Abstract
We have demonstrated that induction of mucosal tolerance to E-selectin, a cytokine-inducible adhesion molecule restricted to activating blood vessels, prevents ischemic and hemorrhagic stroke in spontaneously hypertensive, genetically stroke-prone (SHR-SP) rats. We now examine whether mucosal tolerance to E-selectin has protective effects in ischemic brain damage after permanent middle cerebral artery occlusion (MCAO) in SHR-SP rats and whether these effects are related to generation of regulatory T cells. Rats were exposed to intranasal administration of E-selectin every other day for 10 days (single tolerization group) or on two tolerization schedules separated by 11 days (booster tolerization group). Control groups received PBS on corresponding schedules. MCAO was performed 48 h after the last dose of E-selectin or PBS. There were 45.8% and 37.9% (P < 0.05) decreases of infarction volume in the E-selectin booster group compared with the PBS group at 6 and 48 h, respectively. Single tolerization with E-selectin had only a slight trend toward a decrease in infarction volume (6.3%). CD8-positive cells were decreased in brains of E-selectin booster animals (46.6%, P < 0.01) compared with controls; splenocyte-culture supernatant levels of IL-10 were increased (59.3%, P < 0.05) in E-selectin booster animals. A decrease of infarction volume (34%, P < 0.05) was also observed in SHR-SP rats subjected to MCAO after adoptive transfer of splenocytes from E-selectin-tolerized compared with PBS-tolerized donors. The results indicate that, in addition to preventing stroke, mucosal tolerance to E-selectin is cytoprotective. Thus, immunomodulation targeted to activated blood vessel segments can both reduce stroke occurrence and attenuate brain damage if a stroke supervenes.
We have reported that nasal instillation of E-selectin, which is specifically expressed on activated endothelium, induced mucosal tolerance to that antigen and potently inhibited the development of ischemic and hemorrhagic strokes in spontaneously hypertensive, genetically stroke-prone (SHR-SP) rats with untreated hypertension (1). Mucosal tolerance is a well established model whereby immunologic tolerance is induced to a specific antigen through nasal instillation or feeding of that antigen (2, 3). Antigen administered nasally encounters nasal-associated lymphoid tissue, and antigen administered orally encounters gut-associated lymphoid tissue, both of which form well developed immune networks. Nasal-associated lymphoid tissue and gut-associated lymphoid tissue evolved to protect the host from invading pathogens and, perhaps by necessity, developed the inherent property of preventing the host from reacting to inhaled or ingested proteins that are not pathogenic. The schedule and amount of antigen administration determine the nature of the tolerance. Clonal deletion or anergy of antigen-reactive T cells can occur after a single feeding of very high-dose antigen (4); active tolerance with production of regulatory T cells occurs after repetitive administrations of low-dose antigen (5, 6). T cells tolerized with a low-dose regimen secrete cytokines such as IL-10 and transforming growth factor (TGF) β1 on antigen restimulation, which suppress cell-mediated, or TH1, immune responses (5). Although activation of these T cells is specific for the tolerizing antigen, the immunomodulatory cytokines secreted in response to activation have nonspecific effects. Thus, wherever the tolerizing antigen is present, local immunosuppression will occur. This phenomenon, known as “active cellular regulation” or “bystander suppression,” leads to relatively organ-specific immunosuppression (7). The nasal route appears equally efficient and, in some instances, even more effective than the oral route in suppressing autoimmune diseases in animal models (3), perhaps because there is less degradation of the proteins before they reach the mucosal surface.
E-selectin (CD62E) is a cell-surface glycoprotein cell-adhesion molecule that is cytokine-inducible. E-selectin expression is not constitutive; it is virtually limited to endothelium that is becoming activated in response to inflammatory stimuli, such as IL-1, tumor necrosis factor, or lipopolysaccharide. Its expression peaks between 3 and 6 h after endothelial stimulation by tumor necrosis factor and decreases thereafter to basal levels within 12-24 h in vitro but may be chronically expressed at the site of local inflammation in vivo (8, 9). As such, it serves as an appropriate molecular target to guide regulatory T cells (that have been tolerized to E-selectin) to activating blood vessels where they can release antiinflammatory cytokines, such as IL-10 and TGF-β; when released locally, they can suppress vessel activation, prevent local thrombosis and hemorrhage, and, consequently, prevent stroke (1).
There is now compelling evidence that inflammation participates in the progression of postischemic brain injury during the acute stroke period (10-12). There is also a strong consensus that appropriate treatment for stroke should be instituted as early as possible (13, 14). Because brain damage generally continues to progress during the early hours of a stroke, therapy tends to confer greater salvage of brain tissue the earlier it can be instituted. If mucosal tolerance to E-selectin could suppress inflammatory and immune mechanisms that contribute to postischemic brain injury progression, therapy would be present the moment a stroke occurs.
Mucosal administration of autoantigens has been shown to suppress inflammation and disease activity in models of stroke (15) and atherosclerosis (16) as well as in several models of autoimmunity such as diabetes, arthritis, and experimental allergic encephalomyelitis (7). Based on the profound reduction in infarct size, the frequency of spontaneous strokes observed in the E-selectin stroke prevention study (1), and the capacity of mucosal tolerization to suppress local inflammatory and immune activity (7), we postulated that prophylactic E-selectin tolerization could reduce brain injury in a preclinical model of stroke induced by permanent middle cerebral artery occlusion (MCAO) in SHR-SP rats. We found that animals tolerized to E-selectin on a booster schedule displayed robust cytoprotection with substantial reduction in brain infarct volume compared with controls, and that similar cytoprotection could be achieved by adoptive transfer of splenocytes from E-selectin-tolerized donors.
Materials and Methods
Animals and Groups. Male and female offspring (8-10 weeks of age) of SHR-SP breeders (a kind gift of Y. Yamori, Kyoto University, Kyoto) were used. The National Institute of Neurological Disorders and Stroke Animal Care and Use Committee reviewed and approved all experiments. Intranasal application of E-selectin was carried out with the animals under brief anesthesia with 5% isoflurane in 30% O2/70% N2O. Blood pressure was used to divide among groups and maintain similar group blood pressures. Intranasal instillation groups were as follows: (i) PBS single-tolerization group (n = 10), (ii) PBS boostertolerization group (n = 8), (iii) recombinant human E-selectin (R & D Systems and Novavax, Rockville, MD) single-tolerization group (n = 9), and (iv) recombinant human E-selectin booster-tolerization group (n = 8).
Tolerization Schedule. The tolerization schedule was as follows: (i) single, PBS (20 μl) or E-selectin (2.5 μg/20 μl) instilled into each nostril every other day for 10 days (total of five administrations); and (ii) booster, intranasal instillations of the same substance at the same volume and concentration and on the same schedule as described above, repeated once after 11 days.
Physiological Variables. During MCAO, mean arterial blood pressure was monitored by using a blood pressure analyzer (Micromed, Louisville, KY). PaO2, PaCO2, pH, and electrolytes (Na+/K+/Ca2+) were measured with a Rapidlab 860 Blood Gas Analyzer (Bayer, Norwood, MA). The rectal temperatures were measured and maintained at 37 ± 0.5°C with a heating blanket (K-20, American Pharmaseal, Valencia, CA) during all surgical procedures and during recovery from anesthesia (i.e., until normal locomotor activity was observed). Daily body weight was also monitored.
Delayed-Type Hypersensitivity (DTH) Reaction. For assessing the DTH reaction, a single-course tolerization schedule with either PBS or E-selectin was conducted (n = 3). Fourteen days later, the animals were immunized (hind footpad) with 75 μg of E-selectin/100 μl of PBS plus 100 μl of complete Freund's adjuvant (Sigma). Fourteen days later, ear thickness was measured as a baseline before the rats were rechallenged with 50 μg of E-selectin/100 μl of PBS injected into the ear. Ear thickness increase over baseline was measured with microcalipers (Mitsutoyo, Tokyo) 2 days later.
MCAO. Rats were anesthetized with 5% isoflurane for induction and 1-1.5% isoflurane for maintenance in 30% O2/70% N2O by means of face mask. The right femoral artery was cannulated (PE-50, Becton Dickinson) for blood-pressure monitoring and serial blood-gas sampling. The rats were placed in the lateral position, and a curved vertical 2-cm skin incision was made in the midpoint between the left orbit and the external auditory canal. A small burr hole (2-3 mm) was made with a high-speed microdrill through the outer surface of the skull at the junction between the medial wall and the roof of the inferotemporal fossa (17). The dura was opened with a 30-gauge needle to expose the middle cerebral artery (MCA), and the MCA was occluded between the inferior cerebral vein and the lateral olfactory tract by bipolar electrocoagulator. The coagulated MCA segment was then transected with microscissors to ensure that the occlusion was permanent. The rats were allowed to recover for 6 and 48 h.
Laser-Doppler Flowmetry. For the cerebral blood-flow measurement, we used the Laser-Doppler Flowmetry monitor (Vasamedics, Minneapolis). The scalp was incised in the midline. The flow was measured at three points at the surface of the cortex. All three points were 1.0 mm posterior to the bregma. Point A was placed in the 6 mm lateral from midline in the contralateral hemisphere, point B was placed 1.5 mm lateral to the midline in the ischemic hemisphere, and point C was placed 6 mm lateral to the midline in the ischemic hemisphere; sites B and C represent the perifocal region and the ischemic core, respectively. At each point, a 2-mm hole was drilled into the skull, and the bone was removed carefully. The dura was left intact to prevent cerebral spinal fluid leakage. The 37°C 0.9% saline was rinsed between the laser-Doppler probe and dura to get a clear optical medium (18).
Adoptive Transfer. Spleens were removed aseptically 48 h after the last dose of tolerizing agent from donor rats that had undergone nasal instillation of either PBS or E-selectin on a booster-tolerization schedule. Single-cell suspensions were prepared and cultured (5 × 106 cells per ml) in 12-well Costar plates (Corning) in RPMI medium 1640 supplemented with 10% FCS, 0.05 M 2-mercaptoethanol, 1% sodium pyruvate, 1% nonessential amino acids, 1% l-glutamine, 1% penicillin, 1% streptomycin, and 1 M Hepes (all cell culture reagents were purchased from GIBCO/Life Technologies). Cells were cultured with the T cell mitogen Con A (Sigma) (2 μg/ml) for 48 h. The cells were harvested and counted, and 1 × 108 cells per ml were injected (i.p.) into naive SHR-SP rats immediately before coagulation of the middle cerebral artery; rats were killed 48 h later.
Assessment of Infarct Volume. At 6 or 48 h after MCAO, rats were anesthetized and killed by guillotine; brains were removed, frozen on dry ice, and sectioned at 20-μm thickness. The sections were postfixed over paraformaldehyde vapors and stained with cresyl violet. A computerized image analysis system (NIH IMAGE 1.62) was used to measured cross-sectional infarct areas in each coronal section (≥13 sections of brain) (19). Regional volumes were calculated by summing cross-sectional areas and multiplying these areas by the distance between sections, followed by correction for brain swelling (edema) as described (20, 21).
ELISA. Spleens were removed aseptically from rats 6 h after MCAO in either PBS or E-selection booster-tolerization groups. Single-cell suspensions were prepared as described above and cultured in 96-well microtiter plates at a concentration of 5 × 106 cells per ml. The supernatants were collected at 40 and 72 h after culture with Con A (2 μg/ml) (22). The commercial serum cytokine assays [OptEIA rat IL-4, IL-10, and IFN-γ (Pharmingen) and DuoSet human TGF-β (R & D Systems)] were used according to the manufacturers' instructions. Briefly, 96-well plates (Nunc) were coated overnight (4°C) with 100 μl of antibodies to IL-4, IL-10, IFN-γ, and TGF-β diluted in coating buffer and treated with blocking buffer for 2 h at room temperature. Splenocyte culture supernatants and serially diluted standards were added (100 μl per well), and plates were incubated for 2 h at room temperature. After washing, 100 μl of biotinylated secondary antibody was added, and plates were incubated for 2 h at room temperature; 100 μl of 1:250 horse-radish-peroxidase-conjugated streptavidin was then added to each well, and plates were incubated for 30 min before stopping the reaction by adding 1 M H2SO4. The plates were read at 450 nm with correction at 570 nm. The values were calculated according to the standard curve measured in each plate. The standards were measured in triplicate, and samples were measured in duplicate.
Immunohistochemistry. Enzyme immunohistochemistry was performed on frozen coronal sections (20 μm). Sections were postfixed with cold acetone for 15 min, washed with PBS, and blocked with 5% normal donkey serum in PBS for 1 h at room temperature. After blocking, sections were incubated overnight at 4°C with the following antibodies against adhesion molecules, cytokines, and inflammatory cell surface markers: antiintercellular adhesion molecule 1, clone 1A29 (R & D Systems); anti-CD4 (Cedarlane Laboratories); anti-CD5 (Pan-T cells) (Seikagaku Kogyo, Tokyo); anti-CD45 (leukocyte common antigen) and anti-CD8 (Pharmingen); antimonocyte/macrophages (clone ED1) (BioSource International, Camarillo, CA); and antigranulocyte and OX42 (microglia) (Serotec). After washing in PBS, sections were incubated for 1 h at room temperature with the appropriate biotinylated secondary antibody raised in donkey against mouse, rabbit, or goat in a concentration of 1:3,000 (Jackson ImmunoResearch). Sections were again washed, and endogenous peroxidase was inactivated by immersing slides in a 0.3% solution of H2O2 in methanol for 20 min. Slides were washed as before and incubated for 1 h in an avidin-biotinperoxidase complex (ABC-Elite Kit, Vector Laboratories) at room temperature. Antigen-antibody binding sites were visualized with diaminobenzidine as substrate (DAB Kit, Sigma). For assessment of nonspecific staining, primary antibodies were omitted or replaced by mouse, rabbit, or goat IgG as appropriate.
Brain sections were examined with an Axioplan microscope (Zeiss). For quantification immunostaining for CD4, CD5, CD8, CD45, E-selectin, and intercellular adhesion molecule 1, images (magnification ×100) of 10 cortical regions in the ipsilateral hemisphere were obtained and analyzed (MetaMorph Image Processing System, Universal Imaging, Media, PA).
Statistical Analysis. Data are expressed as mean ± SEM. Data were statistically analyzed with an unpaired Student's t test and a Wilcoxon signed-rank test. Differences were considered significant at P < 0.05.
Results
Body Weight. In all experimental groups, the animals lost weight during the 48 h after surgery. There were no significant differences among groups in the percentage of baseline weight (i.e., weight of animals at the start of each experiment). In actively tolerized animals, the weights fell to 96.1 ± 0.8% (PBS) and 97.0 ± 1.3% (E-selectin) at 48 h after MCAO. In the adoptive transfer groups, weights fell to 97.9 ± 3.9% (PBS) and 99.1 ± 1.2% (E-selectin) at 48 h after MCAO.
Physiological Variables. Except for pH, physiological variables described in Materials and Methods remained in the normal range and did not differ among groups. The pH values were noted to decrease after operation compared with pre-MCAO values, and there were no significant differences between PBS and E-selectin (both singular and booster) tolerization groups (data not shown).
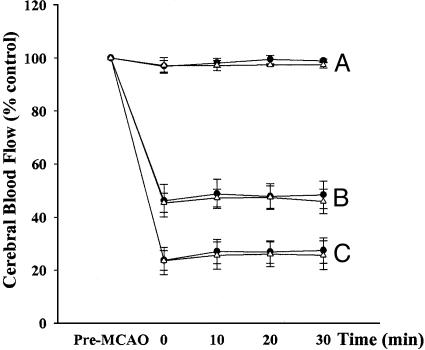
Laser-Doppler Flowmetry. In single and booster tolerization groups, local cerebral blood flow decreased to ≈25% of baseline and 45% of baseline in the infarct core and perifocal region, respectively, after MCAO. There were no differences between PBS and E-selectin groups (Fig. 1).
Fig. 1.
Relative blood flow in the contralateral and ipsilateral hemispheres. Data are presented as percentage of baseline blood-flow value (n = 5 per group). Line A, the corresponding core site in the contralateral hemisphere; lines B and C, the ischemic perifocal and core region, respectively, in the ischemic hemisphere.
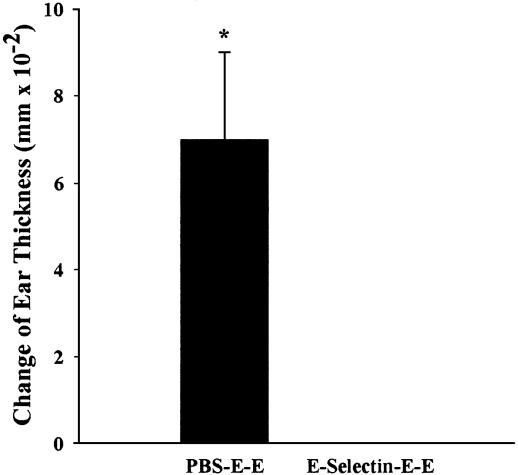
DTH. Ear swelling in the rats that received E-selectin (-0.010 ± 0.009 mm; mean ± SEM) tolerization was significantly less than that in the rats that received PBS (0.070 ± 0.020 mm) (Fig. 2), indicating suppression of TH1-mediated immune responses and confirming tolerization to E-selectin.
Fig. 2.
DTH reaction in E-selectin-tolerized compared with PBS-tolerized SHR-SP rats. Ear thickness determination was as described in Materials and Methods; rat treatment consisted of tolerization to PBS or E-selectin (E) after immunization and sensitization with E. An unpaired t test revealed a significant difference between the two groups (n = 3; *, P < 0.05). Data are presented as mean ± SEM.
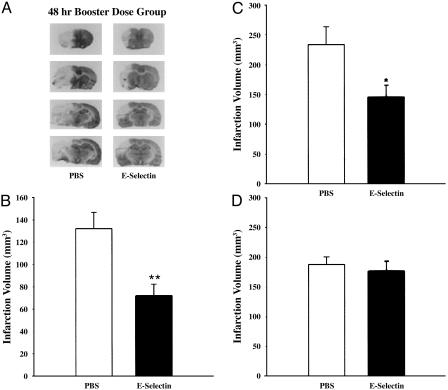
Infarct Size. At 48 h after MCAO, infarct volume in E-selectin (single) tolerized animals (176.4 ± 16.9 mm3; mean ± SEM) averaged 6.3% smaller than in PBS-treated animals (188.2 ± 12.7 mm3), which was not significant (Fig. 3D). In booster-tolerized animals, however, infarct volume was decreased by 45.8% and 37.9%, respectively, at 6 h (PBS = 132.2 ± 14.5 mm3 vs. E-selectin = 71.6 ± 10.6 mm3) (Fig. 3B) and 48 h (PBS = 234.1 ± 29.9 mm3 vs. E-selectin = 145.3 ± 20.3 mm3) (Fig. 3 A and C) after MCAO. These levels of infarct reduction were statistically significant (6 h, P < 0.01; 48 h, P < 0.05).
Fig. 3.
Infarct volumes (corrected for edema) of ischemic hemisphere in PBS- and E-selectin-tolerized animals. (A) Coronal brain sections from the 48-h ischemia group stained with cresyl violet at levels of bregma 2.70, 1.6, -2.12, and -2.56 mm. Pale areas represent infarction. Summary data for these representative sections are displayed in C. Infarct volumes (mean mm3 ± SEM) for single-tolerization SHR-SP rats at 48 h (D) and booster-tolerization SHR-SP rats at 6 h (B) and 48 h (C) after MCAO are compared with simultaneously prepared PBS-treated controls. An unpaired t test revealed a significant difference between booster-tolerization groups (n = 5-10; *, P < 0.05; **, P < 0.01).
Immunohistochemistry. The number of CD8-positive cells per high-power field in the E-selectin booster group was significantly decreased compared with PBS-tolerized animals 48 h after MCAO (P < 0.05). No significant differences were observed between PBS and E-selectin groups in CD3, CD4, CD5, CD45, ED1, OX42, antigranulocyte, or intercellular adhesion molecule 1 staining, but there was a trend toward a decrease in the number and area of E-selectin-positive vessels in E-selectin booster-tolerized animals (Table 1) as measured by computer-aided image processing (metamorph, Universal Imaging).
Table 1. The effect of E-selectin tolerance on total area and number of E-selectin-positive vessels, and CD4-, CD5-, CD8-, CD45-, and ED1-positive cells per high-power field from ischemic hemisphere 48 h after MCAO in SHR-SP rats.
| Booster tolerization group
|
||
|---|---|---|
| Control | E-selectin | |
| E-selection area (μm2) | 11,358 ± 6,086 | 7,999 ± 2,157 |
| E-selectin no. | 142 ± 69 | 87 ± 18 |
| CD4 | 6 ± 1 | 7 ± 1 |
| CD5 | 41 ± 7 | 53 ± 11 |
| CD8 | 48 ± 6 | 30 ± 5* |
| CD45 | 782 ± 195 | 733 ± 153 |
| ED1 | 426 ± 95 | 449 ± 84 |
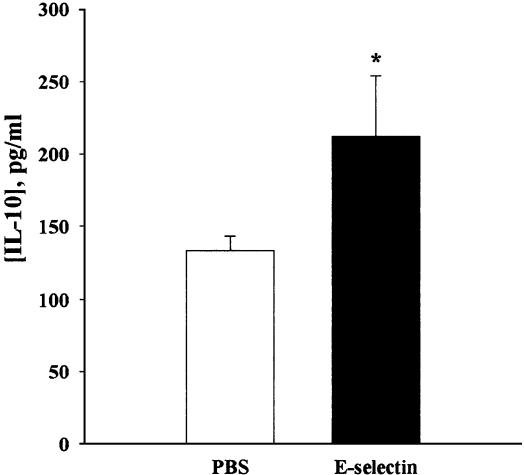
ELISA. The level of IL-10 in the splenocyte culture supernatants at 40 h after Con A stimulation was significantly increased in the booster E-selectin-tolerized group (212.3 ± 42.0 pg/ml) compared with the PBS group (133.3 ± 10.1 pg/ml) (P < 0.05) (Fig. 4). Significant differences were also observed after 72 h of stimulation (results not shown). There were no statistically significant differences between the two groups in the levels of IL-4, IFN-γ, or TGF-β at the 40- or 72-h time points either with or without Con A stimulation (data not shown).
Fig. 4.
The level of IL-10 in the supernatants of splenocyte cultures. A Wilcoxon signed-rank test revealed a significant difference between the two groups (n = 5; *, P < 0.05). Data are presented as mean ± SEM.
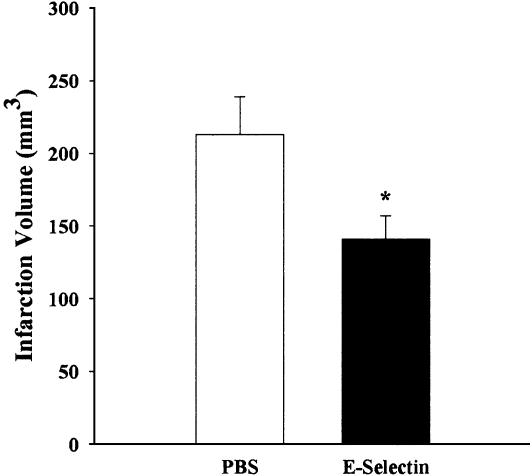
Adoptive Transfer. In adoptive transfer experiments, there was a 33.8% reduction in infarct volume in rats that received spleen cell suspensions from E-selectin booster-tolerized donors (141 ± 16 mm3) compared with rats that received spleen cell suspensions from PBS tolerization donors (213 ± 26 mm3; P < 0.05) (Fig. 5).
Fig. 5.
Infarct volumes (corrected for edema) of ischemic hemisphere in adoptive transfer experiments. Infarct volume is presented (mean mm3 ± SEM) as measured 6 h after MCAO. An unpaired t test revealed a significant difference between the two groups (n = 6-7; *, P < 0.05).
Discussion
In our previous study, we found that booster tolerization with intranasal E-selectin dramatically decreased the infarct number and infarction size and also eliminated intraparenchymal hemorrhage in spontaneous stroke in SHR-SP rats (1). To further investigate whether mucosal tolerance to E-selectin has a cytoprotective effect in focal brain ischemia in addition to its demonstrated stroke prevention effects, we examined its capacity to reduce damage after permanent MCAO in SHR-SP rats. We found that E-selectin mucosal booster tolerization significantly decreased infarct size measured in the brains of these animals at both 6 and 48 h after onset of ischemia (Fig. 3 B and C). Single mucosal tolerization with E-selectin, however, had only a slight trend toward benefit in stroke outcome (Fig. 3D). The requirement of booster tolerization for suppression of ischemic brain damage agrees with our previous stroke prevention study, which suggests that repeated low-dose tolerization is needed to produce an effective cohort of regulatory T cells in this model (23). The smaller lesion size in the PBS group at 6 h (Fig. 3B) compared with 48 h (Fig. 3C) accords with the well described maturation of permanent MCAO infarcts between 12 and 24 h (24).
TH1 cells are generally regarded as mediators of DTH reactions (25). In the cutaneous DTH reaction elicited by 2,4-dinitrofluorobenzene, TH1 cells migrated into the DTH region in the sensitized ear over a period of 24 h, whereas the level of TH2 cells remained low (26). In attempts to identify possible roles of regulatory cells in the tolerance phenomenon observed here, we examined the possible presence of DTH to the tolerogen, E-selectin. We found that E-selectin-specific DTH responses were significantly suppressed in E-selectin-tolerized animals (Fig. 2). Suppression of the TH1-mediated DTH reaction to E-selectin provides evidence for the generation of antigen-specific regulatory T cells in rats tolerized to E-selectin. These findings implicate cell-mediated immunomodulation as the basis for the observed cytoprotection in this MCAO stroke model.
Further evidence that tolerization was a cell-mediated effect was observed in experiments involving adoptive transfer of tolerance. It was observed that adoptive transfer of splenocytes from tolerized rats resulted in a level of protection similar to that seen with booster tolerization (Fig. 5). The demonstration that the effect of mucosal tolerization with E-selectin on MCAO stroke can be replicated in naive SHR-SP rats by adoptive transfer of splenocytes from tolerized rats supports the possibility that this phenomenon is cell-mediated (27). The finding that these splenocytes (which induced tolerance) secreted increased amounts of IL-10 compared with control splenocytes further substantiated the involvement of regulatory T cells in this cytoprotection.
Repetitive mucosal tolerization with low-dose antigen generates regulatory T cells (5, 6), in contrast to intranasal administration of high-dose antigen that leads to clonal anergy or clonal deletion of T cells (4, 7). Dendritic cells and macrophages are subjacent to the mucosa of the nasal passages and act as antigen-presenting cells in the nasal-associated lymphoid tissue. They are embedded in a milieu of prostaglandins (PGE2) and cytokines (TGF-β and IL-10) that are conducive to tolerization rather than immune induction (28-30). Nasally instilled E-selectin is absorbed and taken up by these antigen-presenting cells, cleaved into 10 30-mer peptides (27), and expressed with MHC class II molecules on their cell membranes. Local T cells can then engage MHC class II/antigen complex on the surface of antigen-presenting cells through their T cell receptors. This receptor interaction with costimulation from B7 molecules on the antigen-presenting cells and cytotoxic T lymphocyte antigen 4 on the T cell leads to the generation of antigen-specific regulatory T cells (7, 28) that produce TGF-β (TH3 cell) (31) or IL-10 (Tr1 cell) (6). After tolerization, these T cells circulate systemically and respond to presentation of the specific antigen to which they were tolerized by locally secreting antiinflammatory cytokines, such as TGF-β and IL-10 (7). Although activation of these T cells is specific for the tolerizing antigen, the immunomodulatory cytokines secreted in response to activation have nonspecific effects (32). Thus, local immunosuppression will occur wherever the tolerizing antigen is present. E-selectin is a suitable choice for the tolerizing antigen because it is not constitutively expressed, and its distribution is essentially confined to vascular endothelium. It becomes expressed on the luminal surface of endothelium only in a vessel segment that is becoming activated (9, 33). As such, it can serve as an antigenic target to guide regulatory T cells preferentially to activating vessel segments (34, 35), where they can suppress local vessel activation as well as surrounding immune and inflammatory processes.
The mechanisms by which E-selectin tolerization suppresses ischemic damage in permanent MCAO are not known. As described previously, immunomodulatory and antiinflammatory cytokine release is targeted to activated blood vessels by the E-selectin antigen specificity of regulatory T cells. This cytokine release could attenuate a variety of injury mechanisms, including effects on (i) expression of adhesion molecules (36-39), (ii) activation of leukocytes (40, 41), and (iii) release of proinflammatory cytokines (42, 43) and many other factors involved in the pathobiology of stroke (11, 12). Recent evidence also supports a role for lymphocytes in ischemic brain injury (44, 45). Perhaps E-selectin tolerization selectively affects lymphocyte activation. We found a reduction of CD8+ lymphocytes in the ischemic injury zones of E-selectin-tolerized animals compared with controls. The traffic signals that direct CD8+ effector cells to inflamed tissue have not been studied as extensively as those for the CD4+ subgroup, but they appear to be similar (35). When locally stimulated by antigen, cytotoxic T lymphocytes that are primarily CD8+ cells can contribute to tissue damage by several mechanisms (46), including release of inflammatory cytokines that recruit neutrophils, monocytes, and TH1 cells (47). Thus, smaller infarction volumes in E-selectin-tolerized animals could be due to decreased numbers of CD8+ cells in the ischemic injury zone. However, it remains to be determined whether cytotoxic T lymphocyte activation and neurotoxicity occur during the time frame of progressing brain damage from acute stroke.
Taken together, the results indicate that mucosal tolerance to E-selectin after booster tolerization plays a cytoprotective role in the permanent MCAO stroke model in SHR-SP rats. There are two previously undescribed aspects to this study. First, we demonstrate that immunomodulation targeted to an antigen, E-selectin, that is expressed only on activated blood vessel segments provides robust cytoprotection in focal brain ischemia. Second, this same order of protection can be adoptively transferred to naive recipients by splenocytes from tolerized donors, indicating that the protection is cell-mediated. Immunomodulation targeted to activated blood vessel segments may also prevent hemorrhage and thrombosis and provide immediate cytoprotection to attenuate damage in other tissues and organs. In addition, the approach may apply to other diseases associated with immune-mediated pathogenic mechanisms. Furthermore, the observed capability to passively transfer this tolerance to naive animals invites speculation regarding novel mechanisms to prevent or ameliorate tissue damage associated with stroke as well as other injuries.
Acknowledgments
We thank Mrs. Sandra Taubenkibel for excellent secretarial assistance.
Abbreviations: MCAO, middle cerebral artery occlusion; SHR-SP, spontaneously hypertensive, genetically stroke-prone; DTH, delayed-type hypersensitivity; TGF, transforming growth factor.
References
- 1.Takeda, H., Spatz, M., Ruetzler, C., McCarron, R., Becker, K. & Hallenbeck, J. (2002) Stroke 33, 2156-2163. [DOI] [PubMed] [Google Scholar]
- 2.Weiner, H. L. (1997) Immunol. Today 18, 335-343. [DOI] [PubMed] [Google Scholar]
- 3.Metzler, B. & Wraith, D. C. (1996) Ann. N.Y. Acad. Sci. 778, 228-242. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., Inobe, J., Marks, R., Gonnella, P., Kuchroo, V. K. & Weiner, H. L. (1995) Nature 376, 177-180. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. (1994) Science 265, 1237-1240. [DOI] [PubMed] [Google Scholar]
- 6.Groux, H., O'Garra, A., Bigler, M., Rouleau, M., Antonenko, S., de Vries, J. E. & Roncarolo, M. G. (1997) Nature 389, 737-742. [DOI] [PubMed] [Google Scholar]
- 7.Faria, A. M. & Weiner, H. L. (1999) Adv. Immunol. 73, 153-264. [DOI] [PubMed] [Google Scholar]
- 8.Kansas, G. S. (1996) Blood 88, 3259-3287. [PubMed] [Google Scholar]
- 9.Bevilacqua, M. P. & Nelson, R. M. (1993) J. Clin. Invest. 91, 379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priller, J. & Dirnagl, U. (2002) Ernst Schering Res. Found. Workshop 39, 133-157. [DOI] [PubMed] [Google Scholar]
- 11.Iadecola, C. & Alexander, M. (2001) Curr. Opin. Neurol. 14, 89-94. [DOI] [PubMed] [Google Scholar]
- 12.Dirnagl, U., Iadecola, C. & Moskowitz, M. A. (1999) Trends Neurosci. 22, 391-397. [DOI] [PubMed] [Google Scholar]
- 13.Adams, H. P., Jr., Adams, R. J., Brott, T., del Zoppo, G. J., Furlan, A., Goldstein, L. B., Grubb, R. L., Higashida, R., Kidwell, C., Kwiatkowski, T. G., et al. (2003) Stroke 34, 1056-1083. [DOI] [PubMed] [Google Scholar]
- 14.Marler, J. R., Tilley, B. C., Lu, M., Brott, T. G., Lyden, P. C., Grotta, J. C., Broderick, J. P., Levine, S. R., Frankel, M. P., Horowitz, S. H., et al. (2000) Neurology 55, 1649-1655. [DOI] [PubMed] [Google Scholar]
- 15.Becker, K. J., McCarron, R. M., Ruetzler, C., Laban, O., Sternberg, E., Flanders, K. C. & Hallenbeck, J. M. (1997) Proc. Natl. Acad. Sci. USA 94, 10873-10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron, R., Sukhova, G., Faria, A. M., Hoffmann, E., Mach, F., Libby, P. & Weiner, H. L. (2002) Circulation 106, 1708-1715. [DOI] [PubMed] [Google Scholar]
- 17.Tamura, A., Graham, D. I., McCulloch, J. & Teasdale, G. M. (1981) J. Cereb. Blood Flow Metab. 1, 53-60. [DOI] [PubMed] [Google Scholar]
- 18.Yang, G. Y. & Betz, A. L. (1994) Stroke 25, 1658-1665. [DOI] [PubMed] [Google Scholar]
- 19.Chen, Y., Ginis, I. & Hallenbeck, J. M. (2001) J. Cereb. Blood Flow Metab. 21, 34-40. [DOI] [PubMed] [Google Scholar]
- 20.Leach, M. J., Swan, J. H., Eisenthal, D., Dopson, M. & Nobbs, M. (1993) Stroke 24, 1063-1067. [DOI] [PubMed] [Google Scholar]
- 21.Lin, T. N., He, Y. Y., Wu, G., Khan, M. & Hsu, C. Y. (1993) Stroke 24, 117-121. [DOI] [PubMed] [Google Scholar]
- 22.Chen, Y., Inobe, J., Kuchroo, V. K., Baron, J. L., Janeway, C. A., Jr., & Weiner, H. L. (1996) Proc. Natl. Acad. Sci. USA 93, 388-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, H. R., Taylor, N., Duncan, L., Dick, A. D. & Forrester, J. V. (2001) Br. J. Ophthalmol. 85, 739-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, R. L., Chopp, M., Chen, H. & Garcia, J. H. (1994) J. Neurol. Sci. 125, 3-10. [DOI] [PubMed] [Google Scholar]
- 25.Cher, D. J. & Mosmann, T. R. (1987) J. Immunol. 138, 3688-3694. [PubMed] [Google Scholar]
- 26.Austrup, F., Vestweber, D., Borges, E., Lohning, M., Brauer, R., Herz, U., Renz, H., Hallmann, R., Scheffold, A., Radbruch, A. & Hamann, A. (1997) Nature 385, 81-83. [DOI] [PubMed] [Google Scholar]
- 27.Abbas, A. K., Lichtman, A. H. & Pobe, J. S. (1997) Cellular and Molecular Immunology (Saunders, Philadelphia).
- 28.Nagler-Anderson, C. (2000) Crit. Rev. Immunol. 20, 103-120. [PubMed] [Google Scholar]
- 29.Kalinski, P., Hilkens, C. M., Snijders, A., Snijdewint, F. G. & Kapsenberg, M. L. (1997) Adv. Exp. Med. Biol. 417, 363-367. [DOI] [PubMed] [Google Scholar]
- 30.De Smedt, T., Van Mechelen, M., De Becker, G., Urbain, J., Leo, O. & Moser, M. (1997) Eur. J. Immunol. 27, 1229-1235. [DOI] [PubMed] [Google Scholar]
- 31.Miller, A., Lider, O., Roberts, A. B., Sporn, M. B. & Weiner, H. L. (1992) Proc. Natl. Acad. Sci. USA 89, 421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, A., Lider, O. & Weiner, H. L. (1991) J. Exp. Med. 174, 791-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevilacqua, M. P., Stengelin, S., Gimbrone, M. A., Jr., & Seed, B. (1989) Science 243, 1160-1165. [DOI] [PubMed] [Google Scholar]
- 34.Mackay, C. R., Marston, W. L., Dudler, L., Spertini, O., Tedder, T. F. & Hein, W. R. (1992) Eur. J. Immunol. 22, 887-895. [DOI] [PubMed] [Google Scholar]
- 35.von Andrian, U. H. & Mackay, C. R. (2000) N. Engl. J. Med. 343, 1020-1034. [DOI] [PubMed] [Google Scholar]
- 36.Frijns, C. J. & Kappelle, L. J. (2002) Stroke 33, 2115-2122. [DOI] [PubMed] [Google Scholar]
- 37.del Zoppo, G. J., Schmid-Schonbein, G. W., Mori, E., Copeland, B. R. & Chang, C. M. (1991) Stroke 22, 1276-1283. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblum, W. I. (1997) J. Vasc. Res. 34, 409-417. [DOI] [PubMed] [Google Scholar]
- 39.Cines, D. B., Pollak, E. S., Buck, C. A., Loscalzo, J., Zimmerman, G. A., McEver, R. P., Pober, J. S., Wick, T. M., Konkle, B. A., Schwartz, B. S., et al. (1998) Blood 91, 3527-3561. [PubMed] [Google Scholar]
- 40.Hallenbeck, J. M., Dutka, A. J., Kochanek, P. M., Siren, A., Pezeshkpour, G. H. & Feuerstein, G. (1988) Stroke 19, 863-869. [DOI] [PubMed] [Google Scholar]
- 41.Stoll, G., Jander, S. & Schroeter, M. (1998) Prog. Neurobiol. 56, 149-171. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell, N. J. & Luheshi, G. N. (2000) Trends Neurosci. 23, 618-625. [DOI] [PubMed] [Google Scholar]
- 43.Hallenbeck, J. M. (2002) Nat. Med. 8, 1363-1368. [DOI] [PubMed] [Google Scholar]
- 44.Becker, K., Kindrick, D., Relton, J., Harlan, J. & Winn, R. (2001) Stroke 32, 206-211. [DOI] [PubMed] [Google Scholar]
- 45.Relton, J. K., Sloan, K. E., Frew, E. M., Whalley, E. T., Adams, S. P. & Lobb, R. R. (2001) Stroke 32, 199-205. [DOI] [PubMed] [Google Scholar]
- 46.Kagi, D., Ledermann, B., Burki, K., Zinkernagel, R. M. & Hengartner, H. (1996) Annu. Rev. Immunol. 14, 207-232. [DOI] [PubMed] [Google Scholar]
- 47.Price, D. A., Klenerman, P., Booth, B. L., Phillips, R. E. & Sewell, A. K. (1999) Immunol. Today 20, 212-216. [DOI] [PubMed] [Google Scholar]