Abstract
Casein kinase I is a group of ubiquitous Serine/Threonine kinases that have been implicated in both normal cellular functions and several pathological conditions including Alzheimer’s disease and cancer. Recent findings in colon and pancreatic cancer have brought tremendous attention to these molecules as potential therapeutic targets in treatment of digestive cancers. In this review, we summarize up to date what is known about this family of kinases and their involvement in carcinogenesis and other pathological conditions. Our emphasis is on their implications in digestive cancers and their potential for cancer screening and therapy.
Keywords: Casein kinase I, Colon cancer, Pancreatic cancer, Gastric cancer, Biomarkers
INTRODUCTION
Casein kinase I (CKI) family is a group of monomeric serine/threonine protein kinases that are ubiquitously found in all eukaryotic organisms. Seven members (encoded by distinct genes) have been identified so far in mammals: α, β, γ1, γ2, γ3, δ, and epsilon. Other eukaryotes seem to have more CKI proteins, for example, Drosophila has 8 and Caenorhabditis elegans has 87[1,2].
CKI family members are involved in many diverse and important cellular functions, such as regulation of membrane transport, cell division, DNA repair, circadian rhythms, apoptosis and cellular differentiation[3,4]. Mutations and deregulation of CKI expression and activity has been linked to various diseases including neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, sleeping disorders and cancer. Recent findings in some digestive cancers provide additional evidence about their critical roles in carcinogenesis and their potential utilization in cancer prevention and therapy.
PREVELANCE OF DIGESTIVE CANCERS IN THE UNITED STATES
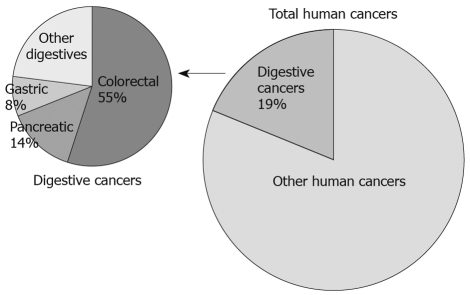
According to the data from the American Cancer Society[5], about 19% of cancer incidence in the United States takes place in the digestive system. Among various digestive cancers (i.e. esophageal cancer, gastric cancer, intestinal cancer, colorectal cancer, liver cancer, gallbladder cancer, pancreatic cancer, etc), colorectal cancer represents > 50% and therefore is the most prevalent one. Despite the high numbers, both incidence and mortality rates for colorectal cancer have been declining steadily since 1975. These declines are mostly thanks to the powerful screening, which makes it possible to remove polyps before they become malignant, or to remove cancerous cells before they develop metastases[5]. Pancreatic cancer is another most deadly digestive cancer, with 34 290 expected deaths in the United States alone for 2008. According to the annual report from the American Cancer Society, only 5% of patients have 5-year survival rate[5]. This is largely due to an insufficiency of early diagnoses and a high resistance to chemotherapy. The comparison between these two cancers in their survival rates signifies the importance of identifying biomarkers that can help in the screening process as well as in the development of therapeutic strategies. Recent findings on CKIepsilon and CKIδ in colon, pancreatic and gastric cancer, which together comprise the three most common digestive cancers in the United States (Figure 1), suggest that the CKI members may hold promise both as screening markers and as therapeutic targets.
Figure 1.
Prevalence and distribution of digestive cancers in USA population. Digestive cancers make up 19% of all cancers in the United States. Colorectal cancer, pancreatic cancer and gastric cancer are the three most common and/or most deadly among them.
BIOCHEMISTRY AND REGULATION OF CKI
The CKI family members have the highest homology in their kinase domains (53%-98% identical) and differ from most other protein kinases by the presence of the sequence Serine-Isoleucine-Asparagine instead of Alanine-Proline-Glutamate in kinase domain VIII[6]. Outside their kinase domains, CKIα and CKIβ are 76% identical, while the CKIγ isoforms are approximate 50% identical in their C-terminal tails. CKIδ and CKIepsilon have long C-terminal extensions with approximate 53% identity.
The CKI kinases appear to have similar substrate specificity in vitro[7], and substrate selection is thought to be regulated in vivo via subcellular localization and docking sites in specific substrates. One consensus phosphorylation site is p-Serine/Threonine-X-X-Serine/Threonine, where X refers to any amino acid[8,9]. This CKI consensus site requires priming by another kinase. CKI also phosphorylates an unprimed site, which optimally contains a cluster of acidic amino acids N-terminal to the target including an acidic residue at n-3 and a hydrophobic region C-terminal to the target[7,10]. A single acidic residue in the n-3 position is not sufficient for CKI phosphorylation. In contrast, in several important targets, such as β-catenin[11,12], CKI does not require n-3 priming, albeit less efficiently than the optimal sites[13].
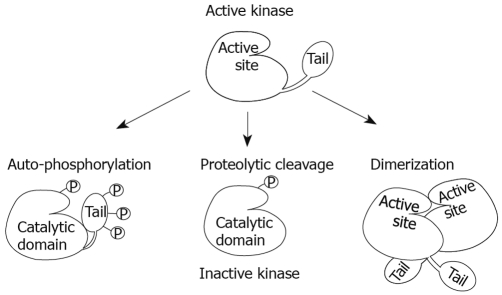
In general, CKI kinases are constitutively active. The long C-terminal extensions of CKIδ and CKIepsilon, however, are autophosphorylated, and this phosphorylation inhibits the activity of the kinase domain, although in vivo phosphatases keep it constitutively active in many cases[14]. Removal of inhibition by proteolytic cleavage or dephosphorylation of the tail is therefore required to fully activate these kinases[15]. Studies of regulation of CKIepsilon and CKIδ in the canonical Wnt signaling pathway indicate that dephosphorylation, rather than proteolytic cleavage, is most likely the way in vivo for their activation[16,17]. This was evidenced by the fact that the activity of wild-type (WT) CKIepsilon, but not of the constitutively active mutant form (MM2) in which all inhibitory phosphorylation sites are altered, was increased 5-fold upon Wnt ligand stimulation[16]. On the contrary, removal of the inhibitory tail actually impaired its proper function in vivo due to failure to form a secondary axis, even though its activity was intact in vitro[17]. Consistent with dephosphorylation as a preferential mode of activation in vivo, Takano et al[18] have identified a naturally occurring CKIepsilon variant (S408N), in which one of the putative auto-inhibitory phosphorylation sites is mutated, leading to increased kinase activity. This was shown to have a protective effect against familial advanced sleep phase syndrome due to its ability to phosphorylate clock proteins, resulting in an elongated circadian rhythm[18].
Even though dephosphorylation has emerged as the predominant mode of activation for CKIepsilon and CKIδ, structural analysis of CKIδ may have revealed an additional mechanism through which CKIepsilon/δ could be regulated in vivo (Figure 2). Since Rivers et al[14] showed that, at the physiological level, constant phosphatase activity appears to keep CKIepsilon and CKIδ in a hypo-phosphorylated state most of the time, which would presumably keep them active, this additional mode of negative regulation could help minimize their activity even in a dephosphorylated state. Based on X-ray crystallography of CKIδ Δ317 and molecular replacement from the published crystal structure of the Schizosaccharomyces pombe homolog Cki1 (Cki1 Δ298)[19,20], Longenecker et al[19] compared the 3-dimentional (3D) structure of the core protein against its amino acid sequence. Through this more accurate comparison they were able to notice a broad surface across the catalytic domain, which is highly conserved between CKIepsilon and CKIδ. Conservation in this region suggests a possible dimerization site, which could block their activations by physically covering the active site. Therefore, dimerization has also been proposed as an alternative mechanism of negative regulation for CKIepsilon and CKIδ[4]. In support of this mechanism, preliminary structural analysis indicates that the CKIepsilon point mutations previously identified in tumor tissue from six breast cancer patients with ductal carcinoma in situ (DCIS) are clustered within this putative dimerization domain (internal communication E. Brumovska and L. Trantirek)[21]. Moreover, based on their position within the 3D structure of the protein, it appears that accumulation of at least three of these point mutations in the same protein would be sufficient to disrupt dimerization (internal communication E. Brumovska and L. Trantirek). These findings could both explain why five of those six patients had multiple mutations in that region, and also suggest that inhibition of dimerization may contribute to tumorigenesis in patients with DCIS through increased overall kinase activity, providing support for dimerization as an additional mechanism of regulation in vivo.
Figure 2.
Models of CKIepsilon/δ regulation. CKIepsilon/δ auto-phosphorylation at their C-terminal tail inhibits their kinase activity through a conformational change that places the tail over the active site, therefore blocking it from potential substrates. Dephosphorylation by phosphatases is the most accepted mechanism for their activation in vivo. In addition, removal of the tail by proteolytic cleavage or mutations has been used in vitro to activate these kinases; however, actual removal of the tail in vivo may have additional effects on proper function. Finally, CKIepsilon/δ dimerization at the active site could inactivate these kinases by physically blocking substrates from entering this region.
GENERAL PATHOLOGY OF CKI
CKI members (α, γ, δ, and epsilon) have been implicated in several pathological conditions, including sleep disorders[18,22], Alzheimer’s Disease (AD)[23] and cancers[4,21,24,25], particularly through identification of specific point mutations and/or changes in expression. Among them, CKIepsilon and δ emerge as the most critical and positive players in these diseases. The recent findings on their active involvement in hyper-phosphorylation of tau protein and accumulation of toxic peptide Amyloid β, two primary characteristics of AD[26,27], further support this notion.
Similarly, the connection between CKIepsilon/δ and cancers has been strengthened through identification of their targets in promotion of cell proliferation and/or inhibition of apoptosis, both of which can contribute to tumorigenesis. For instance, CKIepsilon, δ as well as γ are found to be positive regulators of the canonical Wnt signaling pathway, which promotes cell proliferation through activation of proto-oncogenes like c-myc and cyclin D1, and which is also up-regulated in several malignancies, particularly colon cancer[4,28,29]. Moreover, our recent study showed that CKIepsilon is a positive regulator of the Akt pathway[30], which is also activated in several cancers, including colon cancer[31] and breast cancer[32]. In addition, Akt signaling was also shown to contribute to resistance to a wide range of chemo-therapeutic drugs, making it a very attractive target for cancer treatment research[33-37]. Following up on the suggestion that CKIepsilon may contribute to breast cancer based on both changes in protein expression and accumulation of CKIepsilon point-mutations in tumor samples from DCIS patients[21], we found that CKIepsilon up-regulates Akt in breast cancer cell lines in an independent manner of Phosphate and tensin homologue deleted on chromosome ten (PTEN)[30], the major inhibitor of the Akt pathway[38,39]. Moreover, inhibition with the CKIepsilon/δ inhibitor IC261 was able to block phosphorylation of both Akt and its downstream target Glycogen Synthase Kinase 3β (GSK3β), suggesting that CKIepsilon function is required for Akt activity[30]. A similar effect with IC261 was also seen in Hs578T breast cancer cells, in which the Akt pathway is normally up-regulated regardless PTEN, suggesting that, at least in breast cancer, CKIepsilon/δ may contribute to a more tumorigenic environment through PTEN-independent Akt activation[30].
The connection between CKIepsilon/δ and cancer has also been strengthened by their role in down-regulating apoptosis, particularly Fas-mediated apoptosis[4]. Stimulation by Fas ligand (CD95/APO-1) or agonistic antibodies leads to caspase 8 activation, which can either result in caspase-3 activation or in mitochondria-mediated cell death signaling through Bid cleavage by caspase-8. Desagher et al[40] showed that over-expression of CKIepsilon stabilizes Bid, resulting in a lower number of apoptotic cells, while inhibition of CKI had the opposite effect. In addition, CKIepsilon/δ have also been suggested to contribute to apoptosis by playing a role in the switch mechanism between canonical and non-canonical Wnt signaling, where they may promote canonical Wnt signaling at the expense of JNK-mediated apoptosis[41,42]. However, new evidence showing that CKIepsilon and CKIδ can be activated not only by canonical Wnt3a[16] but also by non-canonical Wnt5a[43] has raised questions about the latter hypothesis.
CKI IN COLON CANCER
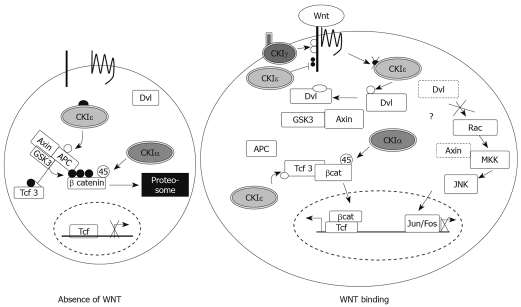
Last year, two independent studies provided strong evidence that CKIepsilon/δ play a role in early stages of tumorigenesis predisposing to colon cancer. Umar et al[44] used the Citrobacter rodentium-induced transmissible murine colonic hyperplasia (TMCH) model, which allows studying changes during these early stages, and showed that CKIepsilon protein levels as well as its activity are increased by 2- to 3-fold after 6 and 12 d of infection, suggesting an association between CKIepsilon up-regulation and colon cancer development. Based on an increase in both Wnt target genes and S45-phosphorylated β-catenin, Umar et al[44] proposed that CKIepsilon up-regulation contributes to colonic hyperplasia through activation of the Wnt pathway, as well as phosphorylation of β-catenin at S45. The notion that CKIepsilon contributes to colon cancer through up-regulation of canonical Wnt signaling is not surprising, given that the pathway itself is activated in a majority of colon cancers, often through inducing mutations in key components such as Adenomatous polyposis coli (APC) and β-catenin[45]. However, their interpretation of increased β-catenin phosphorylation at S45 is questionable. First, the reported increase in β-catenin phosphorylation at S45 in TMCH is actually only subtle after normalizing to total β-catenin levels and therefore more likely due to “a proportional increase in overall β-catenin abundance”[44]. Moreover, even though two reports originally attributed priming phosphorylation of β-catenin at S45 to CKIepsilon[11,46], Liu et al[12] convincingly showed that it is CKIα, not CKIepsilon, that phosphorylates β-catenin at S45 in vivo. They further demonstrated that RNAi against CKIα rather than CKIepsilon, inhibited S45 phosphorylation of β-catenin in 293T cells[12], making CKIα the most likely CKI member responsible for priming phosphorylation of β-catenin. In the absence of Wnt signaling, GSK3β can associate with the β-catenin degradation complex and further phosphorylate β-catenin. This additional phosphorylation targets β-catenin for degradation, therefore preventing it from activating Wnt-specific target genes. After Wnt stimulation, which results in full activation of CKIepsilon[16], dephosphorylated CKIepsilon disrupts the β-catenin degradation complex and prevents GSK3β from further phosphorylating β-catenin. Lack of additional GSK3β-mediated phosphorylation will result in stabilization and accumulation of β-catenin in the cytoplasm, which is required for its subsequent translocation to the nucleus to activate Wnt target genes (Figure 3). Therefore, the reported phosphorylation of β-catenin at S45 seen in TMCH is more consistent with CKIα phosphorylating β-catenin at S45, but still consistent with the authors’ conclusions that CKIepsilon up-regulation contributes to colonic hyperplasia through up-regulation of the Wnt pathway and subsequent increased transcription of Wnt target genes.
Figure 3.
CKI members in canonical Wnt signaling. In the absence of Wnt ligand, β-catenin is phosphorylated by GSK3β after priming at S45 by CKIα and becomes degraded. In this state, CKIepsilon phosphorylates APC and stabilizes the β-catenin degradation complex. Upon Wnt ligand binding to its receptor, CKIepsilon is fully activated through dephosphorylation and contributes to the disassembly of the β-catenin degradation complex by phosphorylating Dishevelled (Dvl) and facilitating its interaction with Axin and GSK3β. Therefore, even though CKIα still primes β-catenin at S45, without additional phosphorylation by GSK3β, β-catenin becomes stabilized and accumulates in the cytoplasm. CKIepsilon also promotes β-catenin stability by phosphorylating Tcf3 which competes against GSK3β for β-catenin. Stabilized β-catenin then translocates into the nucleus, where it contributes to activation of Wnt target genes. CKIepsilon also negatively regulates the Wnt pathway by phosphorylating the LRP-co-receptor, which is positively phosphorylated by CKIγ. Since epistasis analysis showed that CKIepsilon-mediated phosphorylation of LRP was downstream of its phosphorylation of Dvl, this is most likely a negative feedback loop to quickly deactivate the pathway after propagation of the signal.
In a separate study based on a clinical family pedigree analysis of colon cancer[47], one patient was diagnosed at age 46 with several large polyps (5 mm or more), including one > 20 mm. This was approximately 10 years earlier compared to his siblings, suggesting a predisposition to early on-set of colon cancer. Screening identified a point mutation in a highly conserved region of CKIδ (R324H), which was correlated with the more severe condition of the patient through both in vivo phenotypic analysis and cell culture transformation potential[47]. This case further indicates that CKIepsilon/δ contribute to early onset of colon cancer. More specifically, in vivo analysis of the R324H mutant in Xenopus revealed the axis duplication phenotype indicative of increased canonical Wnt signaling as well as an additional gastrulation phenotype that significantly contributed to the aggressiveness of CKIδ -related polyps[47]. Taken together, these results suggest that canonical Wnt signaling is not the only pathway that activates CKIepsilon and CKIδ in colon cancer. The non-canonical Wnt/PCP and Wnt/Ca2+ pathways, which are involved in gastrulation, are also regulated by CKIepsilon and δ[48-50]. Consequently, Tsai et al[47] tested known downstream components of these pathways, namely JNK, RhoA and NF-AT. However, they were not able to detect any changes in either of these pathways when using the R324H mutant compared to WT, suggesting that the Wnt/PCP and Wnt/Ca2+ pathways are not likely affected by the CKIδ mutation.
CKI IN PANCREATIC CANCER
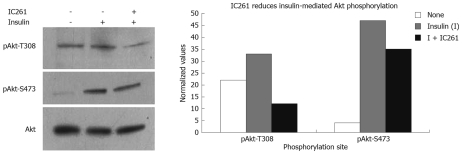
Earlier this year, CKIepsilon and δ were also found in association with another gastrointestinal cancer, pancreatic ductal adenocarcinoma (PDAC), which is the most common type of pancreatic cancer[24]. Brockschmidt et al[24] showed that CKIepsilon and δ are strongly expressed in both PDAC cell lines and actual tumors tissues. Importantly, inhibition of CKIepsilon/δ by IC261 could effectively re-sensitize cells to apoptosis in vitro and reduce tumor growth in vivo. More specifically, prolonged treatment of low doses of IC261 in combination with the agonistic anti-Fas antibody CH11 increased cell death by 50% in pancreatic cancer Panc89 cells, which are normally resistant to Fas -mediated apoptosis. This was also correlated with increased Fas-mediated cleavage of caspase-8 and Bid, as well as activation of caspase-3 as measured by cleavage of its substrate PARP[24]. Moreover, IC261 treatment of SCID mice, which had been previously implanted with PancTu-1 cancer cells to develop pancreatic tumors, lead to a significant reduction in tumor size[24]. While IC261 was as effective as gemcitabine, a drug currently used to treat pancreatic cancer, combined treatment did not result in any additional benefit[24]. Real-time PCR analysis of tumor tissues treated with either drug revealed changes in expression of various genes[24]. Most notably, CKIepsilon and CKIδ were decreased with both drugs, even though the change was greater with IC261 for CKIδ. Moreover, while most genes followed similar patterns between the two treatments, FASLG was strongly increased with IC261 while it was decreased with gemcitabine. Taken together, their data strongly suggests that CKIepsilon/δ promote PDAC through their known anti-apoptotic role in Fas-mediated regulation. However, analysis of their tumor samples revealed that the reduction in tumor size was associated not only with an increase in apoptosis but also with a decrease in cell proliferation. This suggests that CKIepsilon/δ contribute to tumorigenesis in PDAC also by promoting cell proliferation. Since we recently showed that CKIepsilon can up-regulate the Akt[30], which is also up-regulated in PDAC[31,51,52], we tested whether IC261 can inhibit Akt signaling in PDAC cells as well. Our preliminary results show a decrease in Akt phosphorylation at both of its activating sites (Figure 4), indicating that CKIepsilon-mediated Akt up-regulation is not only restricted to breast cancer. However, more studies would be required to determine how much the decrease in Akt signaling is contributing to the decrease in cell proliferation seen by Brockschmidt et al[24] in SCID mice treated with IC261.
Figure 4.
Pharmacological inhibition of CKIepsilon/δ reduces insulin-induced Akt phosphorylation in PDAC cells. Cells were serum starved overnight and then incubated in 10 μg/mL insulin-containing media for 20 min in the presence or absence of the CKIepsilon/δ inhibitor IC261 (40 μmol/L). Akt activation was measured by phosphorylation at T308 and S473. Bands were quantified using Image J software and values were normalized against total Akt.
CKI IN GASTRIC CANCER
In addition to their role in colon cancer and pancreatic cancer, preliminary data from different research labs strongly suggest that CKIepsilon and CKIδ may also be involved in gastric cancer, the next most common gastrointestinal cancer in the United States after pancreatic cancer[5]. Recently, von Blume et al[53] showed that gastrin, a major stimulator of gastric acid secretion, increases CKIepsilon/δ activity. This ultimately results in de-repression of HDAC7-regulated genes such as nur77. Nur77 has been shown as a potent oncogenic survival factor in several types of cancer, including lung, prostate, breast and colon cancer[54]. Therefore, while the focus of the study was more at the mechanistic level of signal transduction, these results indicate that CKIepsilon/δ could play a role in gastric cancer as well. Further evidence in support of this comes from two separate studies on H-prune and its binding partner nm23-H1, which are key inducers of cell motility in breast cancer. Oue et al[55] recently showed that increased expression of H-prune and nm23-H1 was strongly correlated with tumor progression and poor survival in gastric cancer, while Garzia et al[56] provided mechanistic evidence on the interaction between H-prune and nm23-H1, implicating CKIepsilon/δ as key mediators of this interaction. Out of the143 gastric cancer cases analyzed by Oue et al[55], 87% were positive for nm23-H1, which was expressed in 98% of H-prune positive gastric cancer cases. Many of the cases in which H-prune and nm23-H1 were co-expressed showed more advanced T grade, N grade and tumor stage, and H-prune positive patients had significantly worse survival rate than H-prune negative patients, clearly supporting an important role for H-prune (and nm23-H1) in gastric cancer progression. Since co-expression of H-prune and nm23-H1 resulted in more aggressive tumor stages, the formation of H-prune/ nm23-H1 complexes seems to play a critical role in their adverse effects, and disrupting those complexes could be potentially therapeutically advantageous. It is in this context that CKIepsilon/δ could be again promising drug targets, since they phosphorylate a critical region within the H-prune binding region on nm23-H1 and their inhibition with IC261 (or competitive binding with phosphorylated nm23-H1 peptide) disrupted complex formation, resulting in inhibited cell motility[56].
CONCLUSIONS AND FUTURE DIRECTIONS
Results from recent studies in gastrointestinal cancers provide strong evidence of an association between CKI and carcinogenesis. However, they also raise important questions as to whether these kinases could be successfully targeted for cancer screening or treatment. For colon cancer, while activation of the canonical Wnt pathway was evidenced, results by Tsai et al[57] also indicate the possibility of some undefined pathway (related to cell migration and morphogenesis during gastrulation) that may be affected by CKI. Identifying this additional pathway is certainly a daunting task due to the complexity of gastrulation[58,59] and the wide number of known (and unknown) targets of CKI. However, at least two new targets of CKIepsilon could be possible alternative candidates for the R324H mutant, given the great similarity between CKIepsilon and δ. The first one is Rap1, an alternative GTPase that was later shown by the same group to promote gastrulation through CKIepsilon[57,60]. The second is Akt, which we recently identified as a new target of CKIepsilon[30]. Akt is also involved in cell migration, even though its role in gastrulation is still unclear[61]. Regardless which pathways are involved, it may be even more interesting to see whether the CKIepsilon/δ-specific inhibitor IC261 could reduce hyperplasia and/or polyp formation effectively.
Several lines of evidence show that the CKIepsilon/δ-specific inhibitor IC261 has potential therapeutic effects for cancer. On the one hand, CKIepsilon/δ inhibition could be successfully achieved within non-toxic levels both in vitro and in vivo[24,27]. Moreover, IC261 was also shown to be more specific, resulting in fewer side-effects compared to some currently used alternatives[27,62,63]. For instance, while it has been shown to cause transient mitotic arrest, its effect is limited to mitosis, unlike other mitotic spindle drugs like nocodazole, which affect microtubule stability in general[62]. This feature could actually be advantageous to cancer therapy by targeting primarily actively dividing cancer cells, as was also shown in vitro by another group[63]. In addition, Behrend et al[62] also provided a more detailed analysis of the potential effects of IC261 in PDAC and showed that it is as effective as gemcitabine, the chemotherapeutic drug most commonly used for pancreatic cancer patients[27]. However, combinatorial treatment did not have any additive advantages[27], possibly because the two drugs for the most part affect the same pathways. This is tentatively supported by their gene expression data showing mostly similar trends in up and down-regulation of genes such as CKIepsilon and CKIδ. These results highlight the importance of understanding at the molecular level which pathways contribute to the disease, how these pathways are regulated and connected, and which components are affected by specific drug targets. In this case, Brockschmidt et al[24] convincingly showed that IC261 promotes apoptosis in PDAC by blocking CKIepsilon/δ-mediated inhibition of Fas-mediated signaling. However, their data also raises questions about which CKIepsilon/δ-mediated cell proliferative signal is also inhibited by IC261. Answering this question would help determine what other drug could be more effective in combinatorial treatment. For instance, Morgan-Lappe et al[64] mentioned that the Akt specific inhibitor A443654 was successful in combination with specific inhibition by siRNA of the CKIγ3 isoform, which is another positive regulator of Wnt signaling[65]. However, this may not be the best candidate in combination with IC261, which is specific to only CKIepsilon/δ, if IC261 is already blocking Akt signaling.
Taken together, results presented by Tsai, Umar, Brockschmidt, von Blume, Oue and Garzia further support a role of CKIepsilon/δ in carcinogenesis, particularly of the gastrointestinal tract, and make them very promising markers for both early detection/risk assessment and cancer therapy. Understanding the mode of regulation of these kinases can provide insight into how naturally occurring CKIepsilon/δ mutations work, and also help identify which portions of the gene are more likely mutated. This is particularly important for conditions like colon cancer, as well as many other cancers including pancreatic cancer, where asymptomatic early detection (i.e. through screening of mutations such as R324H) could be critical for better prognosis. More studies are required to further explore this area and also determine whether CKIepsilon/δ can be pharmacological targets for treatment as well, particularly in light of the promising results with the CKIepsilon/δ inhibitor IC261 against pancreatic cancer[24] and AD[27], both of which currently do not offer many successful options.
Footnotes
Supported by The Merit Review grant (the Department of Veterans Affairs of the United States) and the Grant-in-Aid (the American Heart Association) to Dr. Chai
Peer reviewer: Ajay Goel, PhD, GI Cancer Research Laboratory, Baylor University Medical Center, 3500 Gaston Avenue, Suite H-250, Dallas, TX 75246, United States
S- Editor Li LF L- Editor Negro F E- Editor Lin YP
References
- 1.Plowman GD, Sudarsanam S, Bingham J, Whyte D, Hunter T. The protein kinases of Caenorhabditis elegans: a model for signal transduction in multicellular organisms. Proc Natl Acad Sci USA. 1999;96:13603–13610. doi: 10.1073/pnas.96.24.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison DK, Murakami MS, Cleghon V. Protein kinases and phosphatases in the Drosophila genome. J Cell Biol. 2000;150:F57–F62. doi: 10.1083/jcb.150.2.f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/s0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 4.Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts & Figures 2008. Available from: URL: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 6.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 7.Pulgar V, Marin O, Meggio F, Allende CC, Allende JE, Pinna LA. Optimal sequences for non-phosphate-directed phosphorylation by protein kinase CK1 (casein kinase-1)--a re-evaluation. Eur J Biochem. 1999;260:520–526. doi: 10.1046/j.1432-1327.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Flotow H, Roach PJ. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I. Implications for hormonal regulation of glycogen synthase. J Biol Chem. 1989;264:9126–9128. [PubMed] [Google Scholar]
- 9.Flotow H, Graves PR, Wang AQ, Fiol CJ, Roeske RW, Roach PJ. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990;265:14264–14269. [PubMed] [Google Scholar]
- 10.Flotow H, Roach PJ. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991;266:3724–3727. [PubMed] [Google Scholar]
- 11.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 13.Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA. 2003;100:10193–10200. doi: 10.1073/pnas.1733909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivers A, Gietzen KF, Vielhaber E, Virshup DM. Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J Biol Chem. 1998;273:15980–15984. doi: 10.1074/jbc.273.26.15980. [DOI] [PubMed] [Google Scholar]
- 15.Cegielska A, Gietzen KF, Rivers A, Virshup DM. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J Biol Chem. 1998;273:1357–1364. doi: 10.1074/jbc.273.3.1357. [DOI] [PubMed] [Google Scholar]
- 16.Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 17.Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc Natl Acad Sci USA. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano A, Uchiyama M, Kajimura N, Mishima K, Inoue Y, Kamei Y, Kitajima T, Shibui K, Katoh M, Watanabe T, et al. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29:1901–1909. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- 19.Longenecker KL, Roach PJ, Hurley TD. Three-dimensional structure of mammalian casein kinase I: molecular basis for phosphate recognition. J Mol Biol. 1996;257:618–631. doi: 10.1006/jmbi.1996.0189. [DOI] [PubMed] [Google Scholar]
- 20.Xu RM, Carmel G, Sweet RM, Kuret J, Cheng X. Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 1995;14:1015–1023. doi: 10.1002/j.1460-2075.1995.tb07082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuja TJ, Lin F, Osann KE, Bryant PJ. Somatic mutations and altered expression of the candidate tumor suppressors CSNK1 epsilon, DLG1, and EDD/hHYD in mammary ductal carcinoma. Cancer Res. 2004;64:942–951. doi: 10.1158/0008-5472.can-03-2100. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptácek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 23.Ghoshal N, Smiley JF, DeMaggio AJ, Hoekstra MF, Cochran EJ, Binder LI, Kuret J. A new molecular link between the fibrillar and granulovacuolar lesions of Alzheimer's disease. Am J Pathol. 1999;155:1163–1172. doi: 10.1016/S0002-9440(10)65219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Bohm B, Henne-Bruns D, et al. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut. 2008;57:799–806. doi: 10.1136/gut.2007.123695. [DOI] [PubMed] [Google Scholar]
- 25.Mishra SK, Yang Z, Mazumdar A, Talukder AH, Larose L, Kumar R. Metastatic tumor antigen 1 short form (MTA1s) associates with casein kinase I-gamma2, an estrogen-responsive kinase. Oncogene. 2004;23:4422–4429. doi: 10.1038/sj.onc.1207569. [DOI] [PubMed] [Google Scholar]
- 26.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007;282:23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 27.Flajolet M, He G, Heiman M, Lin A, Nairn AC, Greengard P. Regulation of Alzheimer's disease amyloid-beta formation by casein kinase I. Proc Natl Acad Sci USA. 2007;104:4159–4164. doi: 10.1073/pnas.0611236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001;235:388–396. doi: 10.1006/dbio.2001.0308. [DOI] [PubMed] [Google Scholar]
- 29.Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 30.Modak C, Bryant P. Casein Kinase I epsilon positively regulates the Akt pathway in breast cancer cell lines. Biochem Biophys Res Commun. 2008;368:801–807. doi: 10.1016/j.bbrc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133–1139. doi: 10.1055/s-2005-858638. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson KM, Streuli CH, Anderson NG. Autocrine signalling through erbB receptors promotes constitutive activation of protein kinase B/Akt in breast cancer cell lines. Breast Cancer Res Treat. 2003;81:117–128. doi: 10.1023/A:1025765215765. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 34.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 35.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 36.Gelardi T, Caputo R, Damiano V, Daniele G, Pepe S, Ciardiello F, Lahn M, Bianco R, Tortora G. Enzastaurin inhibits tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008;99:473–480. doi: 10.1038/sj.bjc.6604493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgillo F, Lee HY. Resistance to epidermal growth factor receptor-targeted therapy. Drug Resist Updat. 2005;8:298–310. doi: 10.1016/j.drup.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.DeGraffenried LA, Fulcher L, Friedrichs WE, Grünwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–1516. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Bagaitkar J, Watabe K. Roles of AKT signal in breast cancer. Front Biosci. 2007;12:4011–4019. doi: 10.2741/2367. [DOI] [PubMed] [Google Scholar]
- 40.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Qiu WJ, Chan SC, Han J, He X, Lin SC. Casein kinase I and casein kinase II differentially regulate axin function in Wnt and JNK pathways. J Biol Chem. 2002;277:2008: 17706–17712. doi: 10.1074/jbc.M111982200. [DOI] [PubMed] [Google Scholar]
- 42.Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007;120:586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- 44.Umar S, Wang Y, Morris AP, Sellin JH. Dual alterations in casein kinase I-epsilon and GSK-3beta modulate beta-catenin stability in hyperproliferating colonic epithelia. Am J Physiol Gastrointest Liver Physiol. 2007;292:G599–G607. doi: 10.1152/ajpgi.00343.2006. [DOI] [PubMed] [Google Scholar]
- 45.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakanaka C. Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem. 2002;132:697–703. doi: 10.1093/oxfordjournals.jbchem.a003276. [DOI] [PubMed] [Google Scholar]
- 47.Tsai IC, Woolf M, Neklason DW, Branford WW, Yost HJ, Burt RW, Virshup DM. Disease-associated casein kinase I delta mutation may promote adenomatous polyps formation via a Wnt/beta-catenin independent mechanism. Int J Cancer. 2007;120:1005–1012. doi: 10.1002/ijc.22368. [DOI] [PubMed] [Google Scholar]
- 48.Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, Barshishat-Küpper M, Rubin JS. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280:777–786. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- 49.Saburi S, McNeill H. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr Opin Cell Biol. 2005;17:482–488. doi: 10.1016/j.ceb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–4195. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–997. [PubMed] [Google Scholar]
- 52.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 53.von Blume J, Knippschild U, Dequiedt F, Giamas G, Beck A, Auer A, Van Lint J, Adler G, Seufferlein T. Phosphorylation at Ser244 by CK1 determines nuclear localization and substrate targeting of PKD2. EMBO J. 2007;26:4619–4633. doi: 10.1038/sj.emboj.7601891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–4743. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 55.Oue N, Yoshida K, Noguchi T, Sentani K, Kikuchi A, Yasui W. Increased expression of h-prune is associated with tumor progression and poor survival in gastric cancer. Cancer Sci. 2007;98:1198–1205. doi: 10.1111/j.1349-7006.2007.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garzia L, D'Angelo A, Amoresano A, Knauer SK, Cirulli C, Campanella C, Stauber RH, Steegborn C, Iolascon A, Zollo M. Phosphorylation of nm23-H1 by CKI induces its complex formation with h-prune and promotes cell motility. Oncogene. 2008;27:1853–1864. doi: 10.1038/sj.onc.1210822. [DOI] [PubMed] [Google Scholar]
- 57.Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM. A Wnt-CKIvarepsilon-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev Cell. 2007;12:335–347. doi: 10.1016/j.devcel.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shook DR, Majer C, Keller R. Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev Biol. 2002;248:220–239. doi: 10.1006/dbio.2002.0718. [DOI] [PubMed] [Google Scholar]
- 59.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Habas R, He X. Cell signaling: moving to a Wnt-Rap. Curr Biol. 2007;17:R474–R477. doi: 10.1016/j.cub.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 62.Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW, Knippschild U. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene. 2000;19:5303–5313. doi: 10.1038/sj.onc.1203939. [DOI] [PubMed] [Google Scholar]
- 63.Yang WS, Stockwell BR. Inhibition of casein kinase 1-epsilon induces cancer-cell-selective, PERIOD2-dependent growth arrest. Genome Biol. 2008;9:R92. doi: 10.1186/gb-2008-9-6-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan-Lappe S, Woods KW, Li Q, Anderson MG, Schurdak ME, Luo Y, Giranda VL, Fesik SW, Leverson JD. RNAi-based screening of the human kinome identifies Akt-cooperating kinases: a new approach to designing efficacious multitargeted kinase inhibitors. Oncogene. 2006;25:1340–1348. doi: 10.1038/sj.onc.1209169. [DOI] [PubMed] [Google Scholar]
- 65.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]