Abstract
Malignant mesothelioma is a highly aggressive neoplasm. The incidence of malignant mesothelioma is increasing worldwide. Diffuse malignant peritoneal mesothelioma (DMPM) represents one-fourth of all mesotheliomas. Association of asbestos exposure with DMPM has been observed, especially in males. The great majority of patients present with abdominal pain and distension, caused by accumulation of tumors and ascitic fluid. In the past, DMPM was considered a pre-terminal condition; therefore attracted little attention. Patients invariably died from their disease within a year. Recently, several prospective trials have demonstrated a median survival of 40 to 90 mo and 5-year survival of 30% to 60% after combined treatment using cytoreductive surgery and perioperative intraperitoneal chemotherapy. This remarkable improvement in survival has prompted new search into the medical science related to DMPM, a disease previously ignored as uninteresting. This review article focuses on the key advances in the epidemiology, diagnosis, staging, treatments and prognosis of DMPM that have occurred in the past decade.
Keywords: Asbestos, Cisplatin, Cytoreductive surgery, Doxorubicin, Intraperitoneal chemotherapy, Mesothelin, Pemetrexed, Peritoneal mesothelioma, Peritonectomy
INTRODUCTION
Malignant mesothelioma is a highly aggressive primary neoplasm of the serosal lining of the pleura, peritoneum, pericardium, or tunica vaginalis[1]. Probably because of extensive use of asbestos in building materials in the past, the incidence of malignant mesothelioma is increasing worldwide and is not expected to peak for another 5 to 20 years[2]. Diffuse malignant peritoneal mesothelioma (DMPM) is the second most common type of mesothelioma[1]. Patients who suffer from this disease usually present with abdominal pain or distension. As the disease progresses, they invariably die from intestinal obstruction or terminal starvation within a year. Due to the infrequent occurrence and the lack of understanding of the natural history of DMPM, traditionally there seemed to be a mutual agreement among medical practitioners that patients with this condition were preterminal. Few therapeutic advances have occurred in the last century since the disease was first described by Miller and Wynn in 1908[3]. Systemic chemotherapy, palliative surgery and/or total abdominal radiation therapy were used selectively, but did not seem to alter the natural history of this disease[4-10].
Recently, a reexamination of this disease including all aspects of diagnostic and treatment strategies has emerged. This is related to the encouraging experiences in numerous centers with cytoreductive surgery and perioperative chemotherapy. This new treatment strategy has consistently demonstrated a markedly improved prognosis achieving a median survival of up to 90 mo and a 5-year survival of 60%[11-19]. The significant improvement achieved by this treatment modality offers a potentially curative option.
In addition, there has been substantial public interest in recent years, because millions of people have been exposed to asbestos in the environment, especially the workplace. The association with malignant mesothelioma, both pleural and peritoneal, has created considerable medical-legal implications involving billions of dollars in compensation costs for industry and government[20-22]. This review article focuses on the advances in the epidemiology, diagnosis, staging, management and prognosis of DMPM that have occurred in the past decade.
EPIDEMIOLOGY
The incidence of malignant mesothelioma has been rising worldwide since the 1970s, with some evidence recently of a slowing of this trend in some countries. In the United States, there was a steep rise in the incidence of mesothelioma through the 1990s, with a recent leveling off in the rate of increase, but no evidence that the peak incidence of mesothelioma has been passed in this country[21,23]. A similar pattern, with a plateauing of the incidence rate, is present in some European countries that reduced asbestos usage in a similar time frame to that in the United States[24-26]. However, the overall incidence of malignant mesothelioma is increasing worldwide and is not expected to peak for another 5 to 20 years[2]. A recent estimate for Great Britain is a peak incidence between 2011 and 2015[27] and the peak incidence in Norway is projected for 2010[28]. La Vecchia et al[29] used death certificates from 8 European countries to predict a peak mortality between 2010 and 2020; a peak incidence in France is expected in 2030[30] and is projected for 2012-2024 in Italy[31]. The incidence is expected to continue to increase in areas of the world where asbestos use has not been curtailed[32-34].
DMPM is the second most common type of malignant mesothelioma[1]. A recent analysis of the Surveillance, Epidemiology, and End Results program of the National Cancer Institute estimated approximately 250 new cases of DMPM in the United States each year[21]. Some studies with an adequate number of cases demonstrated a strong association between the estimated occupational exposure to asbestos and the risk of DMPM, especially in males[20-22]. The overall incidence of this disease is higher in males than females, which may be related to a higher incidence of asbestos-related occupations in men[11]. DMPM has also been reported following radiation therapy, mica exposure, recurrent peritonitis and administration of thorium dioxide[35-39].
CLINICAL PRESENTATION
The initial symptoms and signs of DMPM are non-specific and due to the rarity of the disease, the level of clinical suspicion is relatively low[11,40]. Approximately 70% of patients have serous ascites, a product of the tumor nodules. This mixture of fluid and tumor buildup under pressure appears to be the major cause of morbidity. Increased abdominal girth (55%), pain (45%), and abdominal or pelvic mass (26%) are the most common initial complaints, which lead the physician to arrange for definitive tests resulting in a diagnosis of DMPM (Table 1)[11,40]. Unlike pleural mesothelioma, pain has not been found to have a significant negative impact on survival in patients with DMPM. Approximately 13% of patients present with new onset abdominal wall hernia, which is related to accumulated ascites and increased intraabdominal pressure. Other constitutional symptoms may also be present, such as weight loss (20%) and febrile episodes (10%), which were both associated with a reduced overall survival[11,40]. Tejido García et al[41] previously reported fever as an initial presentation of DMPM in 3 patients and hypothesized that fever constitutes the initial clinical presentation only when the disease remains asymptomatic until it is far advanced. In females, approximately 25% seek medical attention as a result of non-specific gynecological symptoms, such as pelvic mass or infertility[11]. Lymphadenopathy or distant organ metastasis is extremely rare in this disease. Five percent of patients may present with concomitant pleural effusion.
Table 1.
Symptoms and signs of DMPM
| Symptoms |
| Abdominal pain (40%) |
| Abdominal distension (40%) |
| Constitutional symptoms, such as weight loss and fever (20%) |
| Incidental finding (10%) |
| Signs |
| Ascites (70%) |
| Abdominal or pelvic mass (30%) |
| Abdominal wall hernia (10%) |
| Guarding and rebound tenderness (10%) |
| Pleural effusion (5%) |
DMPM: Diffuse malignant peritoneal mesothelioma.
DIAGNOSIS
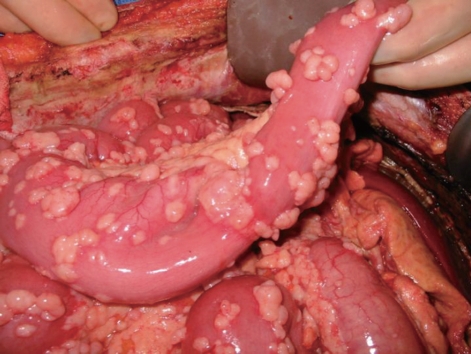
Macroscopically, DMPM is characterized by thousands of whitish tumor nodules of variable size and consistency. These nodules may coalesce to form plaques or masses or layer out evenly to cover part, or the entirety of the peritoneal surface (Figure 1).
Figure 1.
Macroscopically, diffuse malignant peritoneal mesothelioma (DMPM) is characterized by thousands of whitish tumor nodules of variable size and consistency that may coalesce to form plaques or masses or layer out evenly to cover the entire peritoneal surface.
Radiology
Evolutionary change has occurred in the technology of computed tomography (CT). With administration of adequate intravenous, oral and rectal contrast media, multi-slice CT is the current mainstay imaging tool for patients with DMPM. It allows more precise identification and evaluation of DMPM than sonography. In the past, several studies described the radiologic appearances of DMPM in small case-series of fewer than 10 patients[42-44].
Yan et al[45] studied preoperative abdominal and pelvic CT scans of 33 DMPM patients in a systematic manner and identified four radiologic characteristics that could be used to distinguish DMPM from other peritoneal carcinomatosis (Table 2). First, the authors studied the distribution of DMPM and determined that this disease was diffuse throughout the peritoneal cavity. The lack of a primary site for this disease distinguished it from peritoneal dissemination from gastrointestinal or gynecologic malignancies. Second, the most heavily disease-involved regions were the mid-abdomen and pelvis. In contrast to pseudomyxoma peritonei or other diseases causing mucinous carcinomatosis, compartmentalization of the small bowel and a large volume of disease beneath the right hemidiaphragm were absent. Third, the presence of serous ascites rather than mucinous ascites was commonly seen in DMPM. Fourth, none of the patients had extra abdominal lymph node or distant organ metastasis. One must raise the clinical suspicion of DMPM in patients with serous ascites, no primary tumors and yet a disease process that remains confined to the abdominopelvic cavity.
Table 2.
CT characterization of DMPM
| CT characterization of DMPM |
| Diffuse involvement of all peritoneal surface, rarely with an epicenter |
| Preponderance of disease in mid-abdomen and pelvic |
| Presence of serous ascites rather than mucoid |
| Absence of metastasis, irrespective of the volume of disease |
CT: Computed tomography.
Magnetic resonance imaging (MRI) provides good contrast resolution, but requires longer scan times during which respiratory motion and bowel peristalsis can interfere with the image resolution. Positron emission tomography (PET) may be useful and provide functional imaging, but the ability to detect diffuse small tumor nodules is limited. However, PET-CT may be able to provide high CT resolution with simultaneous PET functional imaging. The efficacy of these radiologic modalities in the assessment of DMPM remains to be evaluated.
Biopsy
Commonly, a long delay in the definitive diagnosis of DMPM is a significant problem for both the physician and the patient. Cytological examination of ascitic fluid removed by paracentesis rarely results in a positive finding[46]. If cells are recovered, they frequently resemble hyperplasic mesothelial cells with insufficient atypia present for a confident diagnosis. The state-of-the-art approach to histological verification of the diagnosis of peritoneal malignancy is a CT-guided biopsy, or a laparoscopy. In a study population of 68 DMPM patients, Sugarbaker et al[11] found very few definitive diagnoses made by paracentesis and cytology. Laparoscopy with biopsy was required in 52%, laparotomy with biopsy in 44% and a radiologic guided biopsy in 4%[11]. Eltabbakh and colleagues performed laparotomy or laparoscopy with biopsy as the definitive test for all 15 DMPM patients[10]. Four of the 15 patients had preoperative paracentesis, but all were reported as adenocarcinoma. The low reliability of cytological results warrants an invasive procedure to obtain a generous sample of peritoneal tumor in patients with peritoneal surface cancer of uncertain etiology.
However, an important caveat must accompany the recommendation for laparoscopy in the diagnosis of DMPM. In a series of 8 patients with DMPM diagnosed by laparoscopy, 6 patients presented with tumor implantation in the lateral abdominal wall around trochar tracts (Figure 2), resulting in extraperitoneal dissemination, which changed the natural history of the disease[47]. Therefore, lateral port sites for laparoscopy must be avoided. Trochars should only be placed within the linea alba, so that port sites can be excised at the time of definitive surgical treatment.
Figure 2.
DMPM implantation in the lateral abdominal wall along previous laparoscopic trochar tracts.
Immunohistochemical stains
A biopsy of the tumor is subjected to a complete histopathological analysis. However, the distinction of DMPM from adenocarcinoma is subtle both macroscopically and on routine microscopic study. A series of immunohistochemical markers are necessary to differentiate DMPM from adenocarcinoma (Table 3)[1]. Calretinin identifies cells as being mesothelial in origin. A positive calretinin, cytokeratins 5/6, WT-1, thrombomodulin, and mesothelin stain, accompanied by a negative B72.3, CEA, CD 15, Leu-M1, and BER-EP4 immunostain is highly suggestive of DMPM.
Table 3.
Immunostains of diffuse malignant peritoneal mesothelioma and adenocarcinoma
| Immunostains | Mesothelioma | Adenocarcinoma |
| BER-EP4 | 0-11 | 90-100 |
| B27.3 | 0-5 | 81 |
| Calretinin | 42-100 | 6-9 |
| CA-125 | 14-94 | 90 |
| CD15 (LEU-MI) | 0-10 | 58-100 |
| CEA | 0-10 | 90-100 |
| EMA | 80-100 | 83 |
| P53 | 45 | 43-53 |
| PAN-Cytokeratin | 100 | 100 |
| PLAP | 0 | 50 |
| VIMENTIN | 40 | 0-6 |
| S-100 | 0-11 | 31 |
Histopathology
DMPM has a diversity of cytoarchitectural characteristics that are almost unique among neoplasms originating from a single cell line. The spectrum embraces tumors that are entirely of epithelial or mesenchymal (sarcomatoid) type to a range of biphasic and intermediate forms, as described by Battifora and McCaughey[1].
75% to 90% of DMPM are of the epithelial type, which are characterized by cuboidal or flattened epithelial-like malignant mesothelial cells with ample cytoplasm with distinct cellular membranes, and a relatively uniform, granular to vesicular nuclei (Figure 3A). The subtypes of epithelial DMPM are categorized by the patterns observed for the malignant epithelial component, which are classified as tubulopapillary, solid, deciduoid, storiform-like, fascicular-like, multicystic, papillary, microcystic and granular.
Figure 3.
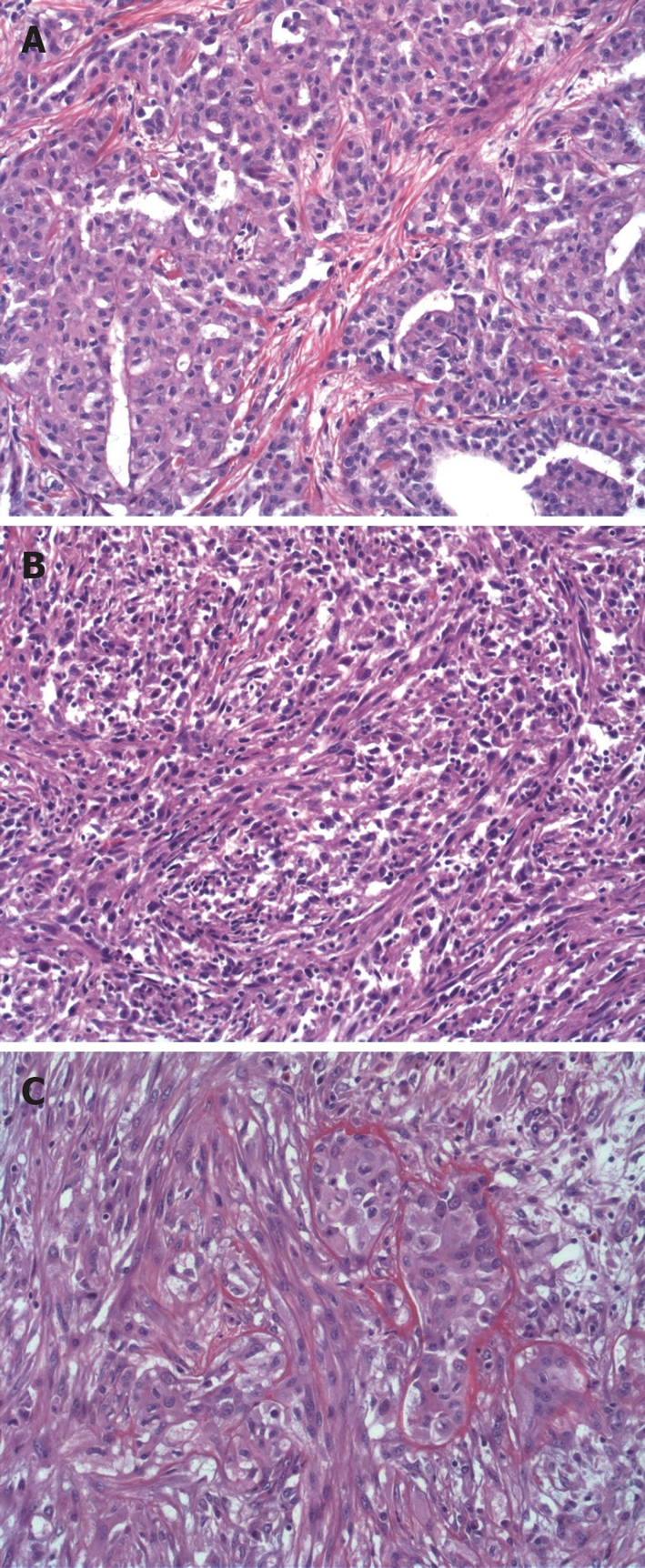
Histopathology of DMPM. A: DMPM-epithelial type, characterized by cuboidal or flattened epithelial-like malignant mesothelial cells (HE, × 20); B: Sarcomatoid type, characterized by sarcomatous spindle-shaped mesothelial cells (HE, × 20); C: Biphasic type, characterized by presence of two phenotypes occurred in same tumor, but sometimes they are intimately admixed (HE, × 20).
The sarcomatoid DMPM is composed only of spindle-shaped mesenchymal type cells (Figure 3B). However, the mesenchymal portion can be as diverse as the epithelial component, in that the sarcomatous elements may morphologically and immunophenotypically resemble any one of the numerous bone and soft tissue tumors by producing malignant osteoid, cartilage or other sarcomatous histologies.
In biphasic DMPM, malignant elements of both epithelial and mesenchymal appearance are present
(Figure 3C). Frequently, the two phenotypes occurred in different parts of the same tumor, but sometimes they are intimately admixed. There is sometimes a high degree of subjectivity involved in the diagnosis of pure sarcomatoid versus biphasic DMPM, which depends on the amount of tissue available and the extent to which it is sampled.
Serum markers
Serum CA-125, a tumor antigen that is present in the majority of patients with ovarian cancer, is also elevated in DMPM. In a study by Kebapci et al[48], CA-125 levels were measured at diagnosis in eight patients with DMPM. Seven patients (6 females and 1 male) had CA-125 values > 32.0 U/mL (normal value 1.2-32 U/mL). In three of these patients serum CA-125 returned to normal levels after chemotherapy. A study by Simsek et al[49] also noted elevated CA-125 in 6 of 7 DMPM patients and in some patients showed a very close correlation with the response to chemotherapy.
Recently, several new tumor markers have been identified to diagnosis mesothelioma. These studies have mostly involved patients with pleural mesothelioma. However, given the similarity between pleural and peritoneal mesothelioma, it is likely that these tests will also be useful in peritoneal mesothelioma. These new tumor markers include mesothelin, soluble mesothelin related proteins (SMRP) and osteopontin. Mesothelin is a cell surface protein highly expressed in mesotheliomas that is attached to the cell surface by a glycosylphosphatidyl inositol (GPI) linkage[50]. Since many GPI-linked proteins are shed into the serum by proteolytic cleavage of the GPI anchor, Hassan and colleagues developed an enzyme-linked immunosorbent assay (ELISA) to determine if mesothelin is shed into the blood. Using this assay an increased serum mesothelin level in 71% of mesothelioma patients was identified[51]. The investigators also looked at serum mesothelin levels before and at several time points after tumor debulking surgery in 6 patients with DMPM; they showed that serum mesothelin levels decreased very rapidly after optimal tumor debulking and may therefore be a useful marker to monitor response to therapy. Another assay that measures SMRP noted elevated levels of SMRP in 37 of 44 patients (84%) with pleural mesothelioma and these levels correlated with tumor bulk[52]. Osteopontin, a glycoprotein that is overexpressed in several cancers, was recently found to be elevated in the serum of patients with pleural mesothelioma. More importantly, serum osteopontin levels were increased in patients with early pleural mesothelioma (stage I) and could therefore be a useful test for early detection[53]. It is very likely that these newly described biomarkers will also be of use for the diagnosis and monitoring of treatment response in patients with DMPM.
MANAGEMENT
Systemic chemotherapy
Traditionally, there has been an agreement among medical practitioners that DMPM was untreatable and thus a preterminal condition with a rapid progression. Patients were managed with systemic chemotherapy and palliative surgery. However, all patients eventually died from the disease as a result of intestinal obstruction and/or terminal starvation[4-10]. The median survival in these patients prior to the year 2000 was less than one year (Table 4) [4-10]. There are now Food and Drug Administration-approved treatment protocols using systemic pemetrexed plus cisplatin. A recent non-randomized study demonstrated a median survival of 13 mo and 1-year survival of 66% in 66 DMPM patients treated with systemic pemetrexed and cisplatin, versus 9 mo and 0% in the respective survival for 32 DMPM patients treated with systemic pemetrexed alone[54]. From the limited data, it is difficult to extrapolate any definitive conclusions regarding the efficacy of this systemic chemotherapy treatment, but further research is warranted.
Table 4.
Median survival of DMPM using traditional treatment modalities
| Authors | n | Median survival (mo) |
| Chailleux et al[4], 1988 | 11/167 | 101 |
| Antman et al[5], 1988 | 37/180 | 151 |
| Sridhar et al[6], 1992 | 13/50 | 9.51 |
| Markman et al[7], 1992 | 19 | 9 |
| Yates et al[8], 1997 | 14/272 | 141 |
| Neumann et al[9], 1999 | 74 | 12 |
| Eltabbakh et al[10], 1999 | 15 | 12.5 |
Combined pleural and peritoneal mesothelioma.
Intraperitoneal chemotherapy
Several studies have evaluated chemotherapy administered via the intraperitoneal route in an attempt to maximize local-regional cytotoxicity and limit systemic side-effects[7,55,56]. However, intraperitoneal chemotherapy penetrates tumor nodules by passive diffusion; therefore the depth of penetration is limited. In addition, the efficacy of intraperitoneal chemotherapy is reduced due to limited chemotherapy distribution in a grossly diseased abdomen. No studies have demonstrated survival benefit for intraperitoneal chemotherapy alone for DMPM.
Cytoreductive surgery and perioperative intraperitoneal chemotherapy
Recently, there has been a reexamination of DMPM treatment, by cytoreductive surgery and perioperative intraperitoneal chemotherapy with intent not to palliate, but to cure[11-19]. There have already been several large studies, including a randomized controlled trial examining the efficacy of this combined procedure for the management of peritoneal carcinomatosis from gastrointestinal and ovarian malignancies[57-62]. DMPM remains confined within the peritoneal cavity throughout its clinical course and these patients experience morbidity and mortality almost exclusively as a result of disease progression in the abdominopelvic cavity. The combined locoregional treatment approach has a strong treatment rationale for DMPM patients.
Cytoreductive surgery is an important first step in the combined treatment; it maximally removes peritoneal tumors together with complete lysis of adhesions between the bowel loops. It consists of a series of peritonectomy procedures including: anterior parietal peritonectomy, greater omentectomy with splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, lesser omentectomy with cholecystectomy, and pelvic peritonectomy with rectosigmoid colonic resection[63]. This provides an optimal situation for adjuvant intraperitoneal chemotherapy, which is given before the formation of any adhesions, allowing direct chemotherapy and tumor-cell contact, without necessarily increasing systemic toxicity[64,65]. Hyperthermia has been known to have direct cytotoxic effects in both a temperature and time-dependent manner[66,67]. It has also been shown to allow a greater depth of penetration of the chemotherapy agents into the tumors and synergize the cytotoxic drugs selected for intraperitoneal use at the time of surgery[68-70].
The most recent phase II study from the National Cancer Institute, Bethesda, USA showed that the median survival of 49 DMPM patients was 92 mo, with a 5-year survival rate of 59%, after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy[14]. The National Cancer Institute of Italy also enrolled 49 patients to undergo the combined treatment and reported that the progression-free survival was 40 mo and the 5-year survival was 57%[16]. Washington Cancer Institute, Washington DC, USA recently published an updated series on 100 DMPM patients who underwent the combined treatment, which demonstrated that overall median survival was 52 mo, with 5- and 7-year survival of 46% and 39%, respectively[19]. Table 5 demonstrates the most recently published updates from all international treatment centers[14,16-19].
Table 5.
Recent updates on cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for DMPM
| Authors | n | Median survival (mo) |
Survival rates (%) |
||||
| 1-yr | 2-yr | 3-yr | 5-yr | 7-yr | |||
| Yan et al[19], 2006 | 100 | 52 | 78 | 64 | 55 | 46 | 39 |
| Feldman et al[14], 2003 | 49 | 92 | 86 | 77 | 59 | 59 | - |
| Deraco et al[16], 2006 | 49 | NR | 88 | 74 | 65 | 57 | - |
| Brigand et al[17], 2006 | 15 | 36 | 69 | 58 | 43 | 29 | - |
| Loggie et al[18], 2001 | 12 | 34 | 60 | 60 | 50 | 33 | 33 |
NR: Median survival was not reached.
Despite the favorable survival data of the combined treatment for DMPM as compared to traditional palliative therapies in the current literature, the results should be interpreted with caution for several reasons. Firstly, the survival benefit is achieved at the expense of moderate to high morbidity and mortality rates, especially at treatment centers in the process of overcoming their initial learning curve. The results achieved by international experts in this field may not be replicated in routine clinical practice. The importance of patient selection is highlighted in the following sections. Second, being a tertiary referral center with multiple patient selection factors operating, the sample studied may not be a valid representation of the targeted population. Thirdly, results of the combined treatment versus traditional therapies should be interpreted with the knowledge that these treatment strategies have not been compared directly. A phase III trial would be ideal. However, this will be difficult to achieve in the current setting, as a comparison between a potentially curative treatment option with a palliative procedure may cause patients to decline randomization. Also, it may be impractical due to the rarity of the disease, as a sufficient number of patients are required. However, a well-designed prospective multi-institutional study may be potentially meaningful.
Radiotherapy
The efficacy of radiotherapy alone for patients with DMPM is unclear. It has been used as an adjunct to other multi-modality treatments in an attempt to achieve aggressive disease control. Taub et al[71] performed a prospective single-institution phase I/II trial on 27 patients with DMPM. The treatment regimen consisted of initial exploratory laparotomy with cytoreductive surgery and placement of indwelling intraperitoneal catheters. Four intraperitoneal courses of doxorubicin (25 mg) alternating with four intraperitoneal courses of cisplatin (100 mg/m2) were administered. Four intraperitoneal doses of gamma interferon were given followed by a second laparotomy with biopsy verification of complete response or attempted resection of residual disease. Intraperitoneal hyperthermic chemotherapy with mitomycin (10 mg/m2) plus cisplatin (75 to 100 mg/m2), followed by whole abdominal radiation was added to the management plan. The overall median survival was 68 mo with a 3-year survival of 67%[71].
Immunotherapy
There is limited data regarding the role of immunotherapy in peritoneal mesothelioma because of a lack of clinical trials specifically targeting this patient population. However, some information is available from Phase I studies that have included DMPM patients. In a phase I study of Flt3 immunotherapy, 15 patients were treated with intraperitoneal or subcutaneous Flt3-L[72]. The treatment was well tolerated. Of the four DMPM patients treated in this study, two had stable disease lasting 8 mo. Another study evaluated intraperitoneal administration of human interleukin-12[73]. Although this trial included only one DMPM patient, the patient had a complete response for 2 years. Investigators at the National Cancer Institute, Bethesda are conducting clinical studies targeting the tumor antigen, mesothelin. Mesothelin is a cell surface protein present on normal mesothelial cells lining the pleura, peritoneum and pericardium that is highly expressed in mesothelioma, ovarian and pancreatic cancer[50]. To target mesothelin they have developed a recombinant immunotoxin, SS1P, consisting of an anti-mesothelin Fv linked to a truncated Pseudomonas exotoxin. In a Phase I study of SS1P, 23 patients with mesothelin-expressing cancers including 8 patients with DMPM were treated[74]. In this study, one patient with DMPM had complete resolution of abdominal ascites lasting more than 3 years and has required no further treatment. Given the rarity of DMPM, efficacy studies of promising immunotherapy agents will require well-designed multi-institutional clinical trials.
PROGNOSTIC FACTORS AND STAGING
In the past, no uniform treatments were suggested for patients with DMPM and the survival was largely dependent upon the indolent versus aggressive biology of the disease. Several studies reported a reduced survival outcome associated with biphasic or sarcomatoid histologic type, as compared to epithelial type[11,14,75]. However, the criterion is not useful as a prognostic indicator, because the majority of DMPM patients are diagnosed with the epithelial type. There was in fact no staging system for DMPM. A lack of prognostic indicators for optimal patient selection is not surprising. As the disease is rare, most centers do not have a sufficient number of patients. Treatments employed in these patients have varied greatly. Most studies in the current literature have a relatively small sample size and the clinical implications of these reports, in terms of their value for patient management, are limited.
In the last 5 years, several international treatment centers have demonstrated a markedly improved survival in DMPM patients after the combined treatment, compared to historical controls[11-19]. As increased numbers of patients are treated with a uniform regimen, a more thorough and precise analysis of clinical, radiologic and histopathologic prognostic parameters are possible.
Gender
Females have been found to show a better prognosis in DMPM, as compared to males[11]. A very real epidemiologic difference between males and females appears to be the likelihood of asbestos exposure. The direct exposure to asbestos was definitely causative in men, but less apparent in women[20,22]. It is possible that this difference in causation is at least in part responsible for the difference in the natural history of DMPM in women. However, other clinical characteristics may contribute to the improved prognosis of females. In the authors’ previous study, women seldom presented with weight loss; a lack of this important poor prognostic symptom suggests less advanced disease[40]. Also, women often sought medical attention earlier with gynecological complaints caused by DMPM. Diagnosis as a result of non-specific gynecological symptoms may have contributed to their improved long-term survival[11]. A recent study showed that females were associated with more favorable histopathologic features, which also might contribute to their better overall outcome[76].
Lymph node metastasis
Lymph node metastasis is uncommon in patients with DMPM, but is associated with an extremely poor prognosis[19]. In 100 DMPM patients treated at the Washington Cancer Institute, seven patients were found to have positive lymph nodes. The most common sites of lymph node involvement were external, internal and common iliac lymph nodes, and ileocolic lymph nodes. Their median survival was 6 mo, with 1- and 2-year survival of 43% and 0%, respectively. Ninety-three patients had absence of lymph node involvement and their median survival was 59 mo, with 5- and 7-year survival of 50% and 43%, respectively[19]. Apparently, lymph node metastasis in DMPM uniformly indicates a guarded prognosis. The crucial importance of lymph node positivity versus negativity in DMPM encourages the surgeon to be diligent in seeking abnormal lymph nodes when performing cytoreductive surgery. Any enlarged or firm lymph nodes should be submitted separately from the rest of the specimens. It should become current surgical practice to sample some iliac lymph nodes and all suspicious lymph nodes in patients with DMPM in order to definitively establish their lymph node status.
Completeness of cytoreduction
Nearly all treatment centers agree that completeness of cytoreduction is one of the most significant prognostic factors for long-term survival[11-19]. It is related to the pretreatment tumor load and the surgeon’s ability to eradicate gross disease. Unlike pseudomyxoma peritonei or other mucinous adenocarcinoma, DMPM does not spare the peritoneal surfaces of the small intestines. This unfortunately limits the ability to achieve a complete cytoreduction. However, even an adequate cytoreduction with a residual tumor < 2.5 cm in diameter, combined with perioperative intraperitoneal chemotherapy can offer some patients long-term benefits. International experience has consistently shown that after an adequate cytoreduction and perioperative intraperitoneal chemotherapy, the 5-year survival ranges from 30% to 60%[11-19].
Radiologic classifications
There are problems with using completeness of cytoreduction for prognostication, as this clinical information is unavailable preoperatively in the patient selection process. Yan et al[77] described interpretive CT classifications of the small bowel and mesentery, which are useful in determining the operability of a patient with DMPM. As indicated in Table 6, characteristic interpretative CT appearances of the small bowel and its mesentery are categorized into four classes (Class 0-III). In Class III disease, configuration of the small bowel and mesentery on CT appears so thickened and grossly distorted that an adequate cytoreduction is almost impossible to achieve[77].
Table 6.
Interpretative CT classification of small bowel and small bowel mesentery for DMPM
| Class | Interpretative CT classification of small bowel and small bowel mesentery |
| 0 | No ascites in the region of the small bowel; no evidence of peritoneal tumor present; the jejunal and ileal vessels appear as round and curvilinear densities within the mesenteric fat |
| I | Free ascites only; mesentery is stranded and stratified as the fluid accumulation outlined the small bowel mesentery; small bowel vessels are easily identified within the mesenteric fat |
| II | Tumor involvement of small bowel and/or its mesentery; peritoneal surface is thickened and enhanced due to the presence of tumor nodules or plaques; there may be an increased amount of ascites and the mesentery may appear stellate or pleated |
| III | Increased solid tumor involvement and adjacent small bowel loops are matted together in some cuts; small bowel mesenteric vessels are difficult to define due to obliteration of mesenteric fat |
Histopathologic staging
In 1995, Goldblum and Hart first described a nuclear grading system according to histomorphologic features of DMPM[78]. In 2001, Kerrigan and colleagues first tested this nuclear grading system in 25 female patients with DMPM who underwent a variety of surgical, chemotherapy or radiotherapy treatments and found that the nuclear grading was not strongly associated with long-term survival[79]. In 2005, Nonaka and co-workers demonstrated that the size of the mesothelioma nucleus was prognostically significant for overall survival in 35 patients who underwent uniform treatment using cytoreductive surgery and perioperative intraperitoneal chemotherapy[15].
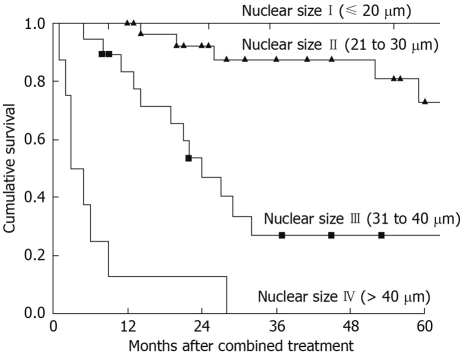
In 2006, with a larger sample size, uniform treatment, longer follow-up and more histopathology sections per patients studied, Yan and collaborators found in multivariate analysis that the nuclear size was the only independent prognostic determinant for overall survival in DMPM[12]. The 3-year survival rates with nuclear size of 10-20 μm, 21-30 μm, 31-40 μm and > 40 μm were 100%, 87%, 27% and 0%, respectively (Figure 4)[12]. The findings may suggest that nuclear size is a surrogate molecular marker of the biological aggressiveness of DMPM. The prognosis of patients with DMPM after maximal attempt of cytoreduction and perioperative intraperitoneal chemotherapy is predominantly governed by the biological aggressiveness of the mesothelioma cells. It seems that this combined treatment offers little survival benefit to patients with a nucleus size of > 40 μm. Consequently, the authors have proposed a histopathologic staging system using nuclear size only, as an objective and quantitative assessment of the predominant nuclear size of DMPM[12].
Figure 4.
Cumulative survival after cytoreductive surgery and perioperative intraperitoneal chemotherapy for DMPM. The prognostic significance of mesothelioma nuclear size was P < 0.001.
Future directions
Asbestos is predicted to cost the economy of the western world around $US 300 billion in compensation in future decades. This is in addition to health-care costs associated with the disease. Many unanswered questions remain regarding the management of DMPM. For example, is there now a role for long-term bi-directional (intravenous and intraperitoneal) chemotherapy as reported in ovarian cancer[62]. This disease, which has always been considered a pre-terminal condition, can now be treated with curative-intent by cytoreductive surgery and perioperative intraperitoneal chemotherapy at a referral center with benefit in terms of long-term survival. This new treatment approach may also result in quality of life benefit in that there is a complete resolution of ascites in most patients. Perhaps it is safe to suggest that this new combined treatment option is a new standard of care with which all other treatment options should now be compared. With more patients undergoing a uniform treatment plan, a deeper understanding can be gained in the diagnosis, radiology and histopathology of this rare disease.
Despite numerous studies demonstrating promising survival advantages and acceptable perioperative outcomes associated with the combined treatment, as shown in the present review, there is a lack of high-level evidence or comparative data on the safety and effectiveness of the combined treatment versus current alternative therapies. Recruitment of adequate numbers of patients for a randomized controlled trial to evaluate the comparative safety and effectiveness of these procedures for DMPM is difficult, as the period of time required to recruit an adequate number of patients to reach any statistical difference is likely to extend over different evolutions of both the intervention and comparator. In addition, there might be unwillingness from both patients and healthcare providers to randomize patients to receive a potentially curative treatment against a palliative approach.
Under these circumstances, high quality prospective observational data collection will be extremely important, as it provides more accurate estimates of safety and efficacy outcomes for the procedure, in addition to evaluating potential prognostic factors associated with favourable outcomes. More importantly, outcomes must be recorded on an intention-to-treat basis. Many centers report outcomes only in patients who are accepted for aggressive management strategies and who received complete cytoreduction. Although this may be predictive of outcome, it only applies to selected patients, usually with favourable prognostic features. This is not useful in terms of patient selection. Ideally, establishment of a multi-institutional registry requiring a minimum data set for all patients and recording treatment outcomes regardless of the intervention they received would provide more reliable estimates of outcomes for patients receiving different therapies over a longer term.
We propose a multi-institutional registry database that would collect the following information: (1) data on all DMPM patients in whom complete cytoreduction and perioperative intraperitoneal chemotherapy is attempted, regardless of whether or not peritonectomy is performed, whether or not optimal cytoreduction is achieved or an open-and-close laparotomy is performed; (2) clinicopathologic information including demographic data, radiological findings, lymph node status, systemic metastases, extent of intraperitoneal disease and histopathological grading; (3) surgical intervention details including components of cytoreductive surgery, types of visceral resections, application of perioperative intraperitoneal chemotherapy, completeness of cytoreduction, operation time and transfusion requirement; (4) short-term outcomes including in-hospital mortality, moderate to severe morbidity, including Grade III/IV chemotherapy-related toxicity, intra-abdominal abscess, fistula, anastomotic leak, sepsis, pulmonary embolism, radiological interventions, complications requiring return to operating room and intensive care unit; (5) delayed complications including post-discharge morbidity and re-admissions and (6) regular patient follow-up performed at set intervals for effectiveness including 5-year overall survival, minimum 2-year disease-free/progression-free survival, quality of life after acute treatment effects.
It is possible that this approach to observational data collection may also provide some information on prognostically similar patients undergoing different management pathways, thereby providing some evidence for a comparison of different treatment options. In addition, the roles of systemic chemotherapy, immunotherapy and targeted treatments in DMPM patients remain to be studied and their integration into the combined therapy has yet to be determined.
Footnotes
Peer reviewers: Dario Conte, Professor, GI Unit - IRCCS Osp. Maggiore, Chair of Gastroenterology, Via F. Sforza, 35, Milano 20122, Italy; Shingo Tsuji, MD, PhD, AGAF, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine (A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Li LF L- Editor Webster JR E- Editor Lin YP
References
- 1.Battifora H, McCaughey WTE. Tumors of the Serosal Membranes. Washington DC: Armed Forces Institute of Pathology; 1994. [Google Scholar]
- 2.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 3.Miller J, Wynn H. A malignant tumour arising from the endothelium of the peritoneum, and producing a mucoid ascitic fluid. J Pathol Bacteriol. 2005;12:267–268. [Google Scholar]
- 4.Chailleux E, Dabouis G, Pioche D, de Lajartre M, de Lajartre AY, Rembeaux A, Germaud P. Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest. 1988;93:159–162. doi: 10.1378/chest.93.1.159. [DOI] [PubMed] [Google Scholar]
- 5.Antman K, Shemin R, Ryan L, Klegar K, Osteen R, Herman T, Lederman G, Corson J. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965-1985. J Clin Oncol. 1988;6:147–153. doi: 10.1200/JCO.1988.6.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Sridhar KS, Doria R, Raub WA Jr, Thurer RJ, Saldana M. New strategies are needed in diffuse malignant mesothelioma. Cancer. 1992;70:2969–2979. doi: 10.1002/1097-0142(19921215)70:12<2969::aid-cncr2820701239>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 1992;118:547–550. doi: 10.1007/BF01225271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in south east England: clinicopathological experience of 272 cases. Thorax. 1997;52:507–512. doi: 10.1136/thx.52.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann V, Müller KM, Fischer M. [Peritoneal mesothelioma--incidence and etiology] Pathologe. 1999;20:169–176. doi: 10.1007/s002920050340. [DOI] [PubMed] [Google Scholar]
- 10.Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Intengen ME. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70:6–12. doi: 10.1002/(sici)1096-9098(199901)70:1<6::aid-jso2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker PH, Welch LS, Mohamed F, Glehen O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am. 2003;12:605–621, xi. doi: 10.1016/s1055-3207(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 12.Yan TD, Brun EA, Cerruto CA, Haveric N, Chang D, Sugarbaker PH. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2007;14:41–49. doi: 10.1245/s10434-006-9169-7. [DOI] [PubMed] [Google Scholar]
- 13.Park BJ, Alexander HR, Libutti SK, Wu P, Royalty D, Kranda KC, Bartlett DL. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP) Ann Surg Oncol. 1999;6:582–590. doi: 10.1007/s10434-999-0582-6. [DOI] [PubMed] [Google Scholar]
- 14.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka D, Kusamura S, Baratti D, Casali P, Cabras AD, Younan R, Rosai J, Deraco M. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer. 2005;104:2181–2188. doi: 10.1002/cncr.21239. [DOI] [PubMed] [Google Scholar]
- 16.Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, Salvatore A, Cabras Ad AD, Kusamura S. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13:229–237. doi: 10.1245/ASO.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, Glehen O. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13:405–412. doi: 10.1245/ASO.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 18.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67:999–1003. [PubMed] [Google Scholar]
- 19.Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:948–953. doi: 10.1016/j.ejso.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Welch LS, Acherman YI, Haile E, Sokas RK, Sugarbaker PH. Asbestos and peritoneal mesothelioma among college-educated men. Int J Occup Environ Health. 2005;11:254–258. doi: 10.1179/107735205800245975. [DOI] [PubMed] [Google Scholar]
- 21.Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol. 2004;159:107–112. doi: 10.1093/aje/kwh025. [DOI] [PubMed] [Google Scholar]
- 22.Spirtas R, Heineman EF, Bernstein L, Beebe GW, Keehn RJ, Stark A, Harlow BL, Benichou J. Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med. 1994;51:804–811. doi: 10.1136/oem.51.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weill H, Hughes JM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–441. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montanaro F, Bray F, Gennaro V, Merler E, Tyczynski JE, Parkin DM, Strnad M, Jechov'a M, Storm HH, Aareleid T, et al. Pleural mesothelioma incidence in Europe: evidence of some deceleration in the increasing trends. Cancer Causes Control. 2003;14:791–803. doi: 10.1023/a:1026300619747. [DOI] [PubMed] [Google Scholar]
- 25.Langård S. Nordic experience: expected decline in the incidence of mesotheliomas resulting from ceased exposure? Med Lav. 2005;96:304–311. [PubMed] [Google Scholar]
- 26.Swuste P, Burdorf A, Ruers B. Asbestos, asbestos-related diseases, and compensation claims in The Netherlands. Int J Occup Environ Health. 2004;10:159–165. doi: 10.1179/oeh.2004.10.2.159. [DOI] [PubMed] [Google Scholar]
- 27.McElvenny DM, Darnton AJ, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79–87. doi: 10.1093/occmed/kqi034. [DOI] [PubMed] [Google Scholar]
- 28.Ulvestad B, Kjaerheim K, Møller B, Andersen A. Incidence trends of mesothelioma in Norway, 1965-1999. Int J Cancer. 2003;107:94–98. doi: 10.1002/ijc.11357. [DOI] [PubMed] [Google Scholar]
- 29.La Vecchia C, Decarli A, Peto J, Levi F, Tomei F, Negri E. An age, period and cohort analysis of pleural cancer mortality in Europe. Eur J Cancer Prev. 2000;9:179–184. [PubMed] [Google Scholar]
- 30.Banaei A, Auvert B, Goldberg M, Gueguen A, Luce D, Goldberg S. Future trends in mortality of French men from mesothelioma. Occup Environ Med. 2000;57:488–494. doi: 10.1136/oem.57.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinaccio A, Montanaro F, Mastrantonio M, Uccelli R, Altavista P, Nesti M, Costantini AS, Gorini G. Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer. 2005;115:142–147. doi: 10.1002/ijc.20820. [DOI] [PubMed] [Google Scholar]
- 32.Dave SK, Beckett WS. Occupational asbestos exposure and predictable asbestos-related diseases in India. Am J Ind Med. 2005;48:137–143. doi: 10.1002/ajim.20198. [DOI] [PubMed] [Google Scholar]
- 33.Kazan-Allen L. Asbestos and mesothelioma: worldwide trends. Lung Cancer. 2005;49 Suppl 1:S3–S8. doi: 10.1016/j.lungcan.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Chang KC, Leung CC, Tam CM, Yu WC, Hui DS, Lam WK. Malignant mesothelioma in Hong Kong. Respir Med. 2006;100:75–82. doi: 10.1016/j.rmed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Antman KH, Corson JM, Li FP, Greenberger J, Sytkowski A, Henson DE, Weinstein L. Malignant mesothelioma following radiation exposure. J Clin Oncol. 1983;1:695–700. doi: 10.1200/JCO.1983.1.11.695. [DOI] [PubMed] [Google Scholar]
- 36.Chahinian AP, Pajak TF, Holland JF, Norton L, Ambinder RM, Mandel EM. Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann Intern Med. 1982;96:746–755. doi: 10.7326/0003-4819-96-6-746. [DOI] [PubMed] [Google Scholar]
- 37.Riddell RH, Goodman MJ, Moossa AR. Peritoneal malignant mesothelioma in a patient with recurrent peritonitis. Cancer. 1981;48:134–139. doi: 10.1002/1097-0142(19810701)48:1<134::aid-cncr2820480124>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Maurer R, Egloff B. Malignant peritoneal mesothelioma after cholangiography with thorotrast. Cancer. 1975;36:1381–1385. doi: 10.1002/1097-0142(197510)36:4<1381::aid-cncr2820360429>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 39.Peterson JT Jr, Greenberg SD, Buffler PA. Non-asbestos-related malignant mesothelioma. A review. Cancer. 1984;54:951–960. doi: 10.1002/1097-0142(19840901)54:5<951::aid-cncr2820540536>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Acherman YI, Welch LS, Bromley CM, Sugarbaker PH. Clinical presentation of peritoneal mesothelioma. Tumori. 2003;89:269–273. doi: 10.1177/030089160308900307. [DOI] [PubMed] [Google Scholar]
- 41.Tejido García R, Anta Fernández M, Hernández Hernández JL, Bravo González J, González Macías J. [Fever of unknown origin as the clinical presentation of malignant peritoneal mesothelioma] An Med Interna. 1997;14:573–575. [PubMed] [Google Scholar]
- 42.Reuter K, Raptopoulos V, Reale F, Krolikowski FJ, D'Orsi CJ, Graham S, Smith EH. Diagnosis of peritoneal mesothelioma: computed tomography, sonography, and fine-needle aspiration biopsy. AJR Am J Roentgenol. 1983;140:1189–1194. doi: 10.2214/ajr.140.6.1189. [DOI] [PubMed] [Google Scholar]
- 43.Ros PR, Yuschok TJ, Buck JL, Shekitka KM, Kaude JV. Peritoneal mesothelioma. Radiologic appearances correlated with histology. Acta Radiol. 1991;32:355–358. [PubMed] [Google Scholar]
- 44.Guest PJ, Reznek RH, Selleslag D, Geraghty R, Slevin M. Peritoneal mesothelioma: the role of computed tomography in diagnosis and follow up. Clin Radiol. 1992;45:79–84. doi: 10.1016/s0009-9260(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 45.Yan TD, Haveric N, Carmignani CP, Bromley CM, Sugarbaker PH. Computed tomographic characterization of malignant peritoneal mesothelioma. Tumori. 2005;91:394–400. doi: 10.1177/030089160509100503. [DOI] [PubMed] [Google Scholar]
- 46.Yu GH, Soma L, Hahn S, Friedberg JS. Changing clinical course of patients with malignant mesothelioma: implications for FNA cytology and utility of immunocytochemical staining. Diagn Cytopathol. 2001;24:322–327. doi: 10.1002/dc.1069. [DOI] [PubMed] [Google Scholar]
- 47.Muensterer OJ, Averbach AM, Jacquet P, Otero SE, Sugarbaker PH. Malignant peritoneal mesothelioma. Case-report demonstrating pitfalls of diagnostic laparoscopy. Int Surg. 1997;82:240–243. [PubMed] [Google Scholar]
- 48.Kebapci M, Vardareli E, Adapinar B, Acikalin M. CT findings and serum ca 125 levels in malignant peritoneal mesothelioma: report of 11 new cases and review of the literature. Eur Radiol. 2003;13:2620–2626. doi: 10.1007/s00330-003-1851-6. [DOI] [PubMed] [Google Scholar]
- 49.Simsek H, Kadayifci A, Okan E. Importance of serum CA 125 levels in malignant peritoneal mesothelioma. Tumour Biol. 1996;17:1–4. doi: 10.1159/000217960. [DOI] [PubMed] [Google Scholar]
- 50.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 51.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, Alexander R, Willingham M, Pastan I, Onda M. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 52.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, Winzell P, Hellstrom KE, Hellstrom I. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 53.Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 54.Jänne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, Mintzer DM, Taylor L, Ashland J, Ye Z, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer. 2005;7:40–46. doi: 10.3816/CLC.2005.n.020. [DOI] [PubMed] [Google Scholar]
- 55.Markman M. Intraperitoneal belly bath chemotherapy. 2nd ed. Chicago: Percept Press; 1990. [Google Scholar]
- 56.Vlasveld LT, Gallee MP, Rodenhuis S, Taal BG. Intraperitoneal chemotherapy for malignant peritoneal mesothelioma. Eur J Cancer. 1991;27:732–734. doi: 10.1016/0277-5379(91)90176-e. [DOI] [PubMed] [Google Scholar]
- 57.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 58.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12:65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 59.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Vignal J, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- 61.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 62.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 63.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz MH, Barone RM. The rationale of perioperative intraperitoneal chemotherapy in the treatment of peritoneal surface malignancies. Surg Oncol Clin N Am. 2003;12:673–688. doi: 10.1016/s1055-3207(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 65.Sugarbaker P, Cunliffe W, Belliveau J, Bruin E, Graves T. Rationale for perioperative intraperitoneal chemotherapy as a surgical adjunct for gastrointestinal malignancy. Reg Cancer Treat. 1988:66–79. [Google Scholar]
- 66.Armour EP, McEachern D, Wang Z, Corry PM, Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993;53:2740–2744. [PubMed] [Google Scholar]
- 67.Los G, Smals OA, van Vugt MJ, van der Vlist M, den Engelse L, McVie JG, Pinedo HM. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res. 1992;52:1252–1258. [PubMed] [Google Scholar]
- 68.Los G, Sminia P, Wondergem J, Mutsaers PH, Havemen J, ten Bokkel Huinink D, Smals O, Gonzalez-Gonzalez D, McVie JG. Optimisation of intraperitoneal cisplatin therapy with regional hyperthermia in rats. Eur J Cancer. 1991;27:472–477. doi: 10.1016/0277-5379(91)90389-u. [DOI] [PubMed] [Google Scholar]
- 69.van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148–154. doi: 10.1016/s0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 70.Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15:79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 71.Taub RN, Hesdorffer ME, Keohan ML. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for malignant periotneal mesothelioma (MPM) J Clin Oncol. 2005;23:664s. doi: 10.1097/COC.0b013e3180684181. [DOI] [PubMed] [Google Scholar]
- 72.Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, Kavanagh JJ, Hicks ME, Levy LB, Folloder JK, et al. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin Cancer Res. 2003;9:5228–5237. [PubMed] [Google Scholar]
- 73.Lenzi R, Rosenblum M, Verschraegen C, Kudelka AP, Kavanagh JJ, Hicks ME, Lang EA, Nash MA, Levy LB, Garcia ME, et al. Phase I study of intraperitoneal recombinant human interleukin 12 in patients with Müllerian carcinoma, gastrointestinal primary malignancies, and mesothelioma. Clin Cancer Res. 2002;8:3686–3695. [PubMed] [Google Scholar]
- 74.Hassan R, Bullock S, Kindler H, Pastan I. Updated results of the phase I study of SS1(dsFv)PE38 for targeted therapy of mesothelin expressing cancers. Eur J Cancer. 2004;2:280A. [Google Scholar]
- 75.Sebbag G, Yan H, Shmookler BM, Chang D, Sugarbaker PH. Results of treatment of 33 patients with peritoneal mesothelioma. Br J Surg. 2000;87:1587–1593. doi: 10.1046/j.1365-2168.2000.01571.x. [DOI] [PubMed] [Google Scholar]
- 76.Yan TD, Popa E, Brun EA, Cerruto CA, Sugarbaker PH. Sex difference in diffuse malignant peritoneal mesothelioma. Br J Surg. 2006;93:1536–1542. doi: 10.1002/bjs.5377. [DOI] [PubMed] [Google Scholar]
- 77.Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;103:839–849. doi: 10.1002/cncr.20836. [DOI] [PubMed] [Google Scholar]
- 78.Goldblum J, Hart WR. Localized and diffuse mesotheliomas of the genital tract and peritoneum in women. A clinicopathologic study of nineteen true mesothelial neoplasms, other than adenomatoid tumors, multicystic mesotheliomas, and localized fibrous tumors. Am J Surg Pathol. 1995;19:1124–1137. doi: 10.1097/00000478-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Kerrigan SA, Turnnir RT, Clement PB, Young RH, Churg A. Diffuse malignant epithelial mesotheliomas of the peritoneum in women: a clinicopathologic study of 25 patients. Cancer. 2002;94:378–385. doi: 10.1002/cncr.10209. [DOI] [PubMed] [Google Scholar]