Abstract
Despite recent advances in medicine, colorectal cancer (CRC) remains one of the greatest hazards for public health worldwide and especially the industrialized world. It has been well documented with concrete data that regular screening colonoscopy aimed at early detection of precancerous polyps can help decrease the incidence of CRC. However, the adherence of the general population to such screening programs has been shown to be lower than that expected, thus allowing CRC to remain a major threat for public health. Various reasons have been suggested to explain the disappointing compliance of the population to CRC screening programs, some of them associated with colonoscopy per se, which is viewed by many people as an unpleasant examination. Governments, medical societies, individual gastroenterologists, as well as the medical industry are working in order to improve endoscopic devices and/or to improve standard colonoscopy. The aim is to improve the acceptance of the population for this method of CRC screening, by providing a painless and reliable examination of the colon. This review focuses on some of the latest improvements in this field.
Keywords: New colonoscopes, Colonoscopy, Colorectal cancer screening, Technology
INTRODUCTION
Despite recent developments in medical research including attempts to use blood, stool samples or imaging techniques (e.g. CT technology) to detect early cancer, colonoscopy remains the examination of choice in colorectal cancer (CRC) prevention. Regular screening colonoscopy aimed at early detection of precancerous polyps seems to reduce the incidence of CRC[1]. Although most studies that have proved the benefits of regular colorectal screening were based only on flexible rectosigmoidoscopy, they show a 60% reduction of CRC-associated deaths, provided screening was done before development of symptoms[2,3]. However, despite the proven benefits of endoscopic colon screening, patients worldwide seem to be unwilling to adhere to screening programs, demonstrating an unacceptably low compliance rate. Studies including first-degree relatives of patients with colorectal cancer showed a compliance rate that ranged from 50% to 80%[4,5]. When examining the general asymptomatic population, compliance rates are even lower. Lack of symptoms, fear of detecting a tumour, embarrassment and discomfort that many patients believe accompany the procedure, difficulty in bowel preparation and even lack of knowledge or awareness of the benefits of regular colorectal screening are some of the main reasons that seem to prevent patients to adhere to screening programs[6]. Introduction of better sedation (including use of propofol) during colonoscopy seems to somewhat improve patient acceptance of colonoscopy[7]. However, the goal of an “easy” examination of the large bowel, that is widely accepted from the public still remains unfulfilled.
Apart from patient compliance, a good-quality of colonoscopy is necessary to provide all the benefits of endoscopic screening. In various studies, conventional colonoscopy seems to have a 5%-6% polyp miss rate for polyps greater than 1 cm, 13%-15% for polyps 5-9 mm and up to 25% for polyps smaller than 5 mm. Factors that influence the quality of colonoscopy in terms of polyp detection are withdrawal time, adequacy of bowel preparation and thorough inspection behind every intestinal fold[8,9]. Moreover, colonoscopy is a procedure that requires endoscopists with sufficient training, technical skills and experience. This, however, is not the case in every hospital, where colonoscopies might be performed by endoscopists of lesser experience. This was displayed in a recent British prospective study, that reviewed data from 9223 colonoscopies performed in 68 centers over a 4 mo period. Here the cecum intubation rates were rather low (76.9%, with an even worse adjusted rate of 56.9%) and definitely far from the expected 90%-94%[10]. The association between screening colonoscopy and reduction in CRC mortality rates seems to be due to reduction in left colon cancer deaths[11]. In two case-control studies, the relative risk of left-sided colon tumours after a negative colonoscopy was less than 0.2, while for right-sided colon tumours the relative risk ranged from 0.4 to 0.67[12,13]. These data point out that even regular colonoscopic screening has limitations in detecting all suspicious lesions. In order to overcome these problems and limitations, technical improvements to conventional colonoscopes and new devices are being developed, which aim to achieve colonoscopies of higher quality and thus to possibly increase the adherence of the public to CRC prevention programs. Initial data[7] seems to support the authors’ view that improvement of the quality of colonoscopies in terms of accuracy and adenoma detection rates, might also contribute to increasing population adherence to CRC prevention programs. This may be either by “convincing” primary health care physicians to refer more patients to these programs or by influencing the public directly with high adenoma detection rates and low percentages of missed polyps, i.e. by showing that colonoscopy is the “gold standard” in colorectal screening, by far superior to alternative methods (e.g. CT- or MRI-colography). Therefore, in the following paragraphs we will focus not only on new endoscopic devices, but also briefly highlight technical innovations which bring improvements in visualization with standard endoscopes, as they might also prove to have a positive impact on the public’s compliance with colorectal screening.
NEW TECHNOLOGY COLONOSCOPES
Aer-O-scope
This is a self-propelled, self-navigating, disposable endoscope. It consists of the following parts: (1) an electro-optical imaging capsule, containing a digital camera, which is implanted inside a balloon, called “scanning balloon”; (2) a workstation which helps the endoscopist inspect and control capsule movement during the examination; and (3) a supply cable that connects the workstation to the electro-optical capsule. This cable contains multiple channels and provides current, water and suction that are necessary during the examination. The examination begins by placing a silicone balloon (through a rectal introducer) into the patient’s rectum. This rectal balloon is then inflated and seals the anus. Immediately after that, the scanning balloon is also inflated and CO2 is introduced between the rectal and the scanning balloon. The pressures inside and behind the scanning balloon are controlled through electronic sensors and adjusted by the workstation computer. The pressure gradient that is created in this way can propel the scanning balloon inside the intestinal lumen. During its movement the scanning balloon adjusts its volume and shape according to the shape of the intestine, preventing patient discomfort. When the scanning balloon reaches the cecum, CO2 behind the balloon is allowed to leave the colon through the rectal inductor, while new CO2 is introduced, but this time between the scanning ballon and the cecum. This creates a pressure gradient in the opposite direction, which allows the endoscope to travel backward and simultaneously distends the colon in front of the camera. The inspection of the colonic mucosa is conducted during the endoscope’s withdrawal, which is controlled by the endoscopist through the workstation’s computer. Aer-O-scope has an omnidirectional imaging system, based on conical lenses and a mirror that provides simultaneous circumferential, backward as well as forward views, allowing inspection even behind mucosal folds. Ex vivo as well as in vivo porcine studies have shown that the Aer-O-scope reaches its maximal cable length (which is equivalent to that of the cecum in humans) in 80%-90% of the cases and has 98% sensitivity for detecting beads (i.e. markers that imitate polyps) greater than 2.5 mm. In another study performed in 12 healthy young volunteers who underwent both conventional colonoscopy and Aer-O-scope endoscopy, cecal intubation was achieved in 83% of the cases with both methods. This new colonoscopy device promises pain reduction during the examination, since Aer-O-scope does not create loops and the pressure inside the colon is kept at lower levels than those of a conventional colonoscopy. Further human studies are, however, needed in order to prove the advantages of this endoscope, keeping in mind that the device is not currently endowed with a working channel[14-16].
Neoguide endoscopy system
Neoguide endoscopy system (NES) is an articulated endoscope comprising 16 segments of the same length. Each segment has the ability to bend in every direction. NES is handled as a conventional endoscope and is equipped with an external position sensor which measures the endoscope’s insertion depth. The main computer combines data from the orientation of the tip of the endoscope and the external position sensor, regulating the shape of its segment to assume the shape of the colon, as it advances through the lumen. This endoscope adjustment to the shape of the colon leads to reduction of loop formation, which together with colon ligament stretching is the source of 90% of episodes of pain during colonoscopy. Eickhoff et al studied the forces that are exerted on the colonic wall and the displacement of the colon during conventional and Neoguide endoscopy, using model colons. NES was found to apply significantly less forces on the colonic wall and caused significantly less colon displacement and loop formation, whilst offering 3-dimentional, real-time imaging of the bowel[17]. In another human trial by the aforementioned team, loops were formed during NES endoscopy in only 4/10 patients and were successfully straightened with the help of the 3D imaging system[18]. However, despite satisfactory results displayed when using the NES-system (verified by both patients and physicians), large scale studies are needed to compare conventional colonoscopy with NES in terms of efficacy and safety.
Invendo colonoscope
The Invendo colonoscope is a single-use, motor-driven colonoscope, where all the push and pull manoeuvres of the endoscopist are replaced by a handheld device (Invendo Medical, Ltd., Kissing, Germany). It is a flexible colonoscope with a working length of 200 cm, endowed with an inner sheath (with a 10mm diameter). An outer sleeve is pulled over this inner sheath and inverted on each of the respective ends (at the biopsy port and just below the endoscope deflection) and attached to a propulsion connector. The connector is then locked into an endoscope-driving unit and the examination can then be started. Under handheld control by the physician, 8 drive-wheels in the endoscope-driving unit start to move in the selected direction. The wheels grip the inner side of the inverted sleeve, causing the inverted sleeve and inner sheath to move either forward or backwards The endoscope tip can be deflected electro-hydraulically 180° (at body temperature) in any direction by moving a joystick on the handheld device. The colonoscope has a working channel of 3.2 mm (therefore allowing use of a biopsy forceps through the channel). In the first pilot volunteer study on 34 patients the examination was performed in all cases without sedation and had to be interrupted in only 2 patients due to pain. The rest of the patients did not mention any significant discomfort during the examination. The cecal intubation rate was 82%, whereas the mean cecal intubation time was 20 min. Only 4/34 patients complained of abdominal bloating after the procedure[19].
Cathcam
Cathcam is a wire-guided, catheter-based method. It consists of a light, 160 cm long catheter (almost half the weight of the shaft of a colonoscope), which is guided by a looped guide-wire. It is also equipped with 6 light-emitting diodes, a 2.8 mm working channel, lens irrigation and air inflation systems. The hinged guide-wire passes through the 2.8 mm working channel of the catheter. A reusable micro-camera is then fitted on the tip of the catheter. A study conducted in live pigs showed 30% to 40% reduction in the peak force exerted on the colonic wall using Cathcam. A pilot safety and efficacy study included 13 volunteers who had failed to complete a conventional colonoscopy. For the first 5 of these patients, colonoscopy could be completed exclusively by Cathcam. However, the prototype Cathcam’s tip could not be angulated and it was found difficult as well as time-consuming to manoeuvre the wire and the catheter through the left colon. For the rest of the patients conventional colonoscopy was performed up to the point where no further advancement of the scope was possible; at this point, the looped guide-wire was then inserted and advanced into the colon. The conventional colonoscope was then removed (leaving the guide-wire in place) and the Cathcam was advanced over the guide-wire, resulting in a rapid completion of the rest of the procedure. Twelve out of a total 13 patients thus completed the Cathcam colonoscopy. The patients were mildly sedated and only 2 complained of pain, while 8/13 mentioned pain in their previous conventional colonoscopy[20,21]. It seems that Cathcam has the potential to become an important tool for completion of difficult colonoscopies although some further modifications in its design will be necessary in order for Cathcam to become optimal for that purpose.
Pill cam colon
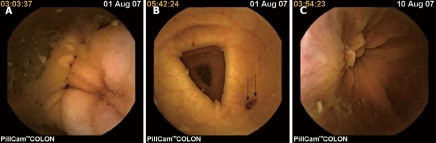
Pill Cam Colon capsule shares the same basic technology with the widely-used small bowel capsule (Pillcam SB). It is an endoscopic capsule of 31 mm length and a diameter of 11 mm. Each end of the capsule is enhanced with a microcamera which acquires images at a frame rate of 4 frames per second (2 images per camera). Its total operating time reaches approximately 10 h. Each camera contains an automatic lighting control and improved optics that provide a broad observation field (twice the coverage area and depth of field compared to those of Pillcam SB) (Figure 1). The colon capsule initially transmits images for only 5 min after its activation and then steps into a “sleeping” (also known as “hibernating”) mode in order to save energy as it travels through the small intestine. Two hours later it is automatically reactivated and starts transmitting images again. By this time the capsule has normally reached the terminal ileum in most patients. The rest of the system (sensors, data recorder and software) are similar to the small bowel capsule. Initial data that were published on the first-generation colon capsule, reported sensitivities in detecting polyps > 6 mm (compared to conventional colonoscopy which was used as gold standard) ranging between 50%-70%, whereas specificities were between 73%-100%[22,23]. A large recent prospective study, which included 328 patients with suspected or known colonic disease, compared colon capsule endoscopy with colonoscopy in detecting lesions of the large intestine. Sensitivity of the colon capsule for diagnosing polyps with a diameter of 6 mm or larger was 64%, whereas its specificity was 84%. In detection of advanced adenomas with a diameter of 10 mm or larger sensitivity and specificity were 64% and 98% respectively[24]. It was also clearly demonstrated in the same study that the sensitivity of the colon capsule depends on good bowel preparation, meaning the presence of clear fluid in the colon, which allows detailed inspection of the mucosa and quick movement of the capsule. This was clearly illustrated in the same study, by the fact that on patients with a good or excellent bowel preparation the sensitivity of the method in detecting advanced adenomas rose significantly up to 88% when compared to that of patients with a fair or poor preparation (here, the corresponding value reached a mere 44%)[24]. Moreover, a previous study had shown that intensive (each capsule examination was read 3 times) and trained capsule data reading improved the sensitivity and specificity from 50% and 83% respectively from the first reading to 70% and 100% after the third (i.e. “trained”) reading[22]. It therefore seems that although the sensitivity of the colon capsule in colorectal screening is lower than that of standard colonoscopy, it can be increased by improving bowel preparation combined with careful reading of the colon capsule examination data. Moreover, an improved, second-generation colon capsule has already been developed. It was recently tested in a feasibility study across 5 centers, involving 104 patients (data from 98 were finally analyzed). Here, sensitivity for detection of polyps ≥ 6 mm was 89%, whereas for polyps ≥ 10 mm it was 88% (specificities were 76% and 89% respectively)[25]. These results suggest a potential for improved accuracy compared with the first-generation colon capsule system and seem to verify the high expectations of those who believe that the colon capsule can indeed be an alternative to conventional colonoscopy in screening for CRC. However, more prospective and comparative studies still need to be performed on this issue.
Figure 1.
Pill cam colon capsule. A: a normal colon (cecum and ileocecal valve); B: A small colonic diverticulum in the colon transversum; C: The rectum with internal haemorrhoids (equivalent to a retroflex view with the standard colonoscope).
ECHNICAL IMPROVEMENTS AND DEVELOPMENT OF COLONOSCOPES
Variable stiffness colonoscopes
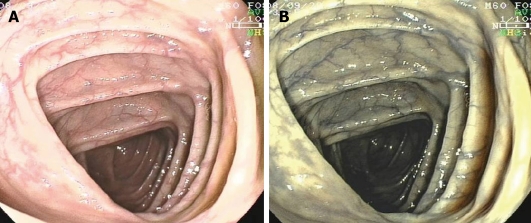
During the last decade colonoscopes with variable stiffness (VSC) have been used in a number of trials. VSCs possess a control ring that adjusts stiffness according to examination conditions (Figure 2). Decreased stiffness gives the endoscope the flexibility needed to traverse sharp angles or a fixed sigmoid colon, while increased stiffness provides adequate rigidity to overcome a loop formation and to straighten the colon. Several studies have been performed, providing conflicting results about the cecal intubation rate, the cecal intubation time, the use of ancillary maneuvers and the need for sedation during colonoscopy with the use of VSCs[26-30]. A recent meta-analysis of randomized controlled trials that compared the pediatric or adult VSCs with standard adult colonoscopes (SAC) in adult patients, showed that VSC-colonoscopy increased cecal intubation rate, required less sedation (whether meperidine or midazolam) and caused less abdominal pain. However, there was no difference in use of ancillary maneuvers and in the time needed for cecal intubation[26]. It is clear that further trials are needed in order to evaluate VSC during procedures without sedation, in patients who previously failed to complete colonoscopy and among inexperienced endoscopists, both being situations where a more efficacious colonoscope is needed. A recent study compared cecal intubation time and patient discomfort using 3 different types of colonoscopes: the pediatric VSC, the non-magnifying adult VSC and the magnifying adult VSC in unsedated patients. Pediatric VCS (in spite of their smaller caliber) did not seem to reduce patient discomfort, but on the other hand had a longer intubation time (possibly due to their decreased rigidity deriving from their smaller diameter, which might make them more floppy). However, pediatric VCS remain important tools in examining a narrowed and fixed colon, especially in diverticular disease and intestinal adhesions[31].
Figure 2.
Variable stiffness colonoscope with the control ring to adjust stiffness (arrow).
Third eye
A new, retrograde-viewing, auxiliary imaging device that can be inserted in the working channel of conventional colonoscopes is the Third Eye (TE). As soon as the examination begins, a transparent cap is positioned on the distal tip of the colonoscope. The TE is then inserted through the colonoscope’s working channel, as soon as the latter has achieved intubation of the cecum. Once in the cecum, the TE extends beyond the tip of the colonoscope. The device is angled and locked in such a way that it does not prohibit the antegrade view of the colonoscope. TE provides a parallel retrograde view during the withdrawal of the colonoscope. In the first safety and efficacy trial of TE, 38 polyps were detected in 24 patients. Thirty polyps were detected only in antegrade view, 4 polyps were detected in both views and 4 more polyps were detected exclusively in the retrograde view. One out of 4 polyps was an adenoma of 0.7 cm. The diagnostic yield of colonoscopy was increased by 11.8%. The device slightly increased the colonoscope withdrawal time (mean withdrawal time was 22 min), mainly because it has to be withdrawn and reinserted every time a polyp needs to be removed. TE is a promising device and a large study comparing it to conventional colonoscopy is expected[32].
Narrow–band imaging
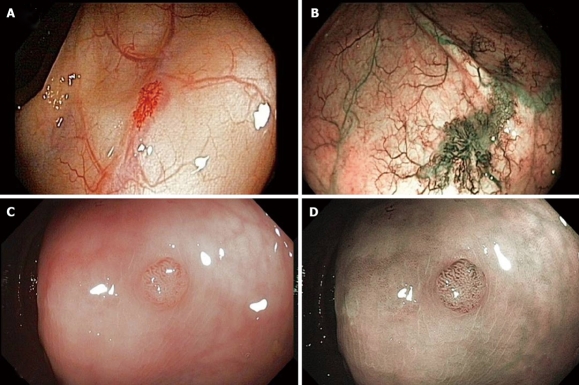
Narrow-band imaging is an innovative optical technology that modifies the center wavelength and bandwidth of an endoscope light into a narrow band illumination of 415 ± 30 nm[33]. This provides a better visualization of the capillary pattern of the mucosa and could thus provide better visualization of colonic adenomas (Figure 3). So far, studies in Western countries have not shown significant differences in detection rate of adenomas between NBI and white light. The value of NBI may be in providing improved detection rates of adenomas for colonoscopists who experience low adenoma detection rates in white light[34]. Moreover, the interpretation of NBI images needs adequate training and its use for screening may be excessively time consuming and cost-ineffective[33]. NBI has shown its efficacy in distinguishing adenomatous from hyperplastic polyps. However, its role in adenoma detection, remains to be fully tested. In a recent prospective randomized study, NBI-assisted colonoscopy was compared to conventional white-light wide-angle colonoscopy in terms of adenoma detection. Here, NBI did not significantly improve adenoma detection but, interestingly, it seemed to induce a learning effect, improving adenoma detection in standard colonoscopy, i.e. helped to “train the eye” of endoscopists in detecting adenomas with standard colonoscopy[35]. Moreover, a recent large (1256 patients), randomized trial, performed in a homogeneous setting (6 private practices, experienced colonoscopists, CRC-screening patients) failed tfo demonstrate an objective benefit of NBI in adenoma detection[36]. Therefore the actual role and usefulness of NBI in screening colonoscopy still seems to require more validation studies.
Figure 3.
Visualization of colonic lesions. A: Angiodysplasias under standard view; B: Angiodysplasias under improved visualization with narrow–band Imaging (NBI); C: A colonic polyp displayed with standard colonoscopy; D: The same polyp with NBI.
Fujinon intelligent chromoendoscopy
Fujinon intelligent chromoendoscopy (FICE), also known as computed virtual chromoendoscopy, is a technique similar to NBI aimed at enhancing tissue surface structures[37] (Figure 4). FICE was used to increase adenoma detection rates during colonoscopy in a German series of 871 patients, comparing it with indigocarmine spraying[38]. However, the results did not differ statistically between the groups in terms of adenoma detection, procedure time or the differentiation between adenomas and non-neoplastic polyps.
Figure 4.
Visualization of the colon transversum. A: Under standard view; B: With Fujinon intelligent chromoendoscopy (FICE). Note the improved visualization of the capillary pattern of the mucosa with FICE.
Based on these and other similar data, it is the personal feeling of the authors that contrast enhancement in conventional imaging techniques will probably not contribute in reducing adenoma miss rates (at least of experienced colonoscopists).
CONCLUSION
The latest developments and variations of endoscopic devices, as well as the aforementioned improvements of conventional colonoscopes, may indeed play an important role in CRC prophylaxis, but a major factor that will judge their actual impact is the feasibility of their implementation in clinical practice, i.e. the question “which of these devises really works?”. In fact, not all of them have yet proven their practicability. Some of them do not have working channels, which may not - currently- be a prerequisite when dealing with a capsule endoscope, but is certainly inacceptable when the devise in question is a “tube” endoscope. Others seem to have other flaws that have not allowed their production up to now. It should be stressed that of all the new devises, the colon capsule seems to be a promising tool in CRC screening, as it is a painless, minimally-invasive method, which requires no bowel insufflation or sedation and could therefore play a significant role as an alternative to standard colonoscopy. Although in the initial studies, its sensitivity in the detection of polyps and advanced adenomas is currently lower than that of conventional colonoscopy, it can be increased by improving bowel preparation, combined with careful reading of the colon capsule examination data. Also, the new, second-generation colon capsule has already shown signs indicating that the capsule is probably the most promising endoscopic devise that can serve as an alternative to classical endoscopy for CRC screening. Another issue that deserves extra caution is costs: Most of these new technologic developments are - for the time being- rather expensive. However, the cost of a device can certainly not be finalized as long as it is still under development or in the experimental phase. By the time the product comes to production, prices can change. Another issue is cost-effectiveness, i.e. a devise might be expensive now but may eventually help reduce costs, e.g. by reducing mortality and morbidity from CRC and by reduction of hospital costs, lost working days etc. Therefore, despite the fact that most of these new devices are currently rather expensive, in the long run they might prove to be cost-effective. Once more, studies - this time questioning the cost-benefit rate of these devises- will be needed.
Finally, another factor that should not be underestimated is the role of the primary health provider. The latter must be well-informed on the benefits that derive from screening colonoscopy in order to encourage the public to participate in CRC screening. Thus, the primary health physician can also act as another extremely effective “tool” to increase population adherence to CRC prevention programs. It is therefore the duty of medical associations, especially gastroenterological organizations to contribute to keeping the public, as well as primary health providers, informed on the benefits of examination of the colon to prevent CRC.
Footnotes
Peer reviewer: Naoki Muguruma, MD, PhD, Department of Gastroenterology and Oncology, the University of Tokushima Graduate School, 3-18-15, Kuramoto-cho, Tokushima 770-8503, Japan
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
References
- 1.Jackson-Thompson J, Ahmed F, German RR, Lai SM, Friedman C. Descriptive epidemiology of colorectal cancer in the United States, 1998-2001. Cancer. 2006;107:1103–1111. doi: 10.1002/cncr.22007. [DOI] [PubMed] [Google Scholar]
- 2.Müller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155:1741–1748. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 3.Müller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–910. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson BM, Murday VA, Finan PJ, Quirke P, Dixon MF, Bishop DT. Feasibility of family based screening for colorectal neoplasia: experience in one general surgical practice. Gut. 1993;34:96–100. doi: 10.1136/gut.34.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson JL, Danley K, Mondrus GT, Deapen D, Mack T. Adherence to screening examinations for colorectal cancer after diagnosis in a first-degree relative. Prev Med. 1995;24:166–170. doi: 10.1006/pmed.1995.1030. [DOI] [PubMed] [Google Scholar]
- 6.Bleiker EM, Menko FH, Taal BG, Kluijt I, Wever LD, Gerritsma MA, Vasen HF, Aaronson NK. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Adler A, Aschenbeck J, Aminalai A, Drossel R, Schröder A, Mayr M, Wettschureck E, Papanikolaou IS, Wiedenmann B, Rösch T. Prospective quality assessment of screening-colonoscopy in Berlin (Berlin colonoscopy project, BECOP-3) Endoscopy. 2008;40 Suppl 1:A197. [Google Scholar]
- 8.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 9.Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990;82:1769–1772. doi: 10.1093/jnci/82.22.1769. [DOI] [PubMed] [Google Scholar]
- 10.Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277–283. doi: 10.1136/gut.2003.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 12.Singh G, Mannalithara A, Wang HJ, Graham DJ, Gerson LB, Triadafilopoulos G. Is protection against colorectal cancer good enough: a comparison between sigmoidoscopy and colonoscopy in the general population. Gastroenterology. 2007;132 Suppl 2:A81. [Google Scholar]
- 13.Brenner H, Chang-Claude J, Seiler CM, Stürmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006;55:1145–1150. doi: 10.1136/gut.2005.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vucelic B, Rex D, Pulanic R, Pfefer J, Hrstic I, Levin B, Halpern Z, Arber N. The aer-o-scope: proof of concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope. Gastroenterology. 2006;130:672–677. doi: 10.1053/j.gastro.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Arber N, Grinshpon R, Pfeffer J, Maor L, Bar-Meir S, Rex D. Proof-of-concept study of the Aer-O-Scope omnidirectional colonoscopic viewing system in ex vivo and in vivo porcine models. Endoscopy. 2007;39:412–417. doi: 10.1055/s-2007-966452. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer J, Grinshpon R, Rex D, Levin B, Rösch T, Arber N, Halpern Z. The Aer-O-Scope: proof of the concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope in a pig model. Endoscopy. 2006;38:144–148. doi: 10.1055/s-2006-925089. [DOI] [PubMed] [Google Scholar]
- 17.Eickhoff A, Jakobs R, Kamal A, Mermash S, Riemann JF, van Dam J. In vitro evaluation of forces exerted by a new computer-assisted colonoscope (the NeoGuide Endoscopy System) Endoscopy. 2006;38:1224–1229. doi: 10.1055/s-2006-945014. [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff A, van Dam J, Jakobs R, Kudis V, Hartmann D, Damian U, Weickert U, Schilling D, Riemann JF. Computer-assisted colonoscopy (the NeoGuide Endoscopy System): results of the first human clinical trial (“PACE study”) Am J Gastroenterol. 2007;102:261–266. doi: 10.1111/j.1572-0241.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 19.Rösch T, Adler A, Pohl H, Wettschureck E, Koch M, Wiedenmann B, Hoepffner N. A motor-driven single-use colonoscope controlled with a hand-held device: a feasibility study in volunteers. Gastrointest Endosc. 2008;67:1139–1146. doi: 10.1016/j.gie.2007.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Long G, Fritscher-Ravens A, Mosse CA, Mills T, Swain P. The Cath-Cam: a new concept in colonoscopy. Gastrointest Endosc. 2006;64:997–1001. doi: 10.1016/j.gie.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Fritscher-Ravens A, Fox S, Swain CP, Milla P, Long G. CathCam guide wire-directed colonoscopy: first pilot study in patients with a previous incomplete colonoscopy. Endoscopy. 2006;38:209–213. doi: 10.1055/s-2006-925138. [DOI] [PubMed] [Google Scholar]
- 22.Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 23.Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971–977. doi: 10.1055/s-2006-944835. [DOI] [PubMed] [Google Scholar]
- 24.Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 25.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 26.Othman MO, Bradley AG, Choudhary A, Hoffman RM, Roy PK. Variable stiffness colonoscope versus regular adult colonoscope: meta-analysis of randomized controlled trials. Endoscopy. 2009;41:17–24. doi: 10.1055/s-0028-1103488. [DOI] [PubMed] [Google Scholar]
- 27.Rex DK. Effect of variable stiffness colonoscopes on cecal intubation times for routine colonoscopy by an experienced examiner in sedated patients. Endoscopy. 2001;33:60–64. doi: 10.1055/s-2001-11179. [DOI] [PubMed] [Google Scholar]
- 28.Howell DA, Ku PM, Desilets DJ. A comparative trial of variable stiffness colonoscopy. Gastrointest Endosc. 2002;52:AB52–AB58. [Google Scholar]
- 29.Odori T, Goto H, Arisawa T, Niwa Y, Ohmiya N, Hayakawa T. Clinical results and development of variable-stiffness video colonoscopes. Endoscopy. 2001;33:65–69. doi: 10.1055/s-2001-11174. [DOI] [PubMed] [Google Scholar]
- 30.Chen PJ, Shih YL, Chu HC, Chang WK, Hsieh TY, Chao YC. A prospective trial of variable stiffness colonoscopes with different tip diameters in unsedated patients. Am J Gastroenterol. 2008;103:1365–1371. doi: 10.1111/j.1572-0241.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 31.Saifuddin T, Trivedi M, King PD, Madsen R, Marshall JB. Usefulness of a pediatric colonoscope for colonoscopy in adults. Gastrointest Endosc. 2000;51:314–317. doi: 10.1016/s0016-5107(00)70361-0. [DOI] [PubMed] [Google Scholar]
- 32.Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy. 2008;40:478–482. doi: 10.1055/s-2007-995811. [DOI] [PubMed] [Google Scholar]
- 33.Emura F, Saito Y, Ikematsu H. Narrow-band imaging optical chromocolonoscopy: advantages and limitations. World J Gastroenterol. 2008;14:4867–4872. doi: 10.3748/wjg.14.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42–47. doi: 10.1053/j.gastro.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59–64. doi: 10.1136/gut.2007.123539. [DOI] [PubMed] [Google Scholar]
- 36.Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B, Rösch T. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410–416.e1; quiz 715. doi: 10.1053/j.gastro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80–83. doi: 10.1055/s-2006-945045. [DOI] [PubMed] [Google Scholar]
- 38.Pohl J, Nguyen-tat M, Lotterer E, Sackmann M, Gossner L, Mayer G, Ell C. A prospective randomized multicenter study on the Fujinon intelligent colour enhancement (FICE) system versus standard colonoscopy with targeted indigocarmine chromoscopy: What is the impact of novel optical imaging techniques on polyp detection rates? Endoscopy. 2008;40 Suppl 1:A1341. [Google Scholar]