Abstract
Endoscopic ultrasound (EUS) is an imaging technique that has consolidated its role as an important tool for diagnosis and therapeutics. In recent years we have seen a dramatic increase in the number of EUS-guided therapeutic indications (celiac plexus neurolysis/block, pseudocyst drainage, etc). Preliminary reports have suggested EUS may also be used to guide vascular access for both imaging and treating different vascular diseases. This review aims to objectively describe the existing evidence in the field.
Keywords: Endosonography, Fine needle aspiration, Endoscopic ultrasound-FNA, Bleeding, Vascular therapy
INTRODUCTION
Although radiology imaging techniques such as computerized tomography (CT) and magnetic resonance imaging (MRI) have dramatically improved their image resolution in recent years and permit detailed anatomic information to be obtained (diagnosis and staging of diseases in a non invasive manner), the endoscopic ultrasound (EUS) technique still has some advantages over them. For example, EUS may allow one to place a biopsy needle into tiny lesions (< 5-10 mm) which are often too small to be identified by these complementary imaging techniques or too well encased by surrounding vascular structures to allow percutaneous biopsy methods to be used[1]. Endoscopic ultrasound guided fine needle aspiration biopsy (EUS FNA) has been classically employed for diagnostic purposes (biopsy of peri-intestinal lymph nodes and masses)[1]. However, in recent years we have seen a dramatic increase in the number of EUS-guided therapeutic indications: celiac plexus neurolysis/block[2], endoscopic drainage of pancreatic pseudocysts[3], common bile duct or pancreatic duct drainage[4], delivery of anti-tumor agents to malignant masses[5] etc. More recently, preliminary reports have suggested EUS may be helpful to guide the vascular access for both imaging and treating different vascular diseases[6]. It remains unclear whether or not this novel therapeutic indication of EUS-FNA is safe and effective. This article attempts to objectively describe the current state of knowledge in the field by presenting the limited data available at present time.
EUS-GUIDED VASCULAR ACCESS AND THERAPY
Justification for this new therapeutic approach
After the initial reluctance from the medical community to this type of intervention, there is a growing interest in this field[7]. It is well known that gastrointestinal bleeding is a frequent event and that hospital admission and endoscopy are usually required in the majority of cases[8]. Endoscopy is effective in identifying the cause of bleeding, permitting the delivery of hemostatic agents and preventing surgery in the majority of cases. However, endoscopic therapy may fail in 15%-20% of cases[9]. Lesions refractory to initial endoscopic therapy may benefit from a second endoscopic treatment or a vascular intervention (angiography, embolization with coils or micro-particles, cyanoacrylate/glue sealing, transjugular intrahepatic portosystemic shunt or TIPS)[7]. Vascular interventions are typically performed by interventional radiotherapists under X-ray guidance. However, this type of interventions may also be conducted under EUS guidance as suggested by some preliminary reports[7]. Another potential use for EUS in this setting is to employ Doppler US (available with the linear echoendoscopes and US miniprobes) to identify culpable vessels of bleeding, to help direct therapy into these bleeding vessels (arteries or varices) and to monitor the efficacy of the endoscopic therapy delivered[10-12]. Although initial reports have shown some promise, there is a paucity of data regarding this issue and its clinical impact and safety have yet to be proved.
Instruments required for EUS-guided interventions
Although EUS-FNA has also been described using the radial echoendoscope (elevated risk of serious complications), EUS-FNA should be performed with the electronic curved linear array echoendoscope which permits real-time visualization of the needle as it is advanced into the target area for biopsy or injection[5]. The linear array echoendoscope allows one to image the target area providing high resolution images on a grey scale and to use the pulse and color Doppler for vascular exam. Specifically designed EUS needles are required for vessel puncture and therapy. These needles are available in different diameters (25, 22 and 19 Gauge) and may be visualized from its exit from the biopsy channel. EUS needles are shown on the ultrasound image as a bright/white line. This type of needle is provided with a central stylet that upon removal permits the suction and injection of substances. Another important point is that the EUS-FNA/injection technique entirely relies on the ultrasound visualization (although X-ray aid may be required for certain therapeutic indications). Although it is well known that EUS-FNA is safe (< 1% rate of complications, usually mild inflammation or self-limiting hemorrhage and fever), little is known regarding safety of EUS-guided vascular interventions[1].
Doppler US
There are limited data regarding usefulness of Doppler US for the management of gastrointestinal bleeding[10-14]. Doppler US is readily available with the linear echoendoscopes (good to deliver substances but difficult to use in an acute bleeding patient and therefore difficult to apply in clinical practice) and some dedicated through-the-scope miniprobes (do not allow the delivery of therapy but permit monitoring treatment efficacy and are more likely to be available in clinical practice).
Doppler US monitored therapy has been reported to be successful for recurrent bleeding from peptic ulcers or Dieulafoy's lesions[10-14]. Doppler US may allow one to directly target the bleeding vessel, deliver therapy in a more effective manner and to monitor if blood flow has disappeared after therapy. This Doppler US monitored therapy has been suggested to be more accurate than endoscopic stigmata to predict patient risk of rebleeding after successful endoscopic therapy[7]. The absence of a Doppler US signal after therapy has been associated with a low risk of rebleeding (regardless of endoscopic stigmata)[15]. On the other hand, the presence of a Doppler US signal post-therapy has been associated with an elevated risk of bleeding even in those ulcers that have no visible vessel or clot on endoscopy image[15].
EUS with Doppler US may delineate the anatomy and identify the presence of gastric varices or Dieulafoy lesions to help direct therapy[10-14]. Several case reports and small uncontrolled case series have suggested the potential usefulness of Doppler US for this indication[10-16]. It has been reported that injection of absolute alcohol (under EUS Doppler US control) is feasible and effective for treating refractory Dieulafoy lesions[14]. Furthermore, EUS (without Doppler) may help identify the feeding vessels in these patients and monitor therapy effectiveness with promising results[16]. Unfortunately, the limited number of patients evaluated in the largest series to date (8 patients) limits its credibility and explains its limited impact in clinical practice at the present time.
EUS-FNA of vessels for therapeutic interventions
As we may visualize and target lymph nodes and tumors under EUS guidance[1], it is conceivable that we may also identify and puncture vascular structures in the gastrointestinal tract and surrounding structures (heart, liver etc).
Although experience on this is limited, EUS-FNA guided treatment of esophageal-gastric varices appears to be relatively well known. There are at least two prospective and controlled studies demonstrating its safety and effectiveness. A prospective study of 54 patients with gastric varices demonstrated that EUS-guided cyanoacrylate injection permits one to achieve a complete obliteration of varices[17]. Another prospective, randomized comparison of 50 consecutive patients with bleeding esophageal varices suggested that EUS-guided sclerosis of perforating veins is more effective than conventional endoscopic sclerosis of esophageal varices[18]. EUS-guided injection of perforating veins with cyanoacrylate has also been reported for gastric varices with promising results in terms of safety and effectiveness[19].
The portal venous system may be difficult to access by standard angiographic methods employed by interventional radiologists. Preliminary studies conducted in an animal model suggest that EUS may permit portal vein access, contrast injection and monitoring the portal vein pressure which may be of interest in patients with portal hypertension in order to assess the risk of bleeding and treatment response[20-23]. Similarly, EUS-guided angiography of celiac trunk and hepatic/splenic veins has been reported in the swine model[24]. Unfortunately, the experience with these exciting indications for EUS-guided therapy is limited and restricted to animal models. Questions regarding safety (infections, risk for uncontrolled and non-treatable bleeding) and clinical effectiveness are yet to be answered before it can be applied in humans.
Preliminary experience in humans has been gained in recent years[25]. Patients presenting with refractory GI bleeding from hemosuccus pancreaticus, Dieulafoy lesions or gastrointestinal stromal tumor have been treated under EUS guidance[25]. These patients presented at least 3 bleeding episodes from the aforementioned conditions, required 14-25 units of packed red blood cells and repeated endoscopic and vascular therapies were ineffective. These difficult and refractory patients were treated by EUS-guided injection of absolute alcohol (99%) and/or cyanoacrylate into the bleeding vessel (one of them was a 30 × 50 mm aneurismatic branch of the superior mesenteric artery responsible for feeding the pancreas). The effectiveness of the EUS-guided angiotherapy in these cases was real-time monitored by Doppler US, concluding the injection therapy when no visible flow could be seen in the bleeding vessel. Although limited to 5 selected patients, EUS-guided injection was able to control the bleeding source in all of these refractory cases and no complications were registered. We believe that it is important to remark that although EUS may allow one to effectively deliver cyanoacrylate into esophageal and gastric varices in bleeding circumstances, we have to be aware of two things: (1) Blood may interfere with US imaging and therefore may preclude EUS-guided therapy; and (2) Cyanoacrylate may damage the expensive echoendoscopes.
In the same line of EUS-guided therapy of bleeding lesions, some authors have suggested that EUS may allow one to deliver microcoils to help control certain refractory bleeding episodes[7,26]. Levy et al[26] reported the case of a 50 year old woman who underwent a total pancreatectomy and presented with severe bleeding after ERCP dilatation of surgical anastomosis. Bleeding was considered not amenable for angiographic therapy or surgery despite the fact that the patient required a total of 10 units of red blood cells[26]. EUS exam identified that the bleeding source was a cluster of choledochojejunal varices at the level of the anastomosis. The bleeding point was needled under EUS-guidance (22 Gauge needle) and a microcoil (18S-8/4 Embolization Microcoil; Cook Inc, Bloomington, Ind) was advanced into the bleeding varices by pushing the needle stylet. The effectiveness of the EUS-guided microcoil embolization was monitored by Doppler US and demonstrated a complete cessation of blood flow 10 min after therapy. A total of 5 choledochojejunal varices were embolized in 2 sessions. No acute or delayed complications were registered. Other groups have also reported the use of EUS-guided microcoil embolization for treating large gastric varices. Romero et al[27] reported their preliminary experience in the field with promising results. For large gastric varices it may be required to place more than 1 coil per varix or to combine coils with cyanoacrylate injection to achieve a complete cessation of flow in the varix[27].
The preliminary experience of our group in this field of EUS-guided microcoil embolization of vessels is also promising and provocative[28]. We have conducted 3 animal experiments with the objective of creating an atrophy of the right hepatic lobe (10 d after EUS-guided selective embolization of the right branch of the portal vein) and a compensatory hypertrophy of the left hepatic lobe. This type of therapy has been classically performed by interventional radiologists in patients who require a right hepatic lobe resection for cure (e.g. large or multiple metastasis in the right hepatic lobe in a colon cancer patient). In these cases, it may be required to increase the residual hepatic mass after surgery (right hepatectomy) and the hypertrophy original of the left hepatic lobe after embolization may be a good alternative. Preliminary results in an animal model suggest EUS-guided embolization with coils of the right portal vein is safe, feasible and produces the intended hypertophy of the left hepatic lobe (Figures 1 and 2). A similar approach was also followed by Matthes et al[22] who reported the injection of a polymer (Enteryx) into the main portal vein resulting in thrombosis of the vessel. These anecdotal reports in animal models give the idea that different compounds may be delivered under EUS guidance causing occlusion of small and large caliber vessels. The potential applications in clinical practice are yet to be demonstrated.
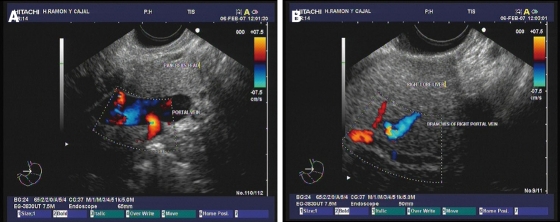
Figure 1.
Doppler ultrasound images. A: The main portal vein show adequate blood flow; B: Vein flow is also evidenced by interrogation of the branches of the right portal vein at the intrahepatic level. Images courtesy of Grupo Español de Protocolos en Endoscopia Digestiva.
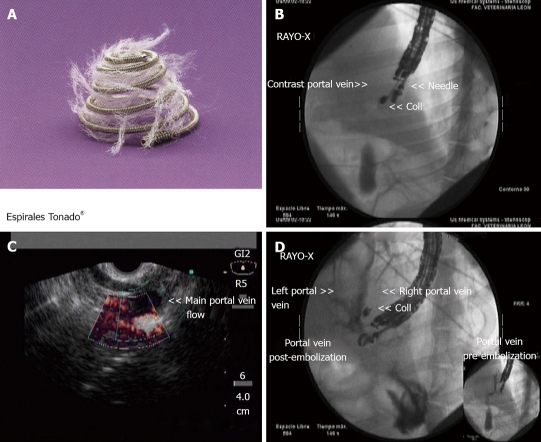
Figure 2.
Endoscopic ultrasound-guided embolization with coils of the right portal vein. A: Image of the microcoil used in the experiment that is amenable for EUS-guided delivery[28]; B: The distal end of the echoendoscope is shown, showing the ultrasound transducer, the needle and the coil being deployed; C: EUS image of the right portal vein showing a grey coloured defect within the vessel that represents the coil after deployment; D: After coil deployment, X-ray fluoroscopy showed no filling of the right portal vein territory, with adequate contrast filling of the left portal vein. Images courtesy of Grupo Español de Protocolos en Endoscopia Digestiva.
In a recent publication, the John Hopkins group reported the first intrahepatic portosystemic shunt (IPSS) performed under EUS-guidance[29]. IPSS may represent an alternative to tranjugular intrahepatic portosystemic shunt (TIPSS) for patients with liver cirrhosis and refractory or ascites. The study was conducted in an animal model (10 animals; 2 of them survived for 2 wk) by using the EUS linear-array and a 19-Gauge needle. The hepatic vein (HV) and then the portal vein (PV) were punctured under EUS guidance, a 0.035-inch guide wire was advanced through the needle into the PV lumen and then the needle was exchanged over the wire and a metal stent was deployed under EUS and fluoroscopic guidance. The distal end of the stent was positioned inside the PV and the proximal end within the HV. EUS-guided portosystemic shunt (IPSS) placement was successful in all animals. Necropsy performed after acute and survival experiments revealed no evidence of bleeding or damage to any intraperitoneal organs. There were no complications during the follow-up period in the 2 animals that were kept alive. Authors concluded that EUS-guided IPSS creation is technically feasible and may become an alternative to the currently used method of TIPSS placement.
CONCLUSION
EUS-guided vascular therapy is a new field that shows some promise for EUS. Preliminary data, most of them in anecdotal case reports or animal models, suggest EUS guided angiotherapy may be feasible, safe and effective. However, data available are still limited and multicenter, prospective controlled studies have to be conducted before we can firmly recommend this provocative indication for therapeutic EUS. Further research in the field is warranted.
Footnotes
Peer reviewers: Carlos Robles-Medranda, MD, Head of the Endoscopy Division, Ecuadorian Institute of Digestive Disease (IECED), San Antonio Clinic, Av. Reales Tamarindo y Tennis Club, Portoviejo, Manabi-Ecuador, Casilla 13-01-266, Ecuador; Naoki Muguruma, MD, PhD, Department of Gastroenterology and Oncology, The University of Tokushima Graduate School, 3-18-15, Kuramoto-cho, Tokushima 770-8503, Japan
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 2.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 3.Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200–203. doi: 10.1016/s0016-5107(98)70165-8. [DOI] [PubMed] [Google Scholar]
- 4.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–230. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Chang KJ, Irisawa A. EUS 2008 Working Group document: evaluation of EUS-guided injection therapy for tumors. Gastrointest Endosc. 2009;69:S54–S58. doi: 10.1016/j.gie.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Saltzman JR. EUS-guided angiography: a future indication for EUS? Gastrointest Endosc. 2007;66:592–595. doi: 10.1016/j.gie.2007.03.1057. [DOI] [PubMed] [Google Scholar]
- 7.Levy MJ, Chak A. EUS 2008 Working Group document: evaluation of EUS-guided vascular therapy. Gastrointest Endosc. 2009;69:S37–S42. doi: 10.1016/j.gie.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population based study (see comment) Am J Gastroenterol. 1997;92:419–424. [PubMed] [Google Scholar]
- 9.Laine L. Endoscopic therapy for bleeding ulcers: room for improvement? Gastrointest Endosc. 2003;57:557–560. doi: 10.1067/mge.2003.163. [DOI] [PubMed] [Google Scholar]
- 10.Wong RC, Farooq FT, Chak A. Endoscopic Doppler US probe for the diagnosis of gastric varices (with videos) Gastrointest Endosc. 2007;65:491–496. doi: 10.1016/j.gie.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Wong RC. Endoscopic Doppler US probe for acute peptic ulcer hemorrhage. Gastrointest Endosc. 2004;60:804–812. doi: 10.1016/s0016-5107(04)02046-2. [DOI] [PubMed] [Google Scholar]
- 12.Kuramochi A, Imazu H, Kakutani H, Uchiyama Y, Hino S, Urashima M. Color Doppler endoscopic ultrasonography in identifying groups at a high-risk of recurrence of esophageal varices after endoscopic treatment. J Gastroenterol. 2007;42:219–224. doi: 10.1007/s00535-006-1992-x. [DOI] [PubMed] [Google Scholar]
- 13.van Leerdam ME, Rauws EA, Geraedts AA, Tijssen JG, Tytgat GN. The role of endoscopic Doppler US in patients with peptic ulcer bleeding. Gastrointest Endosc. 2003;58:677–684. doi: 10.1016/s0016-5107(03)02033-9. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro A, Vazquez-Sequeiros E, Wiersema MJ. Doppler EUS-guided treatment of gastric Dieulafoy’s lesion. Gastrointest Endosc. 2001;53:807–809. doi: 10.1067/mge.2001.113913. [DOI] [PubMed] [Google Scholar]
- 15.Kohler B, Riemann JF. Endoscopic injection therapy of Forrest II and III gastroduodenal ulcers guided by endoscopic Doppler ultrasound. Endoscopy. 1993;25:219–223. doi: 10.1055/s-2007-1010296. [DOI] [PubMed] [Google Scholar]
- 16.Fockens P, Meenan J, van Dullemen HM, Bolwerk CJ, Tytgat GN. Dieulafoy’s disease: endosonographic detection and endosonography-guided treatment. Gastrointest Endosc. 1996;44:437–442. doi: 10.1016/s0016-5107(96)70096-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168–174. doi: 10.1067/mge.2000.107911. [DOI] [PubMed] [Google Scholar]
- 18.de Paulo GA, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophageal varices: a randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006;63:396–402; quiz 463. doi: 10.1016/j.gie.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Castro R, Pellicer-Bautista F, Ortiz-Moyano C, Marcos-Sanchez F, Jimenez-Saenz M, Gomez-Parra M, Leria-Yebenes V, Herrerias-Gutierrez JM. EUS-Guided injection of cyanoacrylate and lipiodol at the feeding veins in gastric varices. Results in four cases. Gastrointest Endosc. 2006;63:AB280. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Caletti G, Brocchi E, Baraldini M, Ferrari A, Gibilaro M, Barbara L. Assessment of portal hypertension by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S21–S27. doi: 10.1016/s0016-5107(90)71011-5. [DOI] [PubMed] [Google Scholar]
- 21.Lai L, Poneros J, Santilli J, Brugge W. EUS-guided portal vein catheterization and pressure measurement in an animal model: a pilot study of feasibility. Gastrointest Endosc. 2004;59:280–283. doi: 10.1016/s0016-5107(03)02544-6. [DOI] [PubMed] [Google Scholar]
- 22.Matthes K, Sahani D, Holalkere NS, Mino-Kenudson M, Brugge WR. Feasibility of endoscopic ultrasound-guided portal vein embolization with Enteryx. Acta Gastroenterol Belg. 2005;68:412–415. [PubMed] [Google Scholar]
- 23.Giday SA, Clarke JO, Buscaglia JM, Shin EJ, Ko CW, Magno P, Kantsevoy SV. EUS-guided portal vein catheterization: a promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008;67:338–342. doi: 10.1016/j.gie.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Magno P, Ko CW, Buscaglia JM, Giday SA, Jagannath SB, Clarke JO, Shin EJ, Kantsevoy SV. EUS-guided angiography: a novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587–591. doi: 10.1016/j.gie.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Levy MJ, Wong Kee Song LM, Farnell MB, Misra S, Sarr MG, Gostout CJ. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;67:572–574. doi: 10.1111/j.1572-0241.2007.01616.x. [DOI] [PubMed] [Google Scholar]
- 26.Levy MJ, Wong Kee Song LM, Kendrick ML, Misra S, Gostout CJ. EUS-guided coil embolization for refractory ectopic variceal bleeding (with videos) Gastrointest Endosc. 2008;67:572–574. doi: 10.1016/j.gie.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, Gomez-Parra M, Herrerias-Gutierrez JM. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007;66:402–407. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez-Sequeiros E, Perez-Miranda M, Juzgado D, Perez-Martinez C, Dolz C, Igea F, Gonzalez-Huix F, Espinel J, Altonaga JR. Embolizacion selectiva de la rama derecha de la vena porta mediante liberacion de coils controlada por ecoendoscopia como tratamiento previo a hepatectomia del lobulo hepatico derecho en un modelo animal. Personal communication. Abstracts Book National Congress of the Spanish Society of Digestive Endoscopy. 2007. [Google Scholar]
- 29.Buscaglia JM, Dray X, Shin EJ, Magno P, Chmura KM, Surti VC, Dillon TE, Ducharme RW, Donatelli G, Thuluvath PJ, et al. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video) Gastrointest Endosc. 2009;69:941–947. doi: 10.1016/j.gie.2008.09.051. [DOI] [PubMed] [Google Scholar]