Abstract
An 80-year-old woman was admitted to our hospital because of tarry stool with iron deficiency anemia. Her past history included autoimmune hepatitis. Esophagogastroduodenal endoscopy was performed to investigate the bleeding source and revealed multiple linear gastric vascular malformations in the antrum and cardia, compatible with Gastric antral vascular ectasia (GAVE). Endoscopic ablation was carried out with the tip of the hot biopsy forceps without opening at soft coagulation mode of 80W. The patient tolerated the procedure well and there were no complications associated with endoscopic therapies. After two sessions of endoscopic ablation her anemia improved to around 10 g/dL, an increase of 3.6 g/dL. Various endoscopic treatments have been described to manage GAVE. The most popular is argon plasma coagulation (APC), although APC is associated with over-distension induced by the argon plasma gas. To avoid over-distension and to reduce the abdominal discomfort/pain of this patient, we have used hot biopsy forceps instead of APC. Our case suggests that this procedure is effective, easy and convenient, as no special equipment or skill is necessary.
Keywords: Hot biopsy forceps, Endoscopic ablation, Gastric antral vascular ectasia
INTRODUCTION
Gastric antral vascular ectasia (GAVE), also named watermelon stomach, is a source of recurrent gastrointestinal hemorrhage and anemia, accounting for about 4% of all non-variceal upper gastrointestinal bleeding. Endoscopically, all patients present with a characteristic antral appearance of either ecstatic vascular stripes radiating out from the pylorus, or multiple cherry-red spots. Marked improvement of both endoscopic and histological features can be achieved with endoscopic treatment using heater probe, Gold probe, Argon plasma coagulation (APC) or laser therapy, which obliterates the vascular ectasia and decreases the degree of bleeding[1-3]. Endoscopic band ligation has also been reported[4]. Surgical intervention includes antrectomy which prevents recurrent bleeding but is usually reserved for patients who fail endoscopic therapies, as it is associated with a high mortality[5]. We herein report a case of GAVE associated with autoimmune hepatitis which was successfully treated with a simple and effective endoscopic ablation with hot biopsy forceps in common use.
CASE REPORT
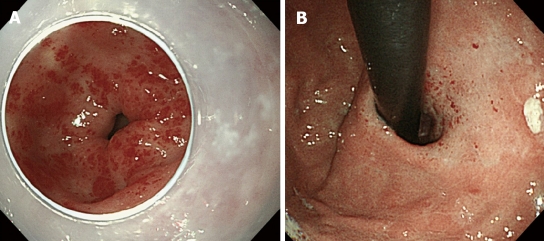
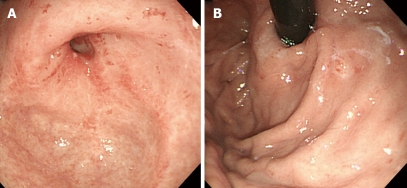
An 80-year-old woman admitted to our hospital because of tarry stool with iron deficiency anemia. Her past history included 13 years of liver dysfunction. She had been referred to our hospital for investigation and treatment of liver cirrhosis three years before this admission. The laboratory data showed that her liver cirrhosis was related to autoimmune hepatitis. Esophagogastroduodenal endoscopy was performed for investigation of the bleeding source and revealed multiple linear gastric vascular malformations in the antrum, compatible with GAVE or watermelon stomach (Figure 1A). Moreover, multiple gastric vascular malformations mimicking GAVE were also detected in the cardia (Figure 1B). A slim, single-channel and high-resolution endoscope with a water-jet system (GIFQ260J; Olympus, Tokyo, Japan) was used for endoscopic treatment. A transparent attachment (D-201-11804; Olympus) was fitted onto the tip of the endoscope mainly to obtain a constant endoscopic view. Endoscopic ablation was carried out with hot biopsy forceps (RADIAL JAW® 3 HOT BIOPSY FORCEPS; BOSTON SCIENTIFIC CORP. MA, USA) and a high-frequency generator (ICC 200; ERBE Elektromedizin, Tübingen, Germany). Contact thermal therapies were conducted with the tip of the hot biopsy forceps without opening at a special coagulation setting (soft coagulation mode 80W). Separate sessions of endoscopic ablation were performed for vascular malformations located in the antrum and cardia, respectively. After endoscopic treatment, a proton pump inhibitor was given orally and iron supplementation intravenously, with food intake started 36 h thereafter. The patient tolerated the procedure well and there were no complications associated with endoscopic therapies. After two sessions of endoscopic ablation, GAVE was markedly diminished (Figure 2A and B). The patient required no further blood transfusions. Her anemia responded well to oral iron supplementation, and her hemoglobin concentration after endoscopic ablation was maintained at around 10 g/dL, an increase of 3.6 g/dL.
Figure 1.
Multiple linear gastric vascular malformations found endoscopically, which is compatible with gastric antral vascular ectasia. A: In the antrum; B: In the cardia.
Figure 2.
Gastric vascular malformations were markedly diminished after 2 sessions of endoscopic ablation with hot biopsy forceps. A: In the antrum; B: In the cardia.
DISCUSSION
GAVE was first described by Rider et al[6] in a patient with severe chronic iron-deficiency anemia in 1953. The ectactic vessels are typically limited to the antrum. Similar to our case, Stoltzer et al[7], however, postulated a high incidence of vascular ectasias located in the cardia as well. Most patients with GAVE suffer from liver cirrhosis, autoimmune disease, chronic renal failure and bone marrow transplantation. The typical initial presentations range from occult bleeding causing transfusion-dependent chronic iron-deficiency anemia to severe acute gastrointestinal bleeding.
Various endoscopic treatments have been described to manage GAVE. Recently, several reports presented the efficacy of endoscopic therapies, such as heater probe coagulation, laser coagulation, and APC in the control of bleeding[1-3]. In particular, APC is the most frequently used endoscopic treatment for GAVE, as it has been reported to be easier to use and to be safer and more effective in comparison to other non-contact treatments[3]. Furthermore, APC has a theoretical safety advantage over other modalities due to its decreased depth of penetration and the tendency for the ionized arc of electrical current to deflect away from coagulated tissue to surrounding areas. However, on the basis of two randomized controlled trials, there is still no evidence to support the suggestion that APC is superior to other endoscopic therapies for acute non-variceal upper gastrointestinal bleeding[8]. Moreover, side-effects of APC may include bleeding, antral scarring, hyperplastic polyps, and gastric outlet obstruction[9]. Symptomatic pneumoperitoneum induced transient abdominal pain, and subcutaneous bubbling of gas have also been observed[10]. It is likely that these problems are caused by over-distension induced by argon plasma gas. To avoid over-distension and to reduce the abdominal discomfort/pain of this patient, we used hot biopsy forceps instead of APC for treatment. Our case suggested that hot biopsy forceps also can be used to ablate GAVE effectively and safely. This technique is similar to that of the heater probe, which is not only more expensive but also less commonly used recently.
Hot biopsy forceps technique involves the use of insulated monopolar electrocoagulating forceps to simultaneously biopsy and electrocoagulate tissue. It is popular for biopsy resection of diminutive polyps during colonoscopy. Furthermore, hot biopsy forceps recently have been used for hemostasis during a newly developed therapeutic endoscopy, named endoscopic submucosal dissection (ESD), for early gastric cancers[11]. Acute bleeding during ESD can be easily stopped by grasping and coagulation of the bleeding vessels using hot biopsy forceps. In personal experience, the tip of the hot biopsy forceps without grasping the mucosa for contact thermal therapies can also be used for hemostasis, especially for bleeding in deep ulcers, during ESD. To avoid deep burn, in our case we used this non-opening hot biopsy forceps contact technique, and furthermore a setting of soft coagulation mode instead of forced coagulation mode was used. Each session of endoscopic therapies was easily finished within 20 min without any complication or discomfort. This procedure is easy, convenient and cost-effective, as the device is available everywhere and no special equipment or skill is needed.
In summary, we have used hot biopsy forceps for complete and successful ablation of GAVE associated with autoimmune hepatitis. This procedure is easy, convenient and cost-effective, as no special equipment or skill is necessary. Further accumulation of cases and follow-up are needed to prove its long term efficacy.
Footnotes
Peer reviewer: Kazuki Sumiyama, MD, PhD, Department of Endoscopy, The Jikei University School of Medicine, 3-25-8 Nishi Shinbashi, Minato-ku, Tokyo 105-8461, Japan
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
References
- 1.Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum. 1996;39:341–346. doi: 10.1002/art.1780390226. [DOI] [PubMed] [Google Scholar]
- 2.Stefanidis I, Liakopoulos V, Kapsoritakis AN, Ioannidis I, Eleftheriadis T, Mertens PR, Winograd R, Vamvaka E, Psychos AK, Potamianos SP. Gastric antral vascular ectasia (watermelon stomach) in patients with ESRD. Am J Kidney Dis. 2006;47:e77–e82. doi: 10.1053/j.ajkd.2006.02.185. [DOI] [PubMed] [Google Scholar]
- 3.Yusoff I, Brennan F, Ormonde D, Laurence B. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002;34:407–410. doi: 10.1055/s-2002-25287. [DOI] [PubMed] [Google Scholar]
- 4.Sinha SK, Udawat HP, Varma S, Lal A, Rana SS, Bhasin DK. Watermelon stomach treated with endoscopic band ligation. Gastrointest Endosc. 2006;64:1028–1031. doi: 10.1016/j.gie.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Mann NS, Rachut E. Gastric antral vascular ectasia causing severe hypoalbuminemia and anemia cured by antrectomy. J Clin Gastroenterol. 2002;34:284–286. doi: 10.1097/00004836-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118–123. [PubMed] [Google Scholar]
- 7.Stotzer PO, Willén R, Kilander AF. Watermelon stomach: not only an antral disease. Gastrointest Endosc. 2002;55:897–900. doi: 10.1067/mge.2002.124558. [DOI] [PubMed] [Google Scholar]
- 8.Havanond C, Havanond P. Argon plasma coagulation therapy for acute non-variceal upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2005:CD003791. doi: 10.1002/14651858.CD003791.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Schmeck-Lindenau HJ, Kurtz W, Heine M. Inflammatory polyps: an unreported side effect of argon plasma coagulation. Endoscopy. 1998;30:S93–S94. doi: 10.1055/s-2007-1001410. [DOI] [PubMed] [Google Scholar]
- 10.Hoyer N, Thouet R, Zellweger U. Massive pneumoperitoneum after endoscopic argon plasma coagulation. Endoscopy. 1998;30:S44–S45. doi: 10.1055/s-2007-1001275. [DOI] [PubMed] [Google Scholar]
- 11.Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]