Abstract
Methionine is the precursor of homocysteine, a sulfur amino acid intermediate in the methylation and transsulfuration pathways. Elevated plasma homocysteine (hyperhomocysteinemia) is associated with occlusive vascular disease. Whether homocysteine per se or a coincident metabolic abnormality causes vascular disease is still an open question. Animals with genetic hyperhomocysteinemia have so far not displayed atheromatous lesions. However, when methionine-rich diets are used to induce hyperhomocysteinemia, vascular pathology is often observed. Such studies have not distinguished the effects of excess dietary methionine from those of hyperhomocysteinemia. We fed apolipoprotein E-deficient mice with experimental diets designed to achieve three conditions: (i) high methionine intake with normal blood homocysteine; (ii) high methionine intake with B vitamin deficiency and hyperhomocysteinemia; and (iii) normal methionine intake with B vitamin deficiency and hyperhomocysteinemia. Mice fed methionine-rich diets had significant atheromatous pathology in the aortic arch even with normal plasma homocysteine levels, whereas mice fed B vitamin-deficient diets developed severe hyperhomocysteinemia without any increase in vascular pathology. Our findings suggest that moderate increases in methionine intake are atherogenic in susceptible mice. Although homocysteine may contribute to the effect of methionine, high plasma homocysteine was not independently atherogenic in this model. Some product of excess methionine metabolism rather than high plasma homocysteine per se may underlie the association of homocysteine with vascular disease.
Methionine is the precursor of homocysteine, a sulfur amino acid intermediate in the methylation and transsulfuration pathways. Homocysteine, a non-protein-forming sulfur amino acid, was first implicated as a cause of occlusive vascular disease by K. S. McCully, who noted the high prevalence of early arteriosclerotic and thromboembolic disease in patients with congenital homocysteinuria (1). Since then, a growing body of epidemiological evidence has shown a strong association of elevated plasma homocysteine with vascular disease in the general population (2-7). The association remains strong after adjustment for major determinants of homocysteine, such as age and renal function, folate, vitamin B12, and vitamin B6, suggesting that homocysteine is an independent risk factor for occlusive vascular disease. Together with the known association of inborn errors of homocysteine metabolism and premature vascular disease in humans, these data provide the basis for a compelling, if still controversial (8-11), hypothesis that elevated blood homocysteine is a cause of vascular disease. This hypothesis has engendered great interest because of the possibility that lowering blood homocysteine through nutritional interventions might prove to be a safe and effective means of reducing the associated risk of disease. However, despite a variety of theoretically plausible mechanisms proposed to underlie this association (12), none has been adequately demonstrated in vivo, and the question remains whether homocysteine is a cause, a fellow traveler, or a marker of pathogenic mechanisms in vascular disease.
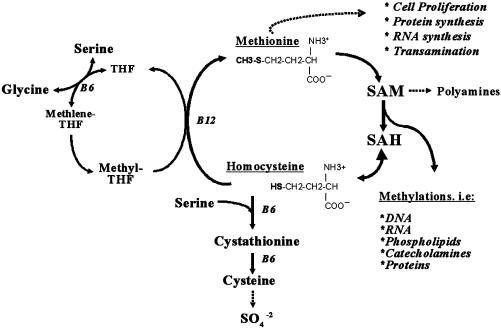
Homocysteine is formed from the essential amino acid methionine, and blood levels are modulated by a complex metabolism that requires the B vitamins folate, methylcobalamin (B12), and pyridoxal 5′-phosphate (B6) (Fig. 1). Excess dietary methionine and B vitamin deficiency can elevate plasma homocysteine, and B vitamin supplementation can lower plasma homocysteine levels.
Fig. 1.
Pathways of homocysteine and methionine metabolism. In cells, homocysteine is derived from methionine after its utilization as a methyl group donor in biological methylation reactions. In this cycle, methionine is activated by condensation with ATP to give the ubiquitous methyl donor, SAM. SAM is transformed into S-adenosylhomocysteine (SAH) by donating its methyl group to the substrates of methylation reactions. Subsequently, SAH gives rise to homocysteine in a reversible reaction that favors SAH over homocysteine production. Because SAH is a potent inhibitor of most methyltransferase enzymes, homocysteine must be constantly removed through the folate- and vitamin B12-dependent remethylation of homocysteine to methionine or by the irreversible vitamin B6-dependent degradation of homocysteine through the transsulfuration pathway. When intracellular homocysteine accumulates, excess homocysteine can be exported into circulation for clearance by kidney and liver. Methionine enters the cycle primarily from diet, but it can also be salvaged from endogenous protein degradation. Methionine can be removed from the cycle for use in protein and polyamine synthesis, or by homocysteine and the transsulfuration pathway. SAM acts as a coregulator of the methylation and transsulfuration pathways by stimulating cystathionine β-synthetase, the first step in the removal of homocysteine and inhibiting methylene tetrahydrofolate reductase, which generates the folate coenzyme used in the synthesis of methionine from homocysteine (67). Saturation of the transsulfuration pathway by excess methionine and B6 deficiency, or by inhibition of homocysteine remethylation due to folate or B12 deficiency, can lead to the production of intracellular homocysteine, which is then exported from cells into plasma. B6, vitamin B6, pyridoxal 5′-phosphate; B12, vitamin B12, methylcobalamin; THF, tetrahydrofolate.
As an endogenously produced metabolic intermediate, homocysteine is never biologically isolated from its determinants, and any rise in plasma homocysteine is necessarily coincident with a metabolic or physiological cause. However, similar levels of blood homocysteine can reflect very different underlying causes. For example, excess dietary methionine drives a transient increase in methionine, S-adenosylmethionine (SAM), and homocysteine (13). In contrast, folate and B12 deficiencies result in low methionine and SAM and in high homocysteine, because they inhibit the regeneration of methionine from homocysteine (14).
Such metabolic complexity has made it difficult to test the hypothesis that elevated homocysteine in blood is vasotoxic. Doing so requires that the pathological consequences of elevated blood homocysteine per se must be distinguished from those of any experimental treatments that are used to induce hyperhomocysteinemia. This difficulty is evident in studies aimed at testing the homocysteine toxicity hypothesis in vivo by raising plasma homocysteine through nutritional means. Such studies have successfully demonstrated that nutritionally induced hyperhomocysteinemia can cause or accelerate arterial lesions in mice (15, 16), rats (17, 18), rabbits (19, 20), and pigs (21, 22). However, because the great majority of these studies used excess dietary methionine to induce hyperhomocysteinemia, the possibility that the observed lesions were due to a harmful effect related to excess methionine intake rather than elevated blood homocysteine per se cannot be ruled out.
In light of this analysis, we hypothesized that if blood homocysteine per se is intrinsically atherogenic, then atherosclerotic pathology should occur in the presence of elevated blood homocysteine regardless of the primary cause of the elevation. If, on the other hand, elevated plasma homocysteine is not directly atherogenic but causes of other types of vascular damage or is an epiphenomenon marking the presence of coincident pathogenic mechanisms, then not all conditions that raise plasma homocysteine would necessarily be associated with atheromatous pathology.
We chose to test our hypothesis in apolipoprotein E (ApoE)-deficient mice (23-25). Mice lacking this gene develop hypercholesterolemia and spontaneous atherosclerotic lesions, which appear in the aortic root and branch points in an age-dependent manner and in a pattern resembling human atheromas (26). When the mice are raised on normal rodent diets, fatty streaks begin to appear within 10 weeks; intermediate lesions containing foam cells, smooth muscle cell proliferation, and endothelial damage appear after 15 weeks; and fibrous plaques appear after 20 weeks (27-32). Although thrombotic lesions are extremely rare in this model, the lesions nevertheless display a range of biochemical, structural, and functional changes that are hallmarks of the human pathology. These include the accumulation of oxidized lipids (33-36) and inflammatory markers (15, 37-39), endothelial dysfunction, and hypertension (40-44). ApoE-deficient mice have proven valuable for determining the role of dietary risk factors for atherosclerosis on disease progression in vivo. Because the mice develop spontaneous vascular lesions, no need exists to assume that the factor of interest is a necessary or sufficient cause of disease. Instead, the effect of dietary modification on the rate and extent of lesion progression can be examined. Such studies have shown that high cholesterol (45, 46) and iron-rich (47, 48) diets can accelerate lesion progression, whereas diets rich in unsaturated fats (49) and antioxidants (35, 50) can slow the disease process.
The present study aimed to distinguish between the atherogenic effects of elevated plasma homocysteine and its nutritional determinants, high methionine intake and B vitamin deficiency. To do so, we fed ApoE-deficient mice methionine-supplemented and B vitamin-deficient or -supplemented diets and determined their effect on plasma homocysteine concentrations and on the progression of genetically induced atheromatous lesions.
Methods
Animals and Diets. Three-week-old weanling male ApoE-deficient (strain B6.129P2-ApoEtm1Unc) mice were purchased from The Jackson Laboratory and maintained at our animal facility. Mice were acclimated on a standard rodent maintenance diet recommended by the American Institute of Nutrition and fed ad libitum for 1 week (AIN-93M) (51). They were then systematically assigned to four groups of similar mean body weights and fed for 10 weeks with control and experimental diets formulated on the basis of Hoffman et al. (15) with vitamin-free, ethanol-precipitated casein and the appropriate vitamin mix (Harlan TEKLAD, Madison, WI). A control group continued eating the control AIN-93M diet. Two diets were formulated to induce hyperhomocysteinemia through combined folate, vitamin B12, and vitamin B6 deficiency with or without methionine enrichment: diet “M+B-,” a methionine-enriched/B vitamin-deficient diet, and diet “B-,” a B vitamin-deficient diet with control levels of methionine. A third, methionine- and B vitamin-enriched diet, “M+B+,” was used to determine the effects of dietary methionine enrichment without hyperhomocysteinemia. Table 1, shows nutrient content per kilogram diet and the number of mice raised on each diet. All diets contained 1% sulfathiozole (10 g/kg diet, Sigma), a nonabsorbed sulfa drug that inhibits folate formation by gut bacteria to ensure that the animal's only source of available folate is from diet. Mice were housed individually, provided with free access to water, and fed by a “group pair-feed protocol” to ensure that all of the mice had similar food intake (52).
Table 1. Methionine, folate, B12, and B6 levels in diets.
| Nutrient content per kg diet
|
|||||
|---|---|---|---|---|---|
| Diet | Mice, n | D/L-Methionine, g | Folate, mg | B12, μg | B6, mg |
| Control (AIN93M) | 10 | 3.3 | 2 | 25 | 7 |
| M+B– | |||||
| Methionine-rich B vitamin-deficient | 12 | 7.7 | 0.11* | 1.8* | 0.15* |
| B– | |||||
| B vitamin-deficient | 13 | 3.3 | 0.11* | 1.8* | 0.15* |
| M+B+ | |||||
| Methionine-rich B vitamin-rich | 12 | 7.7 | 6 | 75 | 21 |
Residual vitamin content assayed in diets. Values represent 5.5, 7.2, and 2.1% of AIN-93M recommended (control) levels of folate, vitamin B12, and vitamin B6, respectively
Blood Biochemistry. Mice were fasted overnight and killed by exsanguination under CO2 anesthesia. Blood was collected by heart puncture into heparinized tubes and kept on ice for <1 h until plasma fractions were separated. Plasma was stored at -70°C until further analysis. Plasma folate was measured by a 96-well plate microbial (Lactobacillus casei) assay (53). Cobalamin (vitamin B12) was measured by a RIA (54). Pyridoxal 5′-phosphate (vitamin B6) was determined by the tyrosine decarboxylase apoenzyme method (55). Plasma total homocysteine and methionine levels were determined by HPLC (56, 57). To achieve adequate volumes for all assays, plasma was pooled by combining equal volumes of plasma from every two mice (on the same diet) into one plasma sample.
Tissue Preparation and Histology. Vascular pathology was evaluated in all 47 mice. After exsanguination, the arterial tree was perfused for 2 min with PBS containing nitroglycerine, followed by perfusion with PBS containing 4% formaldehyde (3 min). The aortic arch, including its main branch points (brachiocephalic trunk, left common carotid artery, and left subclavian artery), was excised and fixed in 1% buffered formaldehyde. The aortic arch including branch points was embedded longitudinally and cut into approximately forty 4-μm sections. Four sections (20 μm apart) of a series of 20 sections, which represented the central area of the arch with an intact morphology of the complete arch and branch points, were analyzed for plaque type and plaque area (hematoxylin/eosin staining).
Atherosclerotic plaques were divided into either initial or advanced lesions. Initial lesions were defined as fatty streaks, containing macrophage-derived foam cells, with intracellular lipid accumulation [American Heart Association (AHA) type II] or with pools of extracellular lipid (AHA type III), whereas advanced lesions contained extracellular lipid, a lipid core (AHA type IV), and/or a fibrous cap (AHA type Va-c) (58). Plaque area was determined by using a microscope coupled to a computerized morphometry system (Quantimet 570, Leica). The evaluator was blind to the experimental treatments.
Statistics. Results were analyzed for differences between the means across dietary treatments by ANOVA with Tukey's honest squares differences post hoc analysis for multiple comparisons.
Results
Weight gain in mice fed the B vitamin-deficient diets with and without supplemental methionine (M+B- and B- diets, respectively) was significantly less during 10 weeks of feeding than in mice fed the control diet. In contrast, mice fed the methionine-rich, high-vitamin M+B+ diet attained the same mean weight as the controls. These differences in growth were observed even though all the mice consumed identical portions of food (mean body weight after 10 weeks on diet in grams ± SD: Control, 22.4 ± 2.6; M+B+, 23.5 ± 1.6; M+B-, 16.1 ± 1.3; B-, 18.6 ± 2.5; P < 0.001 by ANOVA).
Severe hyperhomocysteinemia was induced by B vitamin-deficient diets, together with a concomitant reduction in plasma folate vitamin B6 and vitamin B12 levels (Table 2). The most severe hyperhomocysteinemia resulted from the normal methionine, B vitamin-deficient (B-) diet (plasma total homocysteine = 243.7 ± 82.0 μM). When combined with B vitamin deficiency in the M+B- diet, methionine enrichment attenuated the rise in plasma homocysteine that was achieved by B vitamin deficiency alone; nevertheless, homocysteine remained significantly elevated (plasma total homocysteine = 86.7 ± 25.3 μM). In contrast, the methionine-rich and B vitamin-supplemented M+B+ diet did not increase homocysteine compared with controls (plasma total homocysteine = 5.1 ± 1.0 μM and 4.6 ± 1.4 μM, respectively).
Table 2. Plasma chemistry after 10 weeks on diet.
| Significance
|
||||||
|---|---|---|---|---|---|---|
| Control | M+B+ | M+B– | B– | F | P | |
| Pooled blood samples from total N animals, n | 5 from 10 | 6 from 12 | 6 from 11 | 7 from 13 | ||
| Folate, ng/ml | 59.4 ± 7.8a | 73.0 ± 26.6a | 6.3 ± 1.3b | 10.0 ± 3.2b | 36.2 | <0.001 |
| B12, pmol/l | 23,877a ± 6,177 | 46,662b ± 1,126 | 7,122c ± 1,841 | 7,491c ± 1,078 | 49.8 | <0.001 |
| PLP, pmol/ml | 160.8 ± 55.3a | 191.4 ± 133.9a | 33.1 ± 6.8b | 16.9 ± 10.5b | 9.3 | <0.001 |
| tHcy, nmol/ml | 4.6 ± 1.4a | 5.1 ± 1.0a | 86.7 ± 25.3b | 243.7 ± 82.0b | 37.4 | <0.001 |
| Methionine, nmol/ml | 13.1 ± 1.6 | 10.9 ± 1.9 | 15.4 ± 5.2 | 11.5 ± 3.5 | 2.0 | 0.145 |
Values given are mean ± SD. Values with the same superscript a, b, or c are not significantly different; values indicated by different letters (a, b, and c) are significantly different from each other at P < 0.05 by ANOVA with Tukey's honest squares differences post hoc test. PLP, pyridoxal 5′-phosphate; tHcy, plasma total homocysteine.
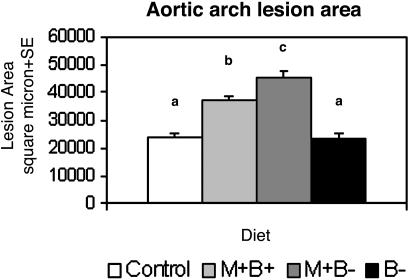
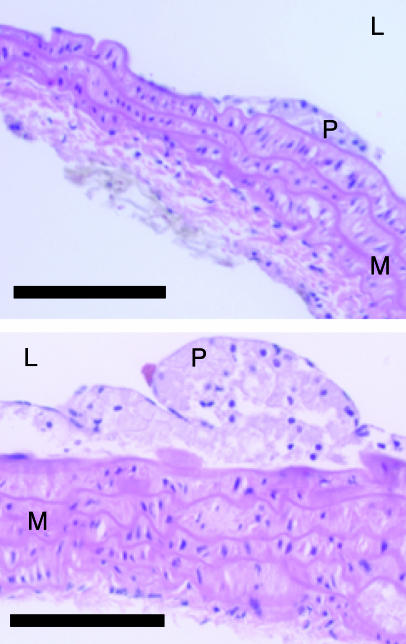
Dietary methionine enrichment significantly increased aortic lesion area beyond the baseline vascular pathology of control mice (Fig. 2). The methionine rich, B vitamin-deficient diet (M+B-) resulted in a nearly 2-fold increase in lesion area compared with controls (lesion area was 45,923 ± 2,804 μm2 vs. 24,557 ± 1,712 μm2, respectively, P < 0.05). B vitamin enrichment (M+B+) only partially mitigated this increase despite completely normalizing homocysteine levels (lesion area was 37,936 ± 1,298 μm2, P < 0.05 vs. controls). In marked contrast, mice with the most severely elevated homocysteine and normal dietary methionine (B- diet) showed no increase in lesion area compared with controls (lesion area was 23,986 ± 1,877 μm2). Fig. 3 illustrates the histological preparations of aortic atheromatous plaques in mice fed the M+B- and B- diets.
Fig. 2.
The effect of the four dietary regimens on the aortic plaque area. Fourteen-week-old ApoE-deficient mice fed control diets develop spontaneous lesions as shown by the white bar. The lesion area increased significantly in mice fed methionine-enriched diets (light and dark gray) in comparison with controls. The highest lesion area was attained in mice that were fed the high-methionine, vitamin-deficient M+B- diet, with a mean lesion area that was nearly twice that seen in the control group (dark gray). The mean aortic lesion area in mice fed the vitamin-deficient normal-methionine B- diet was not significantly different from the mean basal lesion area in controls (white vs. black), despite the fact that the B- group had the highest plasma homocysteine levels (see Table 2). In contrast, the lesion area was significantly higher in mice fed the high-methionine, vitamin-supplemented “M+B+” diet than in controls, despite the fact that homocysteine levels in this group were normal (Table 2). Error bars represent standard errors. a, b, and c are significantly different; P < 0.05.
Fig. 3.
Ateromatous plaques in the aortic arch of ApoE-deficient mice. Hematoxylin/eosin staining of the aortic arch of an ApoE-null mouse fed the B vitamin-deficient diet (B-, Upper) and the methionine-rich and B vitamin-deficient diet (M+B-, Lower). Although both lesions (P) are classified as fatty streak (initial) lesions, lesions in the M+B- group were significantly larger. L, lumen; M, media; P, plaque. (Scale bar = 100 μm.)
Discussion
In the present study, we compared the extent of spontaneous atheromatous lesion progression in genetically susceptible ApoE-deficient mice raised under three conditions: (i) high methionine intake with normal blood homocysteine; (ii) high methionine intake with B vitamin deficiency and hyperhomocysteinemia; and (iii) normal methionine intake with B vitamin deficiency and hyperhomocysteinemia. These conditions enabled us to dissociate the effect of elevated plasma homocysteine on atheromatous vascular pathology from that of excess dietary methionine in this model.
Combined folate, vitamin B12, and vitamin B6 deficiency resulted in severely elevated plasma homocysteine levels in ApoE-deficient mice that were similar to those typically seen in humans with congenital defects of homocysteine and methionine metabolism. The elevated plasma homocysteine, by itself, did not increase the extent of genetically caused vascular pathology as determined in mice fed control diets. However, it augmented the capacity of excess dietary methionine to accelerate the progression of vascular pathology in this model, even though concurrent methionine supplementation during B vitamin deficiency significantly reduced fasting homocysteine levels. The paradoxical ability of high methionine intake to partially lower homocysteine is explained by the stimulation of transsulfuration activity by methionine-derived SAM to remove some homocysteine by the liver and other tissues (14). In contrast, excess methionine accelerated plaque formation independently, even at normal levels of plasma homocysteine.
Our findings agree with two studies that examined the relation of dietary hyperhomocysteinemia to vascular lesions in ApoE-deficient mice (15, 16). Hoffman and colleagues (15) first showed that feeding young ApoE-deficient mice a combined methionine-supplemented (7.7 g/kg diet) and B vitamin-deficient diet for 8 weeks resulted in severe hyperhomocysteinemia accompanied by a doubling of aortic plaque size and increased lesion complexity (15). Zhou and colleagues (16) compared aortic plaque acceleration by supplementing food with methionine (22-44 g/kg diet) or drinking water with homocysteine (0.9-1.8 g/liter). Both methionine and homocysteine supplementation resulted in hyperhomocysteinemia and in lesion acceleration. Dietary methionine enrichment was toxic and resulted in death within 8 months. Although homocysteine was better tolerated, by 12 months, the vascular lesions had progressed to their maximum extent in both supplemented animals and controls such that no additional contribution of homocysteine to the pathology was detected (16).
These studies demonstrate that the dietary manipulation of both methionine and homocysteine metabolism can accelerate the preexisting pathological process in ApoE-deficient mice. However, in contrast to the present study, neither of the previous two studies could exclude the possibility that the excess methionine promoted pathology through processes other than driving homocysteine production, or conversely, that supplementary homocysteine did not serve as an alternative form of dietary methionine.
Whether excess of dietary methionine intake in humans is atherogenic remains to be determined; however, considerable evidence from animal experiments suggests that these findings are not limited to the ApoE-deficient mouse model. A similar experimental dissociation of hyperhomocysteinemia and atherosclerosis has been observed in pigs and primates fed atherogenic diets (21, 59). Ambrosi et al. (21) showed that feeding pigs a methionine-rich diet for 4 months induced hyperhomocysteinemia and atherosclerosis. Folate supplementation of the methionine-rich diet successfully normalized plasma homocysteine levels but did not reduce methionine-induced vascular lesions. Similarly, when Lentz et al. (59) fed cynomolgus monkeys a high-fat and -cholesterol diet for 13-26 months, it induced not only hypercholesterolemia and atherosclerosis, but also hyperhomocysteinemia and B vitamin deficiency. When the same diet was supplemented with folic acid, vitamin B12, and vitamin B6, blood vitamin and homocysteine levels were normalized, but no attenuation of atherosclerosis or vascular dysfunction occurred in supplemented monkeys (59).
It is difficult to reconcile these data with the observation that rare congenital defects in homocysteine metabolism are associated with premature thrombotic and arteriosclerotic disease, whether the defects are in genes of the transsulfuration pathway (which are associated with elevated methionine) or of the methylation pathway (which are associated with low methionine) (20). This observation has often been cited as key evidence supporting the view that vascular disease in hyperhomocysteinemia and particularly atherosclerosis is due to homocysteine per se rather than an effect of its metabolic determinants. Nevertheless, attempts to mimic these human conditions in knockout mice that are defective in transsulfuration or in the remethylation of homocysteine have so far not induced either arteriosclerosis or atherosclerosis in these models despite achieving hyperhomocysteinemia (26, 60). This finding leaves unanswered which aspects of vascular pathology might be causally linked to homocysteine or whether additional factors might be necessary for the putative vasotoxic properties of homocysteine to become apparent (10, 11).
Although the role of homocysteine in atherosclerosis remains to be determined (10, 11), the atherogenic potency of excess methionine intake is a well documented, if often overlooked, phenomenon. Abundant data on dietary amino acid imbalances describe “methionine toxicity” that leads to growth retardation and histopathologic changes in liver, kidney, and spleen, at methionine intake as low as 2% of diet (61). Numerous studies show high methionine intake to produce atherosclerotic changes in mice (15, 16) rats (17, 18, 62-64), rabbits (19, 65), and pigs (21, 22), even if the results have often been attributed to a coincident rise in plasma homocysteine. Further evidence of methionine's vascular toxicity can be derived from studies that examine the vascular effect of dietary protein content and composition. For example, high methionine casein based diets promote vascular pathology in ApoE-deficient mice to a greater extent than low methionine isoflavone-free soy-protein-based diets even within the range of normal dietary methionine intake and similar total protein content (66). Together, these observations demonstrate that methionine toxicity is not limited to the ApoE-deficient mouse model. Additional studies are needed to determine the mechanism that underlies this atherogenic property of excess dietary methionine and whether this mechanism could at least in part explain the epidemiological association between elevated homocysteine levels and cardiovascular disease.
Acknowledgments
We thank Dr. Sheldon Rothenberg, Ms. Marie Nadeau, and Mr. Kenneth Bergami for assistance with methionine, vitamin, and homocysteine measurements, and Dr. Robert Salomon for advice on histopathology. This project was supported by Alzheimer's Association Grant NIRG-02-4119 and by U.S. Department of Agriculture under Agreement 58-1950-4-401. E.L. is a postdoctoral research fellow of the Dr. E. Dekker program (2000T41) of the Dutch Heart Foundation.
Abbreviations: ApoE, apolipoprotein E; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
References
- 1.McCully, K. S. (1969) Am. J. Pathol. 56, 111-128. [PMC free article] [PubMed] [Google Scholar]
- 2.Bautista, L. E., Arenas, I. A., Penuela, A. & Martinez, L. X. (2002) J. Clin. Epidemiol. 55, 882-887. [DOI] [PubMed] [Google Scholar]
- 3.Boushey, C. J., Beresford, S. A., Omenn, G. S. & Motulsky, A. G. (1995) J. Am. Med. Assoc. 274, 1049-1057. [DOI] [PubMed] [Google Scholar]
- 4.Homocysteine Studies Collaboration. (2002) J. Am. Med. Assoc. 288, 2015-2022. [Google Scholar]
- 5.Eikelboom, J. W., Lonn, E., Genest, J., Jr., Hankey, G. & Yusuf, S. (1999) Ann. Intern. Med. 131, 363-375. [DOI] [PubMed] [Google Scholar]
- 6.Klerk, M., Verhoef, P., Clarke, R., Blom, H. J., Kok, F. J. & Schouten, E. G. (2002) J. Am. Med. Assoc. 288, 2023-2031. [DOI] [PubMed] [Google Scholar]
- 7.Wald, D. S., Law, M. & Morris, J. K. (2002) Br. Med. J. 325, 1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meleady, R. & Graham, I. (1999) Nutr. Rev. 57, 299-305. [DOI] [PubMed] [Google Scholar]
- 9.Cleophas, T. J., Hornstra, N., van Hoogstraten, B. & van der Meulen, J. (2000) Am. J. Cardiol. 86, 1005-1009, A8. [DOI] [PubMed] [Google Scholar]
- 10.Brattstrom, L. & Wilcken, D. E. (2000) Am. J. Clin. Nutr. 72, 315-323. [DOI] [PubMed] [Google Scholar]
- 11.Ueland, P. M., Refsum, H., Beresford, S. A. & Vollset, S. E. (2000) Am. J. Clin. Nutr. 72, 324-332. [DOI] [PubMed] [Google Scholar]
- 12.Durand, P., Prost, M., Loreau, N., Lussier-Cacan, S. & Blache, D. (2001) Lab. Invest. 81, 645-672. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein, J. D. & Martin, J. J. (1986) J. Biol. Chem. 261, 1582-1587. [PubMed] [Google Scholar]
- 14.Miller, J. W., Nadeau, M. R., Smith, J., Smith, D. & Selhub, J. (1994) Biochem. J. 298, 415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann, M. A., Lalla, E., Lu, Y., Gleason, M. R., Wolf, B. M., Tanji, N., Ferran, L. J., Jr., Kohl, B., Rao, V., Kisiel, W., et al. (2001) J. Clin. Invest. 107, 675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou, J., Moller, J., Danielsen, C. C., Bentzon, J., Ravn, H. B., Austin, R. C. & Falk, E. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1470-1476. [DOI] [PubMed] [Google Scholar]
- 17.Morita, H., Kurihara, H., Yoshida, S., Saito, Y., Shindo, T., Oh-Hashi, Y., Kurihara, Y., Yazaki, Y. & Nagai, R. (2001) Circulation 103, 133-139. [DOI] [PubMed] [Google Scholar]
- 18.Matthias, D., Becker, C. H., Riezler, R. & Kindling, P. H. (1996) Atherosclerosis 122, 201-216. [DOI] [PubMed] [Google Scholar]
- 19.Toborek, M., Kopiecznagrzebieniak, E., Drozdz, M. & Wieczorek, M. (1995) Atherosclerosis 115, 217-224. [DOI] [PubMed] [Google Scholar]
- 20.McCully, K. S. & Wilson, R. B. (1975) Atherosclerosis 22, 215-227. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosi, P., Rolland, P. H., Bodard, H., Barlatier, A., Charpiot, P., Guisgand, G., Friggi, A., Ghiringhelli, O., Habib, G., Bouvenot, G., et al. (1999) J. Am. Coll. Cardiol. 34, 274-279. [DOI] [PubMed] [Google Scholar]
- 22.Rolland, P. H., Friggi, A., Barlatier, A., Piquet, P., Latrille, V., Faye, M. M., Guillou, J., Charpiot, P., Bodard, H., Ghiringhelli, O., et al. (1995) Circulation 91, 1161-1174. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk, K. W., Hofker, M. H. & Havekes, L. M. (1999) Curr. Atheroscler. Rep. 1, 101-107. [DOI] [PubMed] [Google Scholar]
- 24.Osada, J., Joven, J. & Maeda, N. (2000) Curr. Opin. Lipidol. 11, 25-29. [DOI] [PubMed] [Google Scholar]
- 25.Fazio, S. & Linton, M. F. (2001) Front. Biosci. 6, D515-D525. [DOI] [PubMed] [Google Scholar]
- 26.Chen, Z., Karaplis, A. C., Ackerman, S. L., Pogribny, I. P., Melnyk, S., Lussier-Cacan, S., Chen, M. F., Pai, A., John, S. W., Smith, R. S., et al. (2001) Hum. Mol. Genet. 10, 433-443. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, S. H., Reddick, R. L., Piedrahita, J. A. & Maeda, N. (1992) Science 258, 468-471. [DOI] [PubMed] [Google Scholar]
- 28.Plump, A. S., Smith, J. D., Hayek, T., Aalto-Setala, K., Walsh, A., Verstuyft, J. G., Rubin, E. M. & Breslow, J. L. (1992) Cell 71, 343-353. [DOI] [PubMed] [Google Scholar]
- 29.Reddick, R. L., Zhang, S. H. & Maeda, N. (1994) Arterioscler. Thromb. 14, 141-147. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L. & Ross, R. (1994) Arterioscler. Thromb. 14, 133-140. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld, M. E., Polinsky, P., Virmani, R., Kauser, K., Rubanyi, G. & Schwartz, S. M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2587-2592. [DOI] [PubMed] [Google Scholar]
- 32.Napoli, C., Palinski, W., Di Minno, G. & D'Armiento, F. P. (2000) Nutr. Metab. Cardiovasc. Dis. 10, 209-215. [PubMed] [Google Scholar]
- 33.Neuzil, J., Christison, J. K., Iheanacho, E., Fragonas, J. C., Zammit, V., Hunt, N. H. & Stocker, R. (1998) J. Lipid Res. 39, 354-368. [PubMed] [Google Scholar]
- 34.Tamminen, M., Mottino, G., Qiao, J. H., Breslow, J. L. & Frank, J. S. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 847-853. [DOI] [PubMed] [Google Scholar]
- 35.Letters, J. M., Witting, P. K., Christison, J. K., Eriksson, A. W., Pettersson, K. & Stocker, R. (1999) J. Lipid Res. 40, 1104-1112. [PubMed] [Google Scholar]
- 36.Hayek, T., Oiknine, J., Brook, J. G. & Aviram, M. (1994) Biochem. Biophys. Res. Commun. 201, 1567-1574. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L. & Ross, R. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 842-851. [DOI] [PubMed] [Google Scholar]
- 38.Sukovich, D. A., Kauser, K., Shirley, F. D., DelVecchio, V., Halks-Miller, M. & Rubanyi, G. M. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1498-1505. [DOI] [PubMed] [Google Scholar]
- 39.Zibara, K., Chignier, E., Covacho, C., Poston, R., Canard, G., Hardy, P. & McGregor, J. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2288-2296. [DOI] [PubMed] [Google Scholar]
- 40.Triguero, A., Barber, T., Garcia, C., Puertes, I. R., Sastre, J. & Vina, J. R. (1997) Br. J. Nutr. 78, 823-831. [DOI] [PubMed] [Google Scholar]
- 41.Yang, R., Powell-Braxton, L., Ogaoawara, A. K., Dybdal, N., Bunting, S., Ohneda, O. & Jin, H. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2762-2768. [DOI] [PubMed] [Google Scholar]
- 42.Wang, Y. X., Halks-Miller, M., Vergona, R., Sullivan, M. E., Fitch, R., Mallari, C., Martin-McNulty, B., da Cunha, V., Freay, A., Rubanyi, G. M. & Kauser, K. (2000) Am. J. Physiol. 278, H428-H434. [DOI] [PubMed] [Google Scholar]
- 43.Detmers, P. A., Hernandez, M., Mudgett, J., Hassing, H., Burton, C., Mundt, S., Chun, S., Fletcher, D., Card, D. J., Lisnock, J., et al. (2000) J. Immunol. 165, 3430-3435. [DOI] [PubMed] [Google Scholar]
- 44.Kauser, K., da Cunha, V., Fitch, R., Mallari, C. & Rubanyi, G. M. (2000) Am. J. Physiol. 278, H1679-H1685. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, S. H., Reddick, R. L., Burkey, B. & Maeda, N. (1994) J. Clin. Invest. 94, 937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ree, J. H., van den Broek, W. J., Dahlmans, V. E., Groot, P. H., Vidgeon-Hart, M., Frants, R. R., Wieringa, B., Havekes, L. M. & Hofker, M. H. (1994) Atherosclerosis 111, 25-37. [DOI] [PubMed] [Google Scholar]
- 47.Lee, T. S., Shiao, M. S., Pan, C. C. & Chau, L. Y. (1999) Circulation 99, 1222-1229. [DOI] [PubMed] [Google Scholar]
- 48.Kirk, E. A., Heinecke, J. W. & LeBoeuf, R. C. (2001) J. Clin. Invest. 107, 1545-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calleja, L., Paris, M. A., Paul, A., Vilella, E., Joven, J., Jimenez, A., Beltran, G., Uceda, M., Maeda, N. & Osada, J. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2368-2375. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, S. R., Leichtweis, S. B., Pettersson, K., Croft, K. D., Mori, T. A., Brown, A. J. & Stocker, R. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 585-593. [DOI] [PubMed] [Google Scholar]
- 51.Reeves, P. G., Nielsen, F. H. & Fahey, G. C. (1993) J. Nutr. 123, 1939-1951. [DOI] [PubMed] [Google Scholar]
- 52.Miller, J. W., Ribaya-Mercado, J. D., Russell, R. M., Shepard, D. C., Morrow, F. D., Cochary, E. F., Sadowski, J. A., Gershoff, S. N. & Selhub, J. (1992) Am. J. Clin. Nutr. 55, 1154-1160. [DOI] [PubMed] [Google Scholar]
- 53.Horne, D. W. & Patterson, D. (1988) Clin. Chem. 34, 2357-2359. [PubMed] [Google Scholar]
- 54.Rothenberg, S. P. (1973) Metabolism 22, 1075-1082. [DOI] [PubMed] [Google Scholar]
- 55.Shin, Y. S., Rasshofer, R., Friedrich, B. & Endres, W. (1983) Clin. Chim. Acta 127, 77-85. [DOI] [PubMed] [Google Scholar]
- 56.Araki, A. & Sako, Y. (1987) J. Chromatogr. 422, 43-52. [DOI] [PubMed] [Google Scholar]
- 57.Blankenship, D. T., Krivanek, M. A., Ackermann, B. L. & Cardin, A. D. (1989) Anal. Biochem. 178, 227-232. [DOI] [PubMed] [Google Scholar]
- 58.Stary, H. C., Chandler, A. B., Dinsmore, R. E., Fuster, V., Glagov, S., Insull, W., Jr., Rosenfeld, M. E., Schwartz, C. J., Wagner, W. D. & Wissler, R. W. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 1512-1531. [DOI] [PubMed] [Google Scholar]
- 59.Lentz, S. R., Piegors, D. J., Malinow, M. R. & Heistad, D. D. (2001) Circulation 103, 1006-1011. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe, M., Osada, J., Aratani, Y., Kluckman, K., Reddick, R., Malinow, M. R. & Maeda, N. (1995) Proc. Natl. Acad. Sci. USA 92, 1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harper, A. E., Benevenga, N. J. & Wohlhueter, R. M. (1970) Physiol. Rev. 50, 428-557. [DOI] [PubMed] [Google Scholar]
- 62.Hladovec, J. (1979) Blood Vessels 16, 202-205. [DOI] [PubMed] [Google Scholar]
- 63.Hladovec, J. (1980) Blood Vessels 17, 104-109. [DOI] [PubMed] [Google Scholar]
- 64.Mrhova, O., Hladovec, J. & Urbanova, D. (1988) Cor Vasa 30, 73-79. [PubMed] [Google Scholar]
- 65.Fujimoto, S., Togane, Y., Matsuzaki, C., Yamashina, S., Nakano, H., Yamazaki, J. & Yoshino, G. (2003) Nutr. Metab. Cardiovasc. Dis. 13, 20-27. [DOI] [PubMed] [Google Scholar]
- 66.Ni, W., Tsuda, Y., Sakono, M. & Imaizumi, K. (1998) J. Nutr. 128, 1884-1889. [DOI] [PubMed] [Google Scholar]
- 67.Finkelstein, J. D., Kyle, W. E., Martin, J. L. & Pick, A. M. (1975) Biochem. Biophys. Res. Commun. 66, 81-87. [DOI] [PubMed] [Google Scholar]