aged 70 to 80 years old (Table 3). In a subgroup analysis, the administration of S-1 showed significant effects for younger cases aged under 60 years old, but there were no statistically significant better therapeutic effects in cases aged 60 or older. A similar tendency has also been observed in the Magic trial[31]. The proportion of elderly patients in recent milestone phase III clinical trials on adjuvant therapy around the world is shown in Table 3. It may be useful to consider this information in making decisions on elderly patients continuing adjuvant chemotherapy if patients have severe side effects.
Table 3.
Phase III milestone trials of adjuvant therapy for gastric cancer after surgical resection and information of elderly patients
| Publication | Trials | Patient eligibility | Successful regimens as adjuvant therapy | Adjuvant effect | Median | Range | Proportion of elderly patients (70 years old or over) | Ref. | Country |
| 2001 | INT-116 | Pathological stage IB/II/III/IVM0 | Surgery + Chemo (5-FU/LV)-Radiation (45Gy) | 9% at 3 years | 60 | 25-87 | No information | [31] | USA |
| Surgery alone | 59 | 23-80 | |||||||
| 2006 | MAGIC | Clinical stage II or more (M0) | Surgery + perioperative EPI/CDDP/5-FU (ECF) | 13.6% at 5 years | 62 | 29-85 | 20.4% | [32] | UK |
| Surgery alone | 62 | 23-81 | 21.3% | ||||||
| 2007 | ACTS-GC | Pathological stage II/IIIA/IIIB | Surgery + S1 | 10% at 3 years | 63 | 27-80 | 25.9% | [34] | Japan |
| Surgery alone | 63 | 33-80 | 22.6% |
The INT-116 study found that in cases undergoing radiation chemotherapy, hematotoxicity exceeding grade 3 was observed in 54% of the cases and digestive toxicity was observed in 33%, while in cases undergoing postoperative ECF therapy in the Magic trial, neutropenia exceeding grade 3 was observed in 28% of the cases, leukopenia in 17% of the cases, nausea in 12% of the cases, and emesis in 10% of the cases. On the other hand, in the ACTS-GC, for cases with toxicities exceeding grade 3 due to S-1 therapy, loss of appetite was observed in only 6% of the cases, nausea in 3.7% of the cases, and diarrhea in 3.1% of the cases, and hematotoxicity was mild. In areas where D2 surgery is not a standard procedure, the results of this study are not directly applicable. For example, we have to allow for differences of overall survival in the surgery alone group of the 3 trials; i.e. 3-year survival of ACTS-GC trial (70.1%), 3-year survival of INT 0116 trial (41%), and 5-year survival of MAGIC trial (23%), in order to interpret the survival data. However, if treatment including locally controlled surgery for gastric cancer is to be performed, postoperative adjuvant chemotherapy with S-1 therapy may be an optimal treatment method that can provide lower toxicity and effects for extending a patient’s survival time.
CLINICAL POTENTIAL AND FUTURE PROSPECTIVE FOR ADVANCED GASTRIC CANCER IN ELDERLY PATIENTS
There has been no meta-analysis describing the effect of age on chemotherapy in gastric cancer, however, several subset analyses of the phase III milestone trials elucidated the effect of age on chemotherapy, such as both the SPIRITS trial[18] and ACTS-GC[34], and recently emerging AIO study examining both FLO versus FLP[23].
The former two trials conducted in Japan demonstrated that S1 as an adjuvant therapy is more effective in younger patients than the elderly, while CDDP may not add benefits for elderly patients with far advanced gastric cancer. These results suggest that the concomitant use of CDDP is not very effective for elderly patients and that S-1 monotherapy might be the best standard therapy for elderly patients. In completely resected, non-small cell lung cancer (NSCLC), however, the effect of adjuvant CDDP-based chemotherapy showed that it should not be withheld from elderly patients with NSCLC because it was deemed similarly effective in elderly patients as younger patients (hazard ratio, 0.87; 95% CI, 0.68-1.11; test for trend: P = 0.42)[35]. These results were different from those of far advanced gastric cancer. Additional CDDP is actually shown to increase hematological and gastrointestinal toxicities in the SPIRITS trials[18], in which grade three-fourths neutropenia (16%), anemia (6%), and thrombocytopenia (0%) in S1 administered patients were increased to 59%, 38%, and 8% in CS therapy, respectively. Furthermore, grade three-fourths anorexia (9%) and nausea (2%) recognized in S1 monotherapy were increased up to 45% and 17% in CS therapy, respectively. Nevertheless, such side effects cannot be a reason for CDDP administration to be withheld in elderly patients if it is effective for far advanced gastric cancer, such as adjuvant administration of CDDP against NSCLC. Further confirmation is needed. On the other hand, newly emerging oxaliplatin as a regimen in the Western world seems to be more effective than CDDP, if limited to elderly patients, suggesting that oxaliplatin might be one of the most recommended regimens for the elderly at present because it also reduced toxicity as compared with CDDP[23].
In analyzing the surgical outcomes of patients with gastric cancer who had undergone a radical operation with D2 dissection, we have shown that the outcome for gastric cancer in elderly patients (aged 60 years old or older) was poorer than younger patients (aged under 60 years old), and the prognostic factor of age was completely independent of cancer progression, even after adjustment for the low degree of lymph node dissection during surgery or differences in the frequency of blood transfusion[36,37]. More intriguingly, diffuse type gastric cancer, which is S-1 sensitive[34], tended to show great differences in prognosis between elderly and young patients, putatively reflecting peritoneal immunity[38]. That is, the poor prognoses for gastric cancer in elderly patients is associated with unspecified factors that cannot be explained with malignancy factors covered by the TNM classification. Candidate factors might include the cancer immunocompetence of the host, since cancer immune system function is well known to deteriorate in the elderly[39], and in the future we can expect the possibility of treatment therapies that take into account the decreased immunocompetence of the host for elderly patients with gastric cancer.
It is believed that the lethal effects of cancer chemotherapy against cancer cells are not simply direct effects but are also affected by the induced immunity derived from dead cancer cells (enhanced cellular immunity due to activated cancer antigens). Considering that adjuvant effects against gastric cancer were stronger in cases of gastric cancer in younger patients in the ACTS-GC[34] and the Magic trial[32], additional treatments that provide improvement of immunity may be important for elderly patients. In a well-controlled, prospective phase II trial, it has been shown that Krestin (PSK), which reduces TGF-β in a host and improves cancer immunity[40,41], drastically improves the prognoses of patients with gastric cancer after a radical operation when it is used concomitantly with 5-FU[42]. This suggests that curative effects for cancer may be enhanced by combining S-1 with Krestin, and the efficacy of such combinations may be expected to be higher in elderly diffuse-type gastric cancer patients. Currently, there is a multi-institutional, prospective, randomized trial being conducted to examine the possibility of using S-1/Krestin and S-1 monotherapy as postoperative adjuvant chemotherapy in gastric cancer (HKIT-GC)[43]. The results of this study, which includes elderly patients, are eagerly anticipated.
CONCLUSION
Regarding chemotherapy treatment for gastric cancer in elderly patients, it is believed that it can be applied as a standard procedure as long as the patient’s general health conditions are good and organ functions are sufficiently maintained. However, it is necessary to take into consideration potential deterioration in organ function caused by aging, while sufficient care must be provided in the follow-up stages of treatment. In addition, because elderly patients are nearing the end of their lives, they may have a different sense of priorities compared to younger or middle-aged patients, and it is necessary to fully understand and take into consideration the views of these patients when selecting a treatment method. To improve the treatment results of gastric cancer in elderly patients, we may expect the development of treatment methods that take into consideration modification of unspecified factors, such as cancer immunocompetence, in the future.
Acknowledgments
The authors are indebted to Professor Patrick Barron of the International Medical Communication Center of Tokyo Medical University for his review of this manuscript.
Footnotes
Peer reviewer: Reigetsu Yoshikawa, MD, PhD, Assistant Professor, Department of Genetics, and Department of Surgery, Hyogo College of Medicine, 1-1, Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan
S- Editor Li LF L- Editor Lutze M E- Editor Yin DH
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Foundation for Promotion Cancer Research. Cancer statistics in Japan 2007. Available from: URL: http://www.fpcr.or.jp/publication/pdf/statistics2008.pdf. Accessed 2008 May 13. [Google Scholar]
- 3.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 5.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist. 2005;10 Suppl 3:49–58. doi: 10.1634/theoncologist.10-90003-49. [DOI] [PubMed] [Google Scholar]
- 7.Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, Conroy T, Fickers M, Leyvraz S, Buyse M. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9:827–831. doi: 10.1200/JCO.1991.9.5.827. [DOI] [PubMed] [Google Scholar]
- 8.Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 11.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 12.Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, Köberle D, Borner MM, Rufibach K, Maibach R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–3223. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 13.Trumper M, Ross PJ, Cunningham D, Norman AR, Hawkins R, Seymour M, Harper P, Iveson T, Nicolson M, Hickish T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–834. doi: 10.1016/j.ejca.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205) J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 15.Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–1720. doi: 10.1016/s0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 17.Boku N, Yamamoto S, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Kimura A, Ohtsu A. Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912). Proceedings of the 43rd American Society of Clinical Oncology Annual Meeting; 2007 Jun 1-5; Chicago (IL) [Google Scholar]
- 18.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 19.Imamura H, IIishi H, Tsuburaya A, Hatake K, Imamoto H, Esaki T, Kato M, Furukawa H, Hamada C, Sakata Y. Randomized phase III study of irinotecan plus S-1 (IRIS) versus S-1 alone as first-line treatment for advanced gastric cancer (GC0301/TOP-002). Proceedings of the 2008 Gastrointestinal Cancers Symposium; 2008 Jan 25-27; Orlando (FL) [Google Scholar]
- 20.Nagashima F, Ohtsu A, Yoshida S, Ito K. Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer. 2005;8:6–11. doi: 10.1007/s10120-004-0306-3. [DOI] [PubMed] [Google Scholar]
- 21.Lenz HJ, Lee FC, Haller DG, Singh D, Benson AB 3rd, Strumberg D, Yanagihara R, Yao JC, Phan AT, Ajani JA. Extended safety and efficacy data on S-1 plus cisplatin in patients with untreated, advanced gastric carcinoma in a multicenter phase II study. Cancer. 2007;109:33–40. doi: 10.1002/cncr.22329. [DOI] [PubMed] [Google Scholar]
- 22.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 (CS) with cisplatin/5-FU (CF) as first-line therapy in patients with advanced gastric cancer (FLAGS). Abstract No. 8 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology Annual Meeting; 2009 Jun 22-24; Sun Francisco (CA) [Google Scholar]
- 23.Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 25.Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 26.Hermans J, Bonenkamp JJ. In reply to the editor. J Clin Oncol. 1994;12:879–880. [Google Scholar]
- 27.Nakajima T, Ohta K, Ohyama S, Hamajima N. Meta-analysis of adjuvant chemotherapy trials for gastric cancer at the Cancer Institute Hospital, Tokyo. In: Nakajima T, Yamaguchi T, editors. Multimodality therapy for gastric cancer. Tokyo: Springer-Verlag; 1999. pp. 27–31. [Google Scholar]
- 28.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–1064. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 29.Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 30.Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, Arcangeli V, Drudi G, Canuti D, Fochessati F, et al. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21–27. [PubMed] [Google Scholar]
- 31.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 33.Japanese classification of gastric carcinoma--2nd English edition--response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer. 2001;4:1–8. doi: 10.1007/s101200100009. [DOI] [PubMed] [Google Scholar]
- 34.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 35.Früh M, Rolland E, Pignon JP, Seymour L, Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV, Douillard JY, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26:3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Transfusion alert for patients with curable cancer. World J Surg. 2007;31:2315–2322. doi: 10.1007/s00268-007-9237-6. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K, Ooki A, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Lymph node metastasis density (ND)-factor association with malignant degree and ND40 as "non-curative factor" in gastric cancer. Anticancer Res. 2008;28:435–441. [PubMed] [Google Scholar]
- 38.Yamashita K, Sakuramoto S, Katada N, Futawatari N, Moriya H, Hirai K, Kikuchi S, Watanabe M. Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology. 2009;56:276–281. [PubMed] [Google Scholar]
- 39.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 40.Harada M, Matsunaga K, Oguchi Y, Iijima H, Ito O, Tamada K, Kimura G, Nomoto K. The involvement of transforming growth factor beta in the impaired antitumor T-cell response at the gut-associated lymphoid tissue (GALT) Cancer Res. 1995;55:6146–6151. [PubMed] [Google Scholar]
- 41.Harada M, Matsunaga K, Oguchi Y, Iijima H, Tamada K, Abe K, Takenoyama M, Ito O, Kimura G, Nomoto K. Oral administration of PSK can improve the impaired anti-tumor CD4+ T-cell response in gut-associated lymphoid tissue (GALT) of specific-pathogen-free mice. Int J Cancer. 1997;70:362–372. doi: 10.1002/(sici)1097-0215(19970127)70:3<362::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/s0140-6736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 43.Hokuriku-Kinki Immunochemotherapy Study Group. Randomized controlled study of postoperative adjuvant therapy for gastric cancer using TS-1 or TS-1+PSK ( NCT00216034) Available from: URL: http://www.clinicaltrials.gov/ct/show/ NCT00216034?order. 2005 May (Study start date) [Google Scholar]
- 44.Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]