Abstract
Diffuse malignant peritoneal mesothelioma (DMPM) is an uncommon and rapidly fatal tumor. Therapeutic options have traditionally been limited and ineffective. The biologic and molecular events correlated with poor responsiveness to therapy are still poorly understood. In recent years, an innovative treatment approach involving aggressive cytoreductive surgery (CRS) and perioperative intraperitoneal chemotherapy has reportedly resulted in improved outcome, as compared to historical controls. Since 1995, at the National Cancer Institute (NCI) of Milan (Italy), patients with DMPM have been treated with CRS and hyperthermic intra-peritoneal chemotherapy (HIPEC). In the present paper, clinical experiences and basic science investigations on DMPM at Milan NCI are reviewed. Peri-operative and long-term outcome results with CRS and HIPEC are presented. Clinico-pathological prognostic factors were investigated by multivariate analysis. The pathologic features and immunohistochemical markers related to DMPM biologic behavior were assessed in a large case-series uniformly treated at our institution. The prevalence and prognostic role of telomere maintenance mechanisms, which account for the limitless cell replicative potential of many malignancies, were studied. The dysregulation of the apoptotic pathways may play a role in the relative chemo-resistance of DMPM and a better understanding of apoptosis-related mechanisms could result in novel targeted therapeutic strategies. On this basis, the expression of survivin and other IAP family members (IAP-1, IAP-2, and X-IAP), the pro-apoptotic protein Smac/DIABLO, and antigens associated with cell proliferation (Ki-67) and apoptosis (caspase-cleaved cytokeratin-18) were analyzed. Finally, analyses of EGFR, PDGFRA and PDGFRB were performed to ascertain if deregulation of RTK could offer useful alternative therapeutic targets.
Keywords: Peritoneal mesothelioma, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Telomerase, Surviving, Apoptosis, Receptor tyrosin kinase
INTRODUCTION
Malignant mesothelioma is an uncommon tumor arising from the serosal layer of pleura, peritoneum, pericardium and tunica vaginalis testis[1]. The incidence of the disease has been rising worldwide since 1970, due to widespread exposure to asbestos during previous decades, and it is not expected to peak before the next 20 years[2]. In the United States, approximately 2500 new cases of mesothelioma are registered each year. Diffuse malignant peritoneal mesothelioma (DMPM) accounts for 10% to 30% of all mesotheliomas[3].
In historical case-series, standard therapy with palliative surgery and systemic or intraperitoneal chemotherapy is associated with a median survival of about one year, ranging from 9 to 15 mo[4-6]. However, the disease tends to remain within the abdominal cavity throughout its clinical course and an autopsy study demonstrated that 78% of patients had died because of complications directly related to local-regional progression[7].
In recent years, this has prompted a few specialized centers to develop an innovative local-regional treatment approach. It involves cytoreductive surgery (CRS) with peritonectomy procedures and multivisceral resections to remove the entire visible tumour. Microscopic residual disease is treated by perioperative intraperitoneal chemotherapy. This comprehensive strategy has reportedly resulted in a median survival of 34-92 mo, which strongly suggests improved outcome as compared to historical controls[8-14].
At the National Cancer Institute (NCI) of Milan (Italy), the first combined procedure of CRS and hyperthermic intra-peritoneal chemotherapy (HIPEC) was performed in February 1995 in a patient with peritoneal carcinomatosis from ovarian cancer. In August 1995, the first patient with peritoneal mesothelioma, which was the tenth of the overall series, was treated. The present paper reviews our institutional experience with a special focus on clinical results, pathological studies and basic science investigations.
DIAGNOSIS OF PERITONEAL MESOTHELIOMA
The histologic features of malignant peritoneal mesothelioma are sub-divided into epithelial, sarcomatoid, and biphasic tumors. Clinical and pathological diagnosis of mesotheliomas can be very difficult. The morphology of the neoplasm is extremely variable and is a major basis for diagnostic dilemma[15]. Malignant mesotheliomas are difficult to distinguish from benign reactive lesions of the pleura as well as from metastatic adenocarcinomas. Immunohistochemical studies represent a very important diagnosis aid. At present, however, an absolutely specific marker for mesothelioma has not yet been recognized and the immunohistochemical diagnosis of this tumor largely depends on the use of panels of markers that combine positive [thrombomodulin (CD141), calretinin, keratin 5/6, D2-40, podoplanin, mesothelin, and Wilms tumor 1 protein (WT1)] with negative markers (carcinoembryonic antigen, MOC-31, B72.3, and Ber-EP4) most commonly present in carcinoma[16]. Thyroid transcription factor 1 (TTF-1) can assist in determining origin from lung carcinoma, CDX-2 origin from colon carcinoma, CK 7 and Claudin 4 origin from ovarian carcinoma. Renal cell carcinoma marker (RCC Ma) may be helpful in establishing renal origin. Claudin 4 is a transmembrane protein component of tight junctions, responsible for cell adhesion. Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma vs mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. D2-40 is a commercially available monoclonal antibody that reacts with a 40 kDa antigen in fetal germ cells and germ cell tumors. Since the antibody reacts with epithelial mesotheliomas, but not with carcinomas, it could be very helpful in discriminating between these malignancies. Podoplanin is an approximately 38 kDa membrane mucoprotein originally detected on the surface of rat glomerular epithelial cells (podocytes) that is specifically expressed in the endothelium of lymphatic capillaries but not in the blood vasculature. Podoplanin, like D2-40, is expressed in mesotheliomas but not in adenocarcinomas. Calretinin is an intracellular calcium-binding protein belonging to the troponin C superfamily. At present, calretinin is regarded as being the most sensitive and one of the most specific mesothelioma markers. Calretinin is frequently expressed in both histologic types of mesothelioma, i.e. epithelial and sarcomatoid.
WT1 was first described in 1990 as a tumour suppressor gene associated with Wilms tumour (nephroblastoma). It encodes a typical transcription factor with four C2-H2 zinc fingers in the C-terminus that is expressed in mesotheliomas but not in adenocarcinomas. Because of its high sensitivity and absolute specificity, WT1 is one of the best positive markers for discriminating between metastatic adenocarcinomas and mesotheliomas. The vast majority of cases of squamous cell carcinoma, basal cell carcinoma, thymoma, salivary gland tumor, and biphasic malignant mesothelioma were positive for CK 5/6. CK 5/6 has been used to distinguish malignant mesothelioma from adenocarcinoma of the lung. Thrombomodulin (CD141) is a glycoprotein of molecular weight 75000 kD that is normally present in restricted numbers of cells, including endothelial and mesothelial cells, and was the first of the positive mesothelioma markers that proved useful in the diagnosis of this tumor.
TREATMENT PROTOCOL
Cytoreductive surgical procedures are performed with the aim of removing all the peritoneal tumor deposits according to the technique described by Sugarbaker[17].
In the first 11 patients, HIPEC was administered with cisplatin (25 mg/L of perfusate/m2) and mitomycin-C (3.3 mg/L of perfusate/m2) for 60 min.
From June 1997 to December 1999, patients with DMPM were included in a multi-institutional phase I study testing the combination of cisplatin and doxorubicin in the local-regional setting[18]. Doxorubicin was chosen due to its clinical activity against mesothelioma (as well as ovarian carcinoma and soft-tissue sarcoma), its favourable plasma/peritoneal ratio and high molecular weight, allowing a more rapid clearance from normal than from tumour tissue[19-21]. Doxorubicin activity is also synergistically enhanced by heat and cisplatin, thus favouring its use in a combination regimen under hyperthermic conditions.
Thirty one patients with liposarcoma (n = 9), leiomyosarcoma (n = 6), other soft-tissue sarcomas (n = 4), ovarian carcinoma (n = 6), and malignant mesothelioma (n = 6) undergoing adequate CRS (residual tumour ≤ 2.5 mm) constituted the study population. HIPEC was performed for 90 min at a mean intraperitoneal temperature of 42.5°C. The drugs were administered to triplets of patients in escalating doses, starting with 5 and 20 mg/L of perfusate for doxorubicin and cisplatin, respectively. The dose was increased by 25% for each subsequent triplet. Accrual was stopped when grade IV loco-regional toxicity was observed in one patient. The maximal tolerated dose (MTD) was considered to be that of the previous triplet and was confirmed after three more patients had been treated uneventfully with the putative MTD.
One patient treated with 19 mg/L of doxorubicin and 43 mg/L of cisplatin experienced Grade IV loco-regional toxicity (persistent ileus) and required reoperation. To confirm that MTD had been reached, we treated three more patients with the previous triplet drug dosages. Because no significant loco-regional toxicity was observed, MTD was established at 15.25 mg/L of doxorubicin and 43 mg/L of cisplatin.
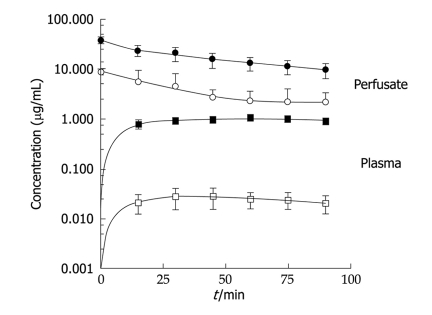
The results of the pharmacokinetic studies are illustrated in Figure 1, which clearly shows how similar perfusate concentrations of doxorubicin and cisplatin gave very different plasma concentrations, with doxorubicin levels being roughly 50-fold lower.
Figure 1.
Time courses of the mean concentrations of doxorubicin and cisplatin in perfusate and plasma. Solid lines obtained by nonlinear least squares regression analyses of data. Open symbols: Doxorubicin (DXR); Filled symbols: Cisplatin (CDDP).
CLINICAL RESULTS
The clinical results from the Milan NCI were published in a preliminary report of the first 20 cases[22], a clinicopathological study[23], an extensive prognostic analysis of potential clinical, pathological and biological variables[24], and an assessment of the pattern of failure[25].
Forty-nine patients with DMPM were enrolled to test the association between potential prognostic variables and survival by multivariate statistical analysi[24]. Patients with low malignant variants (multicystic and papillary well-differentiated mesothelioma) were excluded. The mean age was 52 years (range 22-74 years). Twenty-six patients had preoperative systemic chemotherapy. Forty-three patients were diagnosed with epithelial and 6 patients with biphasic DMPM; 43 patients underwent complete cytoreduction (residual tumor ≤ 2.5 mm) and 6 patients underwent grossly incomplete cytoreduction.
At a mean follow-up of 20.3 mo (range 1-89 mo), the 5-year overall survival (OS) and progression-free survival (PFS) were 57% and 31%, respectively. The median PFS and OS were 39.7 mo and not reached, respectively. There were no treatment-related deaths. Grade 3-4 (NCI CTCAE v.3) surgical complications occurred in eight cases (15%) and grade 3-4 toxicities in six cases (12%).
Potential prognostic variables with P values < 0.20 at univariate analysis (log-rank test), were included in the Cox proportional hazard model (Table 1). The backward-elimination method identified the completeness of cytoreduction and mitotic count (MC) > 5/50 HPF as independent predictors of OS, performance status and MC correlated to PFS.
Table 1.
Clinicopathologic variables with prognostic significance according to univariate (log-rank) and multivariate (Cox proportional hazard model) analyses
| Variable |
Overall survival |
Progression-free survival |
||||
|
Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| P value | HR (95% CI) | P value | P value | HR (95% CI) | P value | |
| Sex | 0.22 | 0.27 | ||||
| Age (< 52 yr vs ≥ 52 yr) | 0.93 | 0.49 | ||||
| Performance status (0 vs 1, 2, or 3) | 0.43 | 0.05 | 0.29 (0.1-0.8) | 0.02 | ||
| Previous surgical score (0 vs ≥ 1) | 0.78 | 0.11 | ||||
| Previous systemic CT | 0.59 | 0.57 | ||||
| PCI (≥ 28 vs < 28) | 0.12 | 0.10 | ||||
| Completeness of cytoreduction (0/1 vs 2/3)a | 0.01 | 8.6 (2.1-36.2) | 0.00 | 0.08 | ||
| IPHP drug schedule (CDDP + DX vs CDDP + MMC) | 0.36 | 0.98 | ||||
| Histological subtype (epithelioid vs biphasic) | 0.09 | 0.07 | ||||
| Mitotic count(< 5 vs ≥ 5) | 0.01 | 10.5 (1.9-55.2) | 0.01 | 0.19 | 3.1 (1.1-8.8) | 0.03 |
| Nuclear grade (high vs low) | 0.02 | 0.10 | ||||
a0/1, minimal residual disease or residual tumor < 2.5 mm; 2/3, residual tumor ≥ 2.5 mm. CI: Confidence interval; PCI: Peritoneal Cancer Index; IPHP: Intraperitoneal hyperthermic perfusion; CDDP: Cisplatin; DX: Doxorubicin; MMC: Mitomycin C; HPF: High-power field.
The estimated hazard rate for patients with grossly incomplete cytoreduction was eight times higher than for those with optimal cytoreduction, after adjustment for other variables. This is in agreement with experimental evidence that intraperitoneal chemotherapy cannot penetrate tumour tissue deeper than a few millimeters. Thus, the volume of residual disease is one the major factors limiting the effectiveness of loco-regional therapy[8-14]. The second variable that remained in the Cox model as a factor influencing OS was MC. Patients with MC > 5/50 HPF presented a hazard rate 10 times higher, as compared with those with lower MC. Available data are conflicting: patients with high MC have been associated with poor prognosis[14], although other authors did not reach the same conclusion[26].
The preoperative clinical conditions have been shown to be a prognostic factor in pleural mesothelioma[1,2]. In the present series, the independent association between MC and PFS emerged after the multivariate analysis even in the absence of significant correlation by univariate analysis. However, the performance status did not correlate with OS. This could be explained by the small number of deaths and the fact that 89% of patients had a good performance status.
Despite encouraging survival results, approximately 40%-60% of patients developed disease progression and died of DMPM following comprehensive treatment[8-14]. However, data on patients who failed to respond to initial treatment are lacking and optimal management of recurrent DMPM has never been defined. Therefore, we analysed the patterns of failure to understand how and possibly why combined treatment failed and to identify the modifications that might improve clinical results in a subset of 38 patients who developed disease progression following CRS and HIPEC[25].
Initial treatment consisted of adequate cytoreduction with residual tumour ≤ 2.5 mm and HIPEC in 28 patients and grossly incomplete CRS in 10 patients. Detailed information regarding progressive disease distribution was prospectively collected by CT scan (n = 26) or both CT-scan and laparatomy (n = 12).
Median time-to-progression was 9 mo. In the individual patients, the pattern of failure was categorized as liver metastases (n = 1), involvement of celiac (n = 1) and retroperitoneal (n = 1) lymph-nodes, isolated seeding of the basal pleura (n = 2) and involvement of both abdominal and pleural cavity (n = 2). In the remaining 31 patients (81.6%), only peritoneal progression was noted: the small bowel and its mesentery were involved in 13 patients, intra-abdominal sites exclusive of small bowel in 4 patients, and both the small bowel and additional intra-abdominal sites in 14 patients. Overall, small bowel was involved in 27 patients (71.1%).
In 28 patients undergoing complete CRS, potential factors determining disease progression were statistically assessed in 13 distinct abdominopelvic regions. At multivariate analysis, only residual tumour up to 2.5 mm vs macroscopically complete CRS correlated to disease progression in the epigastric region, upper jejunum, lower jejunum and upper ileum. Taken together, these data strongly suggest that failure to remove the entire visible tumor in critical areas where cytoreductive surgery is technically difficult may be the leading cause of treatment failure. Therefore, maximal surgical efforts aiming at leaving behind no residual disease are an absolute requirement.
Progressive disease was treated with second HIPEC (n = 3), debulking (n = 4), systemic chemotherapy (n = 16), and supportive care (n = 15). Median survival from progression was 8 mo. At multivariate analysis, time-to-progression < 9 mo, poor performance status, and supportive care correlated with reduced survival from progression. Furthermore, operative treatment (i.e. cytoreduction or cytoreduction with HIPEC) showed a trend toward better outcomes, compared with systemic chemotherapy. Since treatment was determined according to patient conditions and disease extent, the different treatment modalities cannot be compared. Nevertheless, encouraging results were obtained with repeated cytoreduction and HIPEC, making aggressive treatment of progressive disease an attractive option.
MULTICYSTIC AND WELL-DIFFERENTIATED PAPILLARY PERITONEAL MESOTHELIOMA (WDPPM)
Multicystic peritoneal mesothelioma (MPM) and WDPPM are exceedingly uncommon lesions with uncertain malignant potential and no uniform treatment strategy[26,27]. From the beginning of our peritoneal malignancies treatment program, MPM and WDPPM were included among the indications to cytoreduction and HIPEC, owing to their known potential to relapse and to evolve into aggressive malignant tumours[28].
Twelve female patients (4 with MPM and 8 with WDPPM) underwent 13 combined procedures at the NCI of Milan. Seven patients had recurrent disease after previous debulking (1 operation in 5 patients, 2 in 1 patient, 4 in 1 patient). Due to their perceived low aggressiveness, small tumour deposits on visceral peritoneum were preferably removed by electrosurgical dissection and organ resections were performed only if massive disease involvement precluded a conservative approach. Accordingly, uteri and ovaries were spared in four reproductive age women and to date no recurrence has involved the pelvis. Optimal cytoreduction with no or minimal (≤ 2.5 mm) residual disease was accomplished in 12 of 13 procedures (92.3%).
After a median follow-up of 27 mo (range 6-94 mo), postoperative disease progression occurred in two patients and tumour-related death in one. The first patient underwent the procedure twice due to loco-regional MPM recurrence and is presently disease-free. Transition of typical WDPPM to malignant biphasic mesothelioma was documented in the second patient who died of disease progression following incomplete cytoreduction and HIPEC. Projected 5-year overall and progression-free survival were 90.0% and 79.7%, respectively. The projected progression-free survival after 11 debulking operations carried out in seven patients before referral to our center was 9.1% (SE = 6.1); the difference was statistically significant (P = 0 .0156).
Based on our findings, definitive tumour eradication by means of peritonectomy procedures and HIPEC is recommended as the optimal treatment to prevent either disease recurrence or transition to a truly aggressive tumour[28].
PATHOLOGICAL EVALUATION
The pathologic features of 35 patients with DMPM uniformly treated with CRS and HIPEC at the Milan NCI were assessed[23]. The hematoxylin and eosin-stained slides of all cases were reviewed and tumors were classified as epithelial, sarcomatoid, and biphasic (mixed epithelial and sarcomatoid)[29]. Nuclear grade (NG) was assessed as follows: Grade 1: small nuclei, uniform chromatin pattern, and small pinpoint-sized nucleoli; Grade 2: larger nuclei, some chromatin irregularity, and more prominent nucleoli; and Grade 3: large nuclei, irregular chromatin pattern with clearing, and prominent nucleoli[30]. Immunohistochemical studies using the avidin-biotin-complex immunoperoxidase technique were performed with the following antibodies: matrix metalloproteinase-2 (MMP-2); MMP-9; calretinin; WT-1; carcinoembryonic antigen (CEA); Ber-EP4; p16; and epidermal growth factor receptor (EGFR).
The immunohistochemical results are summarized in Table 2. EGFR was diffusely and strongly expressed in a membranous pattern in all but 2 cases (94%). Conversely, p16 was completely negative in 14 cases (40%) and only focally positive in 11 (31%). MMP-2 was expressed in all cases in a diffuse and strong fashion, whereas MMP-9 was expressed in 30 cases but with only variable intensity and distribution. Calretinin and WT-1 were expressed in all cases to a variable degree. Expression of polyclonal CEA and Ber-EP4 were negative in all cases.
Table 2.
Immunohistochemical results
| Score |
No. of patients (n) |
|||||||
| Calretinin | WT-1 | pCEA | Ber-Ep4 | EGFR | p16 | MMP-2 | MMP-9 | |
| 0 | 0 | 0 | 35 | 35 | 2 | 14 | 0 | 5 |
| +1 | 0 | 5 | 0 | 0 | 1 | 11 | 2 | 9 |
| +2 | 1 | 6 | 0 | 0 | 3 | 6 | 3 | 8 |
| +3 | 6 | 5 | 0 | 0 | 7 | 2 | 7 | 8 |
| +4 | 28 | 19 | 0 | 0 | 22 | 2 | 23 | 5 |
The immunohistochemistry stains were scored as 0 (negative), +1 (< 25%), +2 (25%-50%), +3 (50%-75%), and +4 (75%-100%). pCEA: Pathologic carcinoembryonic antigen; EGFR: Epidermal growth factor receptor; MMP: Matrix metalloproteinase.
In agreement with previous reports, p16 was absent or reduced in most DMPM cases[29]. Alterations of the p16INK4 locus in patients with mesothelioma are relatively common. The recent molecular genetic study of 45 cases of primary mesothelioma revealed alterations of p16 in 31% of cases, promoter methylation in 9%, deletion in 22%, and point mutation in 2%[31]. Similar to many other cancers, DMPM exhibits altered cell-growth regulation involving the loss of pRb and p53 function. Inhibition of the p53-dependent and pRb-dependent growth regulatory pathways may occur through mechanisms involving either homozygous loss of the CDKN2A (p16INK4a/p14ARF) locus at chromosome 9p21 or expression of SV40 Tag[32].
In the current study, 63% of cases demonstrated diffuse and strong immunoreactivity for EGFR, analogously to a previous report[33]. EGFR, a receptor tyrosine kinase, is reportedly over-expressed in a wide variety of malignancies. EGFR signaling leads to an increase in cellular proliferation, cell motility, angiogenesis, the inhibition of apoptosis, and the expression of extracellular matrix proteins. High levels of EGFR expression are associated with a poor prognosis in some malignancies.
Asbestos, which is associated with the development of mesothelioma, was reported to stimulate the EGFR auto-phosphorylation in mesothelial cells, trigger the extracellular-regulated kinase (ERK) cascade, and lead to increases in AP-1 activity[34]. In addition, DMPM cell lines are reported to express EGFR and transforming growth factor-α (TGF-α), suggesting an autocrine role for EGFR in DMPM[35].
Proteolytic degradation of the extracellular matrix and basement membranes by proteases is a key component of tumour cell invasion and metastasis. Over-expression of MMPs, particularly MMP-2 (gelatinase A), MMP-9 (gelatinase B), and MMP-11 (stromelysin-3), is related to tumor progression and metastasis in various malignancies[36,37]. MMP-2 and MMP-9 are key enzymes for degrading Type IV collagen, a major component of basement membranes, and are particularly expressed in mesenchymal-derived tumor cells[38]. To our knowledge, only a few studies have investigated MMP immunohistochemically on surgical specimens of DMPM[39].
BIOLOGIC PROGNOSTIC FACTORS
One of the hallmarks of cancer cells is their limitless replicative potential[40]. In a high percentage of tumors, the attainment of immortality is due to the re-activation of telomerase, an RNA-dependent DNA-polymerase that stabilizes telomeres and allows tumour cells to avoid senescence[41]. Some tumors, however, do not have telomerase activity (TA) and maintain their telomeres by one or more mechanisms referred to as alternative lengthening of telomeres (ALT)[42]. No information is available thus far concerning the presence of telomere maintenance mechanism (TMM) in DMPM[43].
The prevalence and prognostic role of the two known TMM, TA and ALT, were investigated for the first time in a series of patients treated at the Milan NCI. Forty-four lesions from 38 patients undergoing CRS and HIPEC (n = 29) or debulking surgery (n = 9) were available[44]. TA was determined using the telomeric-repeat amplification protocol (TRAP) assay[45] and ALT by detecting ALT-associated promyelocytic leukemia (PML) nuclear bodies (APB). APB are sub-nuclear structures containing telomeric DNA, telomere-specific binding proteins and proteins involved in DNA recombination and replication[46].
Thirty-eight lesions (86.4%) expressed at least one TMM. Specifically, 28 lesions (63.6%) were TA+/ALT-, 8 (18.2%) were TA-/ALT+, and 2 (4.6%) were ALT+/TA+. The remaining 6 lesions (13.6%) did not express any TMM.
After a median follow-up of 38 mo (range 2-94 mo), TA correlated at multivariate analysis to both disease-free [TA+ vs TA-: 10% vs 64%; hazard ratio (HR) = 3.30; 95% Confidence Interval (CI): 1.23-8.86; P = 0.018] and cancer-related survival (TA+ vs TA-: 32% vs 79%; HR = 3.56; 95% CI: 1.03-12.51; P = 0.045). These results were confirmed also for the 29 patients who underwent CRS and HIPEC: patients with TA+ tumours had a significantly lower probability of being disease-free than patients with TA- tumours (HR = 3.32; 95% CI: 1.09-10.12; P = 0.03), and showed a trend toward better overall survival (HR = 3.69; 95% CI: 0.79-17.13; P = 0.09) (Figure 1). ALT failed to significantly affect clinical outcome both in the overall series and in the subset of patients undergoing CRS and HIPEC (Figure 2).
Figure 2.
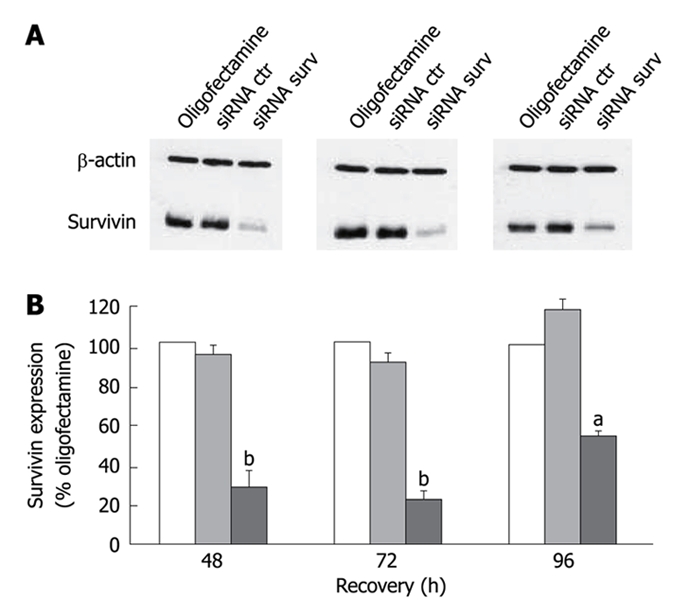
Representative Western blotting experiments illustrating survivin expression in STO cells exposed to oligofectamine alone or transfected with control siRNA and survivin siRNA. A: β-actin was used as a control for protein loading; B: Densitometric quantification of survivin band intensities in oligofectamine-exposed cells (empty column) and cells transfected with the control siRNA (gray column) or the survivin siRNA (black column). Data represent mean ± SD of 3 independent experiments. aP < 0.02; bP < 0.01; Student’s t test; such inhibition was highest (around 80%; P < 0.01) at 48 h and 72 h after transfection and still appreciable, although to a lesser extent (around 50%; P < 0.02), at 96 h.
MOLECULAR THERAPEUTIC TARGETS
Apoptotic cell death is the main mode by which chemical and physical anticancer agents kill tumor cells. Dysregulation of the apoptotic pathways may play a role in the relative chemo-resistance of DMPM, as already demonstrated for pleural mesothelioma[47]. Better understanding of the biological mechanisms underlining the apoptosis-resistant phenotype could result in novel targeted therapeutic strategies. For this purpose, the expression of survivin and other IAP family members, including IAP-1, IAP-2, and X-IAP, were analyzed by immunohistochemistry in surgical specimens of 32 patients with DMPM uniformly treated by CRS and HIPEC at the Milan NCI[48]. The staining characteristics of the pro-apoptotic protein Smac/DIABLO, of the Ki-67 antigen associated with cell proliferation and of the caspase-cleaved cytokeratin 18 associated with apoptosis were also studied.
The results of the immunostaining studies are shown in Table 3. Survivin was expressed in the cytoplasm in 19 DMPM cases (59%), at the nuclear level in 2 DMPM cases (6%) and at both cytoplasmic and nuclear levels in 5 DMPM cases (16%). In the remaining 6 cases, no survivin immunoreactivity was seen. IAP-2 and IAP-1 were expressed in 100% of cases. X-IAP was expressed in 22/32 cases (68.7%) and Smac/DIABLO in 11/32 cases (34.4%). The CK18-caspase cleavage product staining was positive in a median of 0.45% cells and Ki-67 was positive in a median of 10% cells. Caspases are the executioners of apoptosis in both intrinsic and extrinsic pathways[49]. The activated caspases are subject to inhibition by IAPs through direct binding[50]. This inhibitory effect can be abrogated by Smac/DIABLO, a pro-apototic factor released from mitochondria that reactivates initiator and effector caspases, by binding to IAPs and relieving IAP-mediated inhibition[51].
Table 3.
Staining characteristics for IAP family members, Smac/DIABLO, apoptotic and proliferation indices in peritoneal mesothelioma
| Positive cases | Median expression | |
| (n) | (range, %) | |
| Survivin, full length1 | 26 | 60 (0-100) |
| Survivin, specific nuclear form | 7 | 1.5 (0-20) |
| IAP-21 | 32 | 90 (30-100) |
| IAP-1 | 32 | 95 (40-100) |
| X-IAP | 22 | 50 (0-100) |
| Smac/DIABLO | 11 | 5 (0-90) |
| Apoptotic index (CK18-caspase cleavage product) | - | 0.45 (0-5.8) |
| Proliferation index (Ki-67) | - | 10 (0-50) |
1Cytoplasmic/nuclear subcellular distribution.
All 4 IAP family members were simultaneously over-expressed in 16/32 cases, while a lack of expression was consistently found in normal peritoneum, suggesting that these anti-apoptotic proteins are heavily dysregulated in DMPM. Furthermore, an inverse association was found between Smac/DIABLO expression and IAPs co-expression.
Such results provide important insights in DMPM biology. Although cell proliferative index (KI-67) was mostly low, an antigen associated to apoptosis, such as CK-18 caspase cleavage product, was poorly expressed. Furthermore, a concurrent over-expression of apoptosis inhibitor factors and poor expression of pro-apoptotic factors was seen in the majority of cases. This pattern suggests that resistance to programmed cell death may contribute to the chemo-insensitivity of DMPM.
In recent years, considerable efforts have been made to develop strategies for modulating apoptosis in cancer[52]. In this context, approaches to counteract survivin aim to inhibit tumor growth and enhance tumor cell responses to apoptosis-inducing agents[53]. An RNA-interference-based strategy was used to down-regulate survivin expression in a human peritoneal mesothelioma cell line (STO) recently established in our laboratory[54]. Cells were transfected with survivin small interfering RNA (siRNA) or control siRNA. The effects of siRNA-mediated survivin down-regulation was evaluated by enhanced chemoluminescence Western blotting, flow cytometry, fluorescence microscopy and at a molecular level.
Western blotting experiments carried out in cells transfected with survivin-specific siRNA showed a significant reduction of survivin, as compared to cells transfected with control siRNA (Figure 2). Silencing of the survivin gene resulted in a significant and time-dependent decline in cell proliferation.
In cells transfected with survivin siRNA, an apoptotic sub-G0/1 peak was observed by flow cytometry and the presence of cells with an apoptotic nuclear morphology was assessed by fluorescence microscopy. At a molecular level, a significantly increased catalytic activity of caspase-9 was seen.
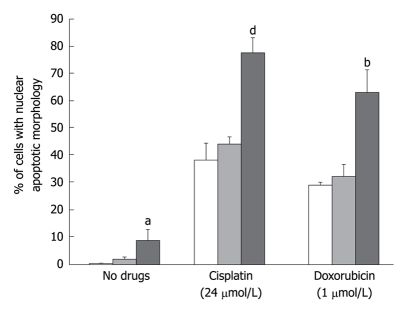
A number of in vitro and in vivo studies indicated that survivin down-regulation was able to sensitize human tumor cells of different histologic origin to conventional chemotherapeutic drugs with distinct mechanisms of action as well as to ionizing radiation[53,55]. To test whether survivin plays a role in the in vitro sensitivity of DMPM cells to anticancer drugs, we examined the effect of survivin down-regulation on the apoptotic response to cisplatin and doxorubicin. Exposure to cisplatin and doxorubicin induced a dose-dependent increase in the percentage of apoptotic cells, which was significantly (P < 0.01 for cisplatin and P < 0.05 for doxorubicin) higher in cells exposed to the survivin siRNA than in those transfected with control siRNA or treated with oligofectamine. A dose-dependent increase in caspase-9 catalytic activity was also observed.
These findings demonstrate that the level of survivin expression influences the in vitro response of DMPM cells to cisplatin and doxorubicin. This has potential clinical implications since it could provide a rational basis for the design of combined therapies, including survivin inhibitors, to improve the responsiveness of DMPM to chemotherapy. However, considering the presence of other anti-apoptotic factors, it is likely that approaches based on the simultaneous targeting of different cytoprotective factors could obtain enhancement of DMPM cell chemo-sensitivity (Figure 3).
Figure 3.
Effects of siRNA-mediated survivin down-regulation on the apoptotic response of STO cells to cisplatin and doxorubicin. The percentage of cells with an apoptotic morphology with respect to the overall population as assessed by fluorescence microscopy in STO cells exposed to oligofectamine alone (empty column) and transfected with control siRNA (gray column) or survivin siRNA (black column) in the absence or presence of different cisplatin or doxorubicin concentrations. Data represent mean ± SD of 3 independent experiments. aP < 0.05; bP < 0.01; dP < 0.001; Student’s t test.
Little is known about receptor tyrosine kinase (RTK) activation in malignant peritoneal mesotheliomas[33,56]. We performed EGFR, PDGFRA and PDGFRB analyses to ascertain if deregulation of RTK could offer useful alternative therapeutic targets in this tumor[57].
EGFR, PDGFRA and PDGFRB expression and phosphorylation were immunohistochemically and biochemically analysed in 15 DMPM cases. The tyrosine kinase domain (exons 18-21) of the EGFR gene were automatically sequenced, as well as the extracellular (exon 10) and juxtamembrane regions (exon 12) and the tyrosine kinase domain (exons 14 and 18) of PDGFRA and PDGFRB. The cognate ligand expression was investigated by real time PCR. Additionally, we explored the status of RTK downstream pathways through mutational and biochemical analysis of the PI3KCA gene (exons 9 and 20)/PTEN/AKT, and ERK, along with mTOR and its effector S6.
Immunohistochemical and immunoprecipitation/Western blotting analyses showed EGFR, PDGFRA and PDGFRB expression and activation in most of the cases. In particular, EGFR and PDGFRA were more frequently phosphorylated than PDGFRB. Autocrine loop activation of these receptors was suggested in all cases by the expression of the related cognate ligands TGF-α, PDGFA and PDGFB, in absence of receptor gain of function mutations. No PI3KCA mutations were found, while all the MPMs showed expression of PTEN and expression/activation of AKT, ERK, as well as of mTOR and S6. These data suggest that EGFR, PDGFRA and PDGFRB seem to be promising molecular targets for tailored treatments in MPM. Furthermore, strong activation of downstream signalling points out a role of mTOR inhibitors or analogous in MPM treatment.
Footnotes
Peer reviewers: Stephen Randolph Grobmyer, MD, Assistant Professor, Division of Surgical Oncology, Department of Surgery, University of Florida, 1600 SW Archer Rd., PO Box 100286, Gainesville, FL 32610, United States; Maria Gazouli, PhD, Department of Biology, School of Medicine, University of Athens, Michalakopoulou 176, Athens 11527, Greece
S- Editor Li LF L- Editor Lutze M E- Editor Lin YP
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 3.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145:211–218. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 1992;118:547–550. doi: 10.1007/BF01225271. [DOI] [PubMed] [Google Scholar]
- 5.Neumann V, Müller KM, Fischer M. [Peritoneal mesothelioma--incidence and etiology] Pathologe. 1999;20:169–176. doi: 10.1007/s002920050340. [DOI] [PubMed] [Google Scholar]
- 6.Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Intengen ME. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70:6–12. doi: 10.1002/(sici)1096-9098(199901)70:1<6::aid-jso2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Antman KH, Blum RH, Greenberger JS, Flowerdew G, Skarin AT, Canellos GP. Multimodality therapy for malignant mesothelioma based on a study of natural history. Am J Med. 1980;68:356–362. doi: 10.1016/0002-9343(80)90103-5. [DOI] [PubMed] [Google Scholar]
- 8.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67:999–1003. [PubMed] [Google Scholar]
- 9.Sugarbaker PH, Welch LS, Mohamed F, Glehen O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am. 2003;12:605–621, xi. doi: 10.1016/s1055-3207(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 10.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 11.Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, Glehen O. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13:405–412. doi: 10.1245/ASO.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Yan TD, Brun EA, Cerruto CA, Haveric N, Chang D, Sugarbaker PH. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2007;14:41–49. doi: 10.1245/s10434-006-9169-7. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Bedard V, Bouzid T, Duvillard P, Kohneh-Sharhi N, Raynard B, Goere D. Malignant peritoneal mesothelioma: treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2007;31:784–788. doi: 10.1016/s0399-8320(07)73964-7. [DOI] [PubMed] [Google Scholar]
- 14.Borczuk AC, Taub RN, Hesdorffer M, Hibshoosh H, Chabot JA, Keohan ML, Alsberry R, Alexis D, Powell CA. P16 loss and mitotic activity predict poor survival in patients with peritoneal malignant mesothelioma. Clin Cancer Res. 2005;11:3303–3308. doi: 10.1158/1078-0432.CCR-04-1884. [DOI] [PubMed] [Google Scholar]
- 15.Husain AN, Colby TV, Ordóñez NG, Krausz T, Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs AR, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 16.King J, Thatcher N, Pickering C, Hasleton P. Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: a systematic review of published reports. Histopathology. 2006;49:561–568. doi: 10.1111/j.1365-2559.2006.02442.x. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi CR, Foletto M, Mocellin S, Pilati P, De SM, Deraco M, Cavaliere F, Palatini P, Guasti F, Scalerta R, et al. Hyperthermic intraoperative intraperitoneal chemotherapy with cisplatin and doxorubicin in patients who undergo cytoreductive surgery for peritoneal carcinomatosis and sarcomatosis: phase I study. Cancer. 2002;94:492–499. doi: 10.1002/cncr.10176. [DOI] [PubMed] [Google Scholar]
- 19.Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987;47:5875–5879. [PubMed] [Google Scholar]
- 20.Roboz J, Jacobs AJ, Holland JF, Deppe G, Cohen CJ. Intraperitoneal infusion of doxorubicin in the treatment of gynecologic carcinomas. Med Pediatr Oncol. 1981;9:245–250. doi: 10.1002/mpo.2950090307. [DOI] [PubMed] [Google Scholar]
- 21.Ozols RF, Young RC, Speyer JL, Sugarbaker PH, Greene R, Jenkins J, Myers CE. Phase I and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res. 1982;42:4265–4269. [PubMed] [Google Scholar]
- 22.Deraco M, Casali P, Inglese MG, Baratti D, Pennacchioli E, Bertulli R, Kusamura S. Peritoneal mesothelioma treated by induction chemotherapy, cytoreductive surgery, and intraperitoneal hyperthermic perfusion. J Surg Oncol. 2003;83:147–153. doi: 10.1002/jso.10255. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka D, Kusamura S, Baratti D, Casali P, Cabras AD, Younan R, Rosai J, Deraco M. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer. 2005;104:2181–2188. doi: 10.1002/cncr.21239. [DOI] [PubMed] [Google Scholar]
- 24.Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, Salvatore A, Cabras Ad AD, Kusamura S. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13:229–237. doi: 10.1245/ASO.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Baratti D, Kusamura S, Cabras AD, Dileo P, Laterza B, Deraco M. Diffuse malignant peritoneal mesothelioma: Failure analysis following cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2009;16:463–472. doi: 10.1245/s10434-008-0219-1. [DOI] [PubMed] [Google Scholar]
- 26.Kerrigan SA, Turnnir RT, Clement PB, Young RH, Churg A. Diffuse malignant epithelial mesotheliomas of the peritoneum in women: a clinicopathologic study of 25 patients. Cancer. 2002;94:378–385. doi: 10.1002/cncr.10209. [DOI] [PubMed] [Google Scholar]
- 27.Sethna K, Mohamed F, Marchettini P, Elias D, Sugarbaker PH. Peritoneal cystic mesothelioma: a case series. Tumori. 2003;89:31–35. doi: 10.1177/030089160308900107. [DOI] [PubMed] [Google Scholar]
- 28.Baratti D, Kusamura S, Nonaka D, Oliva GD, Laterza B, Deraco M. Multicystic and well-differentiated papillary peritoneal mesothelioma treated by surgical cytoreduction and hyperthermic intra-peritoneal chemotherapy (HIPEC) Ann Surg Oncol. 2007;14:2790–2797. doi: 10.1245/s10434-007-9475-8. [DOI] [PubMed] [Google Scholar]
- 29.Weiss SW. World Health Organization, International Histological Classification of Tumours. Histological typing of soft tissue tumours. 2nd edition. Berlin: Springer-Verlag; 1994. [Google Scholar]
- 30.Goldblum J, Hart WR. Localized and diffuse mesotheliomas of the genital tract and peritoneum in women. A clinicopathologic study of nineteen true mesothelial neoplasms, other than adenomatoid tumors, multicystic mesotheliomas, and localized fibrous tumors. Am J Surg Pathol. 1995;19:1124–1137. doi: 10.1097/00000478-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hirao T, Bueno R, Chen CJ, Gordon GJ, Heilig E, Kelsey KT. Alterations of the p16(INK4) locus in human malignant mesothelial tumors. Carcinogenesis. 2002;23:1127–1130. doi: 10.1093/carcin/23.7.1127. [DOI] [PubMed] [Google Scholar]
- 32.Testa JR, Giordano A. SV40 and cell cycle perturbations in malignant mesothelioma. Semin Cancer Biol. 2001;11:31–38. doi: 10.1006/scbi.2000.0344. [DOI] [PubMed] [Google Scholar]
- 33.Trupiano JK, Geisinger KR, Willingham MC, Manders P, Zbieranski N, Case D, Levine EA. Diffuse malignant mesothelioma of the peritoneum and pleura, analysis of markers. Mod Pathol. 2004;17:476–481. doi: 10.1038/modpathol.3800067. [DOI] [PubMed] [Google Scholar]
- 34.Robledo R, Mossman B. Cellular and molecular mechanisms of asbestos-induced fibrosis. J Cell Physiol. 1999;180:158–166. doi: 10.1002/(SICI)1097-4652(199908)180:2<158::AID-JCP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Jänne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res. 2002;62:5242–5247. [PubMed] [Google Scholar]
- 36.Karakiulakis G, Papanikolaou C, Jankovic SM, Aletras A, Papakonstantinou E, Vretou E, Mirtsou-Fidani V. Increased type IV collagen-degrading activity in metastases originating from primary tumors of the human colon. Invasion Metastasis. 1997;17:158–168. [PubMed] [Google Scholar]
- 37.Cox G, Jones JL, O'Byrne KJ. Matrix metalloproteinase 9 and the epidermal growth factor signal pathway in operable non-small cell lung cancer. Clin Cancer Res. 2000;6:2349–2355. [PubMed] [Google Scholar]
- 38.Sato H, Kida Y, Mai M, Endo Y, Sasaki T, Tanaka J, Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992;7:77–83. [PubMed] [Google Scholar]
- 39.Lumb PD, Suvarna SK. Metastasis in pleural mesothelioma. Immunohistochemical markers for disseminated disease. Histopathology. 2004;44:345–352. doi: 10.1111/j.1365-2559.2004.01844.x. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 42.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 43.Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10:399–406. doi: 10.1006/scbi.2000.0339. [DOI] [PubMed] [Google Scholar]
- 44.Villa R, Daidone MG, Motta R, Venturini L, De Marco C, Vannelli A, Kusamura S, Baratti D, Deraco M, Costa A, et al. Multiple mechanisms of telomere maintenance exist and differentially affect clinical outcome in diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2008;14:4134–4140. doi: 10.1158/1078-0432.CCR-08-0099. [DOI] [PubMed] [Google Scholar]
- 45.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 46.Yeager TR, Neumann AA, Englezou A, Huschtscha LI, Noble JR, Reddel RR. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 1999;59:4175–4179. [PubMed] [Google Scholar]
- 47.Xia C, Xu Z, Yuan X, Uematsu K, You L, Li K, Li L, McCormick F, Jablons DM. Induction of apoptosis in mesothelioma cells by antisurvivin oligonucleotides. Mol Cancer Ther. 2002;1:687–694. [PubMed] [Google Scholar]
- 48.Zaffaroni N, Costa A, Pennati M, De Marco C, Affini E, Madeo M, Erdas R, Cabras A, Kusamura S, Baratti D, et al. Survivin is highly expressed and promotes cell survival in malignant peritoneal mesothelioma. Cell Oncol. 2007;29:453–466. doi: 10.1155/2007/456839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 51.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 52.Fischer U, Schulze-Osthoff K. New approaches and therapeutics targeting apoptosis in disease. Pharmacol Rev. 2005;57:187–215. doi: 10.1124/pr.57.2.6. [DOI] [PubMed] [Google Scholar]
- 53.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 54.Zaffaroni N, Costa A, Pennati M, Daidone MG. Potential of survivin as a new therapeutic target in diffuse malignant mesothelioma of the peritoneum. Abstract n° 4613 presented at the 97th AACR Annual Meeting. 2006 Washington, United States, 2006 April 1-5. [Google Scholar]
- 55.Pennati M, Binda M, Colella G, Folini M, Citti L, Villa R, Daidone MG, Zaffaroni N. Radiosensitization of human melanoma cells by ribozyme-mediated inhibition of survivin expression. J Invest Dermatol. 2003;120:648–654. doi: 10.1046/j.1523-1747.2003.12082.x. [DOI] [PubMed] [Google Scholar]
- 56.Foster JM, Gatalica Z, Lilleberg S, Haynatzki G, Loggie BW. Novel and existing mutations in the tyrosine kinase domain of the epidermal growth factor receptor are predictors of optimal resectability in malignant peritoneal mesothelioma. Ann Surg Oncol. 2009;16:152–158. doi: 10.1245/s10434-008-0206-6. [DOI] [PubMed] [Google Scholar]
- 57.Perrone F, Jocollè G, Brich S, Cabras AD, Deraco M, Baratti D, Pilotti S. Analysis of EGFR, PDGFRA, PDGFRB and related pathways in malignant peritoneal mesothelioma. The 9th International Conference of the International Mesothelioma Interest Group [Abstract 217] Published on the Conference abstract Book: Amsterdam. The Netherlands; 2008. pp. September 25–27. [Google Scholar]