Abstract
A balance between survival and apoptotic signals regulates B cell development. These signals are tightly regulated by a host of molecules, including IL-7. Abnormal signaling events may lead to neoplastic transformation of progenitor B cells. Signal transduction inhibitors potentially may modulate these abnormal signals. Inhibitors of the mammalian target of rapamycin (mTOR) such as rapamycin have been used as immunosuppressive agents. We hypothesized that rapamycin might demonstrate activity against B-precursor acute lymphoblastic leukemia. We have found that rapamycin inhibited growth of B-precursor acute lymphoblastic leukemia lines in vitro, with evidence of apoptotic cell death. This growth inhibition was reversible by IL-7. One candidate as a signaling intermediate cross-regulated by rapamycin and IL-7 was p70 S6 kinase. Rapamycin also demonstrated in vivo activity in Eμ-ret transgenic mice, which develop pre-B leukemia/lymphoma: Eμ-ret transgenic mice with advanced disease treated daily with rapamycin as a single agent showed a >2-fold increase in length of survival as compared with symptomatic littermates who received vehicle alone. These results suggest that mammalian target of rapamycin inhibitors may be effective agents against leukemia and that one of the growth signals inhibited by this class of drugs in precursor B leukemic cells may be IL-7-mediated.
Keywords: acute lymphoblastic leukemia, mTOR, rapamycin, IL-7
Many childhood malignancies are lymphoid in origin and arise from transforming events that occur in early B cell progenitors (1). Understanding normal B cell development affords the opportunity to learn how the transformation process subverts normal B cell signaling mechanisms and then to use this information to design and select signal transduction inhibitors. In normal B cell development, rearrangement of the Ig heavy chain gene occurs during pro-B cell stages (reviewed in ref. 2). Pre-B cells are identified by the expression of cytoplasmic μ protein and assembly of the pre-B cell receptor complex (3). Survival, apoptotic, and differentiation signals, provided by a host of molecules, such as the pre-B cell receptor complex (4), adhesion molecule receptors, and cytokine receptors (5), are tightly regulated to maintain B-lymphocyte homeostasis. An imbalance in these signals can lead to malignant transformation. The B cell at the late pro-B-early pre-B transition is a common target of transformation. In the clinical setting, acute lymphoblastic leukemia (ALL) cells derived from early B lineage cells are loosely referred to as “B-precursor ALL” (pre-B-ALL), although the majority of these cells are defined more correctly as pro-B cells with no cytoplasmic μ protein expression. Pre-B-ALL is the most common form of childhood leukemia.
Transgenic mouse models have been useful tools for studying leukemia and lymphoma in vivo (1, 6). The RET protein is a tyrosine kinase expressed during the development of pro-B cells, and RET expression is down-regulated when cytoplasmic μ protein is expressed during the pre-B cell stages of B cell development (7). Eμ-ret transgenic mice express activated RET tyrosine kinase constitutively under the control of the Eμ (μ enhancer), driving B lineage-restricted expression of the activated RET protein. Between 3 and 8 months of life, Eμ-ret transgenic mice develop ALL, manifested by massive adenopathy, splenomegaly, and bone marrow replacement (8-11). The malignant cells are B220+, CD43lo, and surface IgM-, and the majority are cytoplasmic μ- (J.F., unpublished data). Thus, the B lymphoid malignancies that arise in Eμ-ret mice are derived from the late pro-B to early pre-B cell stage of development (12). The Eμ-ret transgenic mouse provides a developmentally targeted model of ALL that is useful in the preclinical evaluation of novel therapeutic strategies.
Cytokines play an important role in promoting and controlling normal B cell development (13, 14) and are involved in malignant transformation of lymphoid precursor cells (15). One such cytokine is IL-7, an important regulator of T and B cell development. IL-7 is absolutely required for normal murine T and B cell development as well as human T cell development (16). Although not absolutely required, IL-7 plays a vital role in human B cell development (17, 18). It provides a survival signal to early B lymphoid precursors (19). When IL-7 engages the IL-7 receptor (IL-7R) on pro-B cells, IL-7R recruits intracellular kinases, resulting in cellular proliferation (20-24). In addition to its role in normal lymphoid development, IL-7 has been associated with certain malignancies (25-28). IL-7 may be associated with Hodgkin's disease (29), Epstein-Barr virus-positive Burkitt's lymphoma (30), and possibly T cell ALL, modulating cell cycle regulators such as p27kip1, cyclins D2 and A, CDK4, and CDK2 (27, 28). Two groups have reported that IL-7 rescues T cell ALL lymphoblasts from apoptosis (25, 26). Although there are several reports of IL-7 stimulating growth of human precursor B cell ALL cells, the role of IL-7 in the development or progression of progenitor B cell lymphoid malignancies is unclear (31).
Rapamycin (sirolimus, Rapamune) is a naturally occurring immunosuppressive macrocyclic lactone that is structurally related to but biochemically distinct from FK506 (tacrolimus, Prograf) (32, 33). Rapamycin inhibits the induction of activation and proliferation of mature T and B cells and is used as an immunosuppressive agent after solid organ transplant (34-38). Also, rapamycin has demonstrated antitumor properties (38-40). There is evidence that mammalian target of rapamycin (mTOR) inhibitors may inhibit the growth of and/or induce apoptosis in a wide variety of tumor types (41-45). Rapamycin inhibits the activation of the mTOR. mTOR is a key regulator of cell growth, protein synthesis, and progression through the cell cycle (46-50). One well described signaling intermediate in the mTOR pathway is p70 S6 kinase (51, 52). By inhibiting mTOR, rapamycin mimics growth-factor withdrawal, characterized by inhibition of protein synthesis and inhibition of cell cycle progression at the G1-S transition (53, 54).
Although not as well studied as in T cells, rapamycin has growth-inhibitory effects in B cells in vitro (33, 35). Rapamycin inhibits secretion of soluble CD23, an autocrine B cell growth factor (55). Crosslinking of B cell receptor leads to p70 S6 kinase activation, triggering protein synthesis by activation of ribosomal proteins (56). These data suggest that rapamycin may affect early B lineage cells, but an effect of mTOR inhibitors and IL-7-mediated signaling on early precursor B cells or on B cell progenitor malignancies has not been described.
In this report, we describe our experiments in which we evaluated the efficacy of rapamycin against progenitor B cell malignancies and the role of IL-7 in response to this mTOR inhibitor by using the Eμ-ret transgenic mouse model. We found that (i) rapamycin caused complete growth inhibition of ALL cell lines in vitro;(ii) symptomatic Eμ-ret transgenic mice treated daily with rapamycin as a single agent showed diminished nodal masses, normalized peripheral white count, and prolonged survival; and (iii) IL-7 reversed this rapamycin-induced inhibition in vitro. The data described here suggest that rapamycin is active in pre-B-ALL and that the mechanism of action of this mTOR inhibitor in malignant progenitor B cells may be controlled by IL-7-mediated signaling.
Materials and Methods
Cell Preparation and Cell Culture Conditions. A single-cell suspension of bone marrow or lymph nodes from leukemic Eμ-ret transgenic mice was prepared (10). These ALL cells were maintained at 37°C under a 5% CO2/95% air atmosphere in RPMI medium 1640, containing l-glutamine, 10 mM Hepes (pH 7.5), 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin (GIBCO/BRL), and 50 μM 2-mercaptoethanol (Fisher Scientific), in addition to C20 medium [10% fetal calf serum/10% calf serum (HyClone)], with 10 units/ml IL-7. Cell-surface markers on these cells were determined by flow cytometry as described (10), and the phenotype was stabilized in culture over time.
Proliferation Assays. Cells were cultured in IL-7-free C20 medium for 24 h. For in vitro culture studies, 1-2 × 104 cells per well were cultured in triplicate in flat-bottom 96-well plates with increasing concentrations of rapamycin (Calbiochem) (0-100 ng/ml) and recombinant mouse IL-7 (Leinco Technologies, St. Louis) (0-30 units/ml) for 3-5 days. Cell growth was assayed by using methylthiazoletetrazolium (MTT; Sigma) as described (57). Absorbance was measured at 595 nm by using a Benchmark microplate spectrophotometer (Bio-Rad). Results are expressed as the mean of absolute absorbance of the treated sample, divided by the mean of absolute absorbance of the control sample. Results >1 indicate proliferation, whereas results <1 indicate growth inhibition.
Apoptosis Assay. Cells (0.5-1 × 105 per ml) were plated in C20 medium with rapamycin and incubated for 3 days. Levels of exposed phosphatidylserine on viable cells were measured by using the ApoAlert annexin V detection kit (Clontech) as described (58). Cells were incubated with FITC-conjugated annexin V, and fluorescence intensity was analyzed by flow cytometry in a FACScan cytometer (Becton Dickinson).
Crude Lysate Preparation and Immunoblotting. Cells (5 × 106) were incubated in C20 medium with or without 10 units/ml IL-7, 100 ng/ml rapamycin, or 10 units/ml IL-7 plus 100 ng/ml rapamycin for 4 h. After harvesting, cells were washed with ice-cold PBS and then lysed in 100 μl of 1% Triton X-100 containing protease and phosphatase inhibitors [1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM Na3VO4, 1 mM NaF, and 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride] at 4°C for 5 min. Crude lysates were obtained by centrifugation to pellet nuclei. Protein concentrations were determined by using a Bio-Rad protein assay kit. Equal amounts of protein were separated by electrophoresis on NuPAGE 4-12% gradient gels and transferred to poly(vinylidene difluoride) membranes (Invitrogen). Specific proteins were detected with anti-phospho-p70 S6 kinase (Thr-389) and anti-total p70 S6 kinase antibodies (Cell Signaling Technologies, Beverly, MA). Immunodetection was performed with horseradish peroxidase-conjugated anti-rabbit IgG and then developed by chemiluminescence (Amersham Biosciences). Immunoblots were stripped for reprobing by incubating in stripping buffer (100 mM 2-mercaptoethanol/2% SDS/62.5 mM Tris·HCl, pH 6.7) for 30 min in 37°C water bath with occasional agitation.
Transgenic Mouse Studies. Eμ-ret transgenic mice express the activated RFP/RET fusion gene under the control of the IgH enhancer (Eμ-ret). Eμ-ret transgenic mice were generated on a C57BL/6 × DBA2 background, and Eμ-ret mice were then bred into BALB/c background as described (10). Mice were assessed three times per week for overt signs of disease (easily palpable adenopathy and splenomegaly). When symptomatic, Eμ-ret mice were randomized to daily i.p. treatment with rapamycin (Wyeth Ayerst Laboratories, Marietta, PA) or the drug vehicle alone (control). Before randomization, easily accessible enlarged lymph nodes were biopsied for cell culture and further in vitro study. Peripheral blood for complete blood count with differential (HemaVet 850FS, CDC Technologies, Oxford, CT) was obtained by eye bleeding at days 0 and 7, and then every 7-14 days until death. Peripheral blood as well as single-cell suspension from bone marrow, lymph nodes, and/or spleen were obtained at death. Weights were monitored three times per week. Rapamycin was administered i.p. at 5 mg/kg daily. Animals were killed when they appeared ill. Event-free survival was determined from the onset of disease until death and analyzed by using stata, Version 7.0 (Stata, College Park, TX).
Results
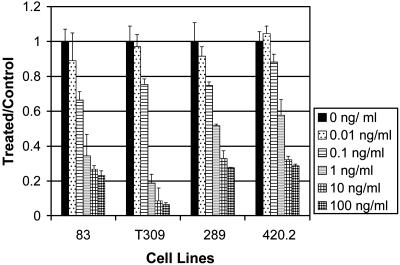
Rapamycin Inhibited Proliferation of ALL Cell Lines. To evaluate the mTOR inhibitor rapamycin in models of progenitor B cell malignancies, we first investigated the effect of rapamycin on ALL cell lines in vitro. The Eμ-ret transgenic mouse-derived cell lines 83, T309, 289, and 420.2 were cultured with 1 unit/ml IL-7 and increasing concentrations of rapamycin (0-100 ng/ml). Cell proliferation was assessed by using MTT. Fig. 1 shows profound inhibition of the growth of ALL lines in culture with various concentrations (ng/ml) of rapamycin. At 1 ng/ml, there was >40% inhibition of growth in all cell lines tested. There was a >70% inhibition of growth at 100 ng/ml. These results were obtained after 3 days of culture, and they allowed for differentiation of the effect of 1 ng/ml and 100 ng/ml rapamycin. By 4-5 days of culture, all of the cells treated (even with the lowest concentration of rapamycin) were dead (data not shown). By comparison, typical serum levels of patients on rapamycin are 8-12 ng/ml, showing that this effect is pharmacologically relevant (59). Rapamycin was tested in several murine and human T and B cell leukemia lines, including Jurkat, BJAB, J558L, Nalm 6, and Nalm 16, and had similar growth-inhibitory effects in each cell line tested (V.I.B., unpublished data). In contrast, the proliferation of the neuroblastoma cell line SKNAS was unaffected by rapamycin at doses up to 100 ng/ml (data not shown).
Fig. 1.
Rapamycin inhibits proliferation of ALL cells. Eμ-ret transgenic mouse-derived cell lines 83, T309, 289, and 420.2 were cultured with 1 unit/ml IL-7 and increasing concentrations of rapamycin (0-100 ng/ml). After 3 days of incubation, cell proliferation was assessed by using MTT. All measurements were performed in triplicate. Bars represent mean of the treated and control mice (relative absorbance of triplicate cultures, as described in Materials and Methods); error bars represent SEM.
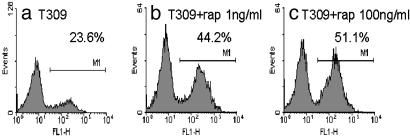
Rapamycin Induced Apoptosis. To determine whether the rapamycin-sensitive cells undergo apoptosis, we treated the pro-B ALL cell line T309 with increasing concentrations of rapamycin for 3 days and then labeled the cells with FITC-conjugated annexin V. The results of these annexin V assays, illustrated in Fig. 2, show that rapamycin induced apoptosis in this ALL cell line. At baseline, 24% of T309 cells were labeled with annexin V, increasing to 44% after treatment with 1 ng/ml rapamycin and to 51% after treatment with 100 ng/ml rapamycin. Characteristic apoptotic DNA laddering also was evident in cell line T309 after treatment with rapamycin in a dose-dependent manner (data not shown). Similar results were seen in the other Eμ-ret transgenic mouse-derived ALL cell lines. These results suggest that at least some of the inhibitory effect of rapamycin is due to induction of apoptosis.
Fig. 2.
Induction of apoptosis by rapamycin in ALL cell line T309. Eμ-ret transgenic mouse-derived pro-B cell line T309 was treated with increasing concentrations of rapamycin (rap) for 3 days. Cells were assessed for apoptotic response by labeling with FITC-conjugated annexin V. Percentages of apoptotic cells are indicated. Flow cytometric histograms show untreated (a), 1 ng/ml rapamycin (b), and 100 ng/ml rapamycin (c). Peak on the right in a-c represents FITC-conjugated annexin V-positive cells.
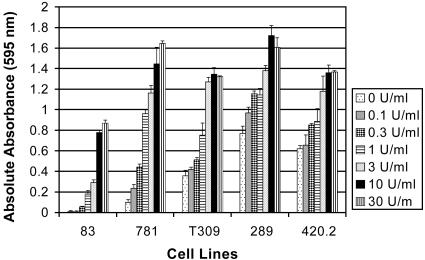
IL-7 Stimulated Proliferation of ALL Cell Lines. We then explored the effect of IL-7 signaling in this system. As shown in Fig. 3, cell lines derived from Eμ-ret transgenic mice were responsive to IL-7. Although all of the cell lines tested were responsive to IL-7, some cells grew in the absence of IL-7, whereas others were IL-7-dependent. Cell lines 83, 781, and T309 were IL-7-dependent, whereas cell lines 289 and 420.2 were responsive to but not dependent on IL-7 (i.e., cells grew in the absence of IL-7 but had increased proliferation when exposed to IL-7). A20, a mature B cell line, showed no response to IL-7 (data not shown). Cell proliferation increased with increasing concentrations of IL-7, ranging from a 2- to 4-fold increase in IL-7-independent cell lines to a 16- to 100-fold increase in IL-7-dependent cell lines. The response to IL-7 plateaued between 10 and 30 units/ml IL-7.
Fig. 3.
IL-7-stimulated proliferation of ALL cell lines in the absence of stroma. Cell lines 83, 781, T309, 289, and 420.2 were cultured with increasing concentrations of IL-7 (0-30 units/ml). After 4-5 days of incubation, cell proliferation was assessed by using MTT. All measurements were performed in triplicate. Bars represent mean of the treated and control mice (absolute absorbance of triplicate cultures, as described in Materials and Methods); error bars represent SEM.
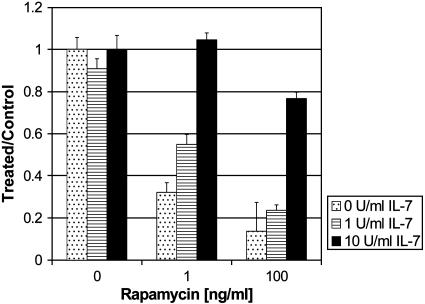
IL-7 Reversed the Inhibitory Effect of Rapamycin. A stroma-independent response to IL-7 is one of the earliest changes noted in pro-B cells from Eμ-ret transgenic mice, an effect that is seen even in fetal development, long before malignant transformation of the cells (J.F., unpublished data). This augmented IL-7 response may provide a “first hit” that may be analogous to the first hit seen in the X-linked severe combined immunodeficiency gene therapy experience (60). Because of these data and the importance of IL-7 in lymphoid development, we hypothesized that IL-7 might antagonize the inhibitory effect of rapamycin. We cultured cells with increasing concentrations of rapamycin (0-100 ng/ml) and IL-7 (0-10 units/ml) for 3 days and then assayed for proliferation. Fig. 4 shows that treatment with IL-7 reversed the rapamycin-induced growth inhibition of cell line 289 in a dose-dependent manner. In the presence of a low dose of rapamycin (1 ng/ml), IL-7 at 10 units/ml reversed rapamycin-induced growth inhibition completely. In the presence of 100 ng/ml rapamycin, IL-7 at 10 units/ml reversed the effect of rapamycin on cell growth only partially to ≈80% of baseline. This reversal was seen both in IL-7-independent/IL-7-responsive and IL-7-dependent/IL-7-responsive cell lines, as well as in cell lines that did not exhibit a response to IL-7 treatment in the absence of concurrent treatment with rapamycin. This reversal effect was not dependent on coadministration of IL-7: growth inhibition of cell lines T309 and 289, pretreated with rapamycin for 24 h, was still reversible with IL-7 (data not shown). Similar results were seen also in human ALL cell lines (V.I.B., unpublished data). These data suggest that this cytokine pathway may be targeted by mTOR inhibitors and that the IL-7 pathway may be a potential therapeutic target for signal transduction inhibitors.
Fig. 4.
IL-7 rescue of rapamycin-induced growth inhibition of ALL cells. Cells were cultured with increasing concentrations of rapamycin (0, 1, or 100 ng/ml) and IL-7 (0, 1, or 10 units/ml) for 3 days. Cell proliferation was assessed by using MTT. All measurements were performed in triplicate. Bars represent mean of the treated and control mice (relative absorbance of triplicate cultures, as described in Materials and Methods); error bars represent SEM.
IL-7 Restored the Rapamycin-Induced Dephosphorylation of p70 S6 Kinase. To study the mechanism of the ability of IL-7 to reverse the inhibitory effect of rapamycin on ALL cells, we used immunoblotting of cell lysates to detect proteins and phosphoproteins downstream of mTOR and the IL-7R. To determine signaling intermediates potentially cross-regulated by rapamycin and IL-7, we detected phospho-p70 S6 kinase (Thr-389) and p70 S6 kinase total protein levels in the ALL cell line 289 after treatment with rapamycin with or without IL-7. After treatment for 4 h with rapamycin, this cell line showed a profound decrease in phospho-p70 S6 kinase. This effect was reversed by IL-7 (Fig. 5 Upper). As expected, p70 S6 kinase total protein levels were unchanged by treatment with rapamycin, IL-7, or the combination of both (Fig. 5 Lower).
Fig. 5.
Effect of rapamycin and IL-7 on phospho-p70 S6 kinase (Thr-389). Line 289 ALL cells (5 × 106) were cultured with 10 units/ml IL-7 and 100 ng/ml rapamycin (Rap) for 4 h. (Upper) Immunoblot of phospho-p70 S6 kinase (Thr-389). (Lower) Total p70 S6 kinase protein from the immunoblot in Upper. Each lane was loaded with 100 μg of protein.
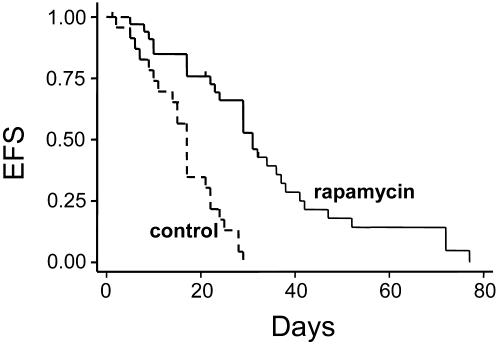
Single Agent Rapamycin Extended Survival and Normalized Elevated Peripheral White Blood Cell Counts (WBCs) in Leukemic Mice with Advanced Disease. To study the effect of rapamycin in vivo,Eμ-ret transgenic mice were treated daily with rapamycin when they manifested signs of advanced disease, including enlarged lymph nodes, hepatosplenomegaly, and elevated WBC. Rapamycin extended survival in rapamycin-treated Eμ-ret transgenic mice with advanced disease compared with control littermates, as shown in the Kaplan-Meier curve in Fig. 6. The average time from diagnosis to death was 15 days for control mice (range 1-29 days; n = 23) vs. 32 days for rapamycin-treated mice (range 5-77 days; n = 34) (P < 0.00001).
Fig. 6.
Prolonged survival in mice with advanced ALL receiving rapamycin as a single agent. Eμ-ret transgenic mice with overt disease were treated daily i.p. with rapamycin (5 mg/kg per dose), as described in Materials and Methods. This Kaplan-Meier analysis of event-free survival (EFS) shows the fraction of animals surviving after the onset of disease. Rapamycin doubled the mean survival time of leukemic mice. The average time from diagnosis to death was 32 days in treated mice (n = 34) and 15 days in untreated mice (n = 23) (P < 0.00001).
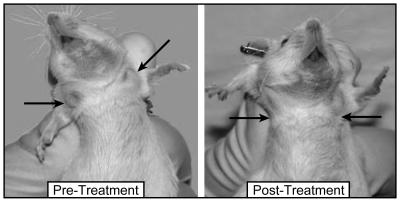
We monitored complete blood counts and weight in these mice. Peripheral complete blood counts were conducted at 0 and 7 days and then every 7-14 days, and weights were monitored three times per week for the duration of the experiment. In addition to extending survival in Eμ-ret transgenic mice, rapamycin normalized the peripheral WBC. In the control group, WBC increased progressively until the animals were killed, whereas WBC in the rapamycin-treated group normalized within 7-10 days of instituting therapy (Table 1). However, by the time of death, the mean WBC of the rapamycin-treated mice was above normal (mean 32.5; range 2.5-164). The mean hemoglobin and platelet counts of the rapamycin-treated group, compared with the mean hemoglobin and platelet counts of the control group, were not significantly different (Table 1). The weights of the treated vs. control groups also were not significantly different. Thus, rapamycin in these mice was well tolerated, reducing tumor burden without causing significant anemia and/or thrombocytopenia. The positive response to rapamycin was clinically apparent also with rapid decreases in nodal masses (Fig. 7). The mouse shown in Fig. 7 represents the typical course seen with mice treated with rapamycin. This mouse began treatment with a WBC of 28.8, which reached a nadir of 3.2 after 14 days of rapamycin treatment.
Table 1. Mean blood counts and weight of control vs. rapamycin-treated mice over time.
| Day 0
|
Day 7
|
Days 14-21
|
Death
|
|||||
|---|---|---|---|---|---|---|---|---|
| Measurement | Control | Treated | Control | Treated | Control | Treated | Control | Treated |
| WBC, × 103 Cells per μl | 48.2 (4.7–200) | 43.1 (2.3–408) | 63.3 (2.2–328) | 18.1 (2.9–200) | 60 (8.3–138) | 15.3 (4.0–65.3) | 67 (8.3–328) | 32.5 (2.5–164) |
| Hgb, g/dl | 12.7 (9.8–16.5) | 12.3 (8.7–15.9) | 11.8 (7.2–17.1) | 12.3 (7.3–15.8) | 10.8 (8.5–12.7) | 12.3 (7.6–16.1) | 10.7 (6.5–15.5) | 12.4 (8.4–16.6) |
| Plt, × 103 Cells per μl | 631 (219–1,203) | 666 (229–1,344) | 652 (156–1,600) | 556 (212–1,663) | 483 (137–795) | 563 (171–881) | 488 (135–768) | 584 (147–1,018) |
| Weight (g) | 29.1 ± 3.4 | 29.14 ± 5.1 | 29.5 ± 3.4 | 28.9 ± 4.3 | 30.1 ± 5.1 | 29.3 ± 3.6 | 29.9 ± 4.5 | 29.4 ± 4.6 |
Ranges of values are given in parentheses. Normal ranges in mice (determined by Cell Technologies and based on ref. 73) are as follows: WBC, 1.8–10.7; Hgb, 11.0–15.1; Plt, 592–972. Weight is given as mean ± SD. Hgb, hemoglobin content; Plt, platelet count.
Fig. 7.
The mTOR inhibitor rapamycin reduces leukemic adenopathy significantly. (Left) Leukemic Eμ-ret transgenic mouse with overt disease. This mouse has significant cervical and axillary adenopathy, as indicated by arrows. (Right) The same mouse 14 days post treatment with rapamycin. The mouse shows significant reduction in visible tumor burden, as indicated by arrows.
Discussion
Leukemia is the most common childhood malignancy. The majority of pediatric ALL arises from transforming events that occur in early precursor B lineage cells. Although the prognosis for pediatric ALL has improved dramatically over the past two decades, the prognosis for refractory and relapsed ALL remains poor (61, 62). Thus, newer, targeted agents need to be identified and integrated into the present cytotoxic chemotherapy regimens. Signal transduction inhibitors show promise in this regard, with STI-571 (imatinib mesylate, Gleevec) as an example. Using the Eμ-ret transgenic mouse as a model for lymphoblastic lymphoma and leukemia, we report three significant findings here. (i) The mTOR inhibitor rapamycin inhibits proliferation of leukemia cells in vitro; (ii) rapamycin is active as a single agent in vivo against ALL; and (iii) rapamycin-induced growth inhibition is reversible by IL-7, suggesting that IL-7-mediated signaling is involved in the response of pre-B-ALL cells to rapamycin.
We investigated the effect of IL-7 in this system for several reasons. (i) IL-7 plays a role in cell survival and differentiation during the early stages of B cell development (2); (ii) malignant transformation occurs at the pro-B-early pre-B cell transition in our leukemia mouse model (10); and (iii) aberrant IL-7 and IL-7R expression has been associated with certain malignancies (25-28). IL-7 causes pro-B cell proliferation (63) and can up-regulate RAG-1 expression in lymphoid precursors (64). IL-7 is essential for the development of normal B and T cells in mice because IL-7 and IL-7Rα knockout mice have profound defects in B cell development (reviewed in ref. 13). In humans, IL-7 causes proliferation of early precursor B cells but is not essential for B cell development (17).
Recently, two cases of T cell leukemia have developed in patients with X-linked severe combined immunodeficiency (SCID-X1) after receiving autologous CD34+ bone marrow cells transduced ex vivo with γc chain gene (60). The remaining eight patients continue to have functioning T, B, and natural killer (NK) cells for >3 years, but they are being closely monitored for the advent of similar disease (65, 66). SCID-X1, manifested by deficient T and NK cell production and abnormal B cell function, occurs as a result of a mutation in the γc chain (reviewed in refs. 67 and 68). This phenotype is mainly a consequence of defective IL-7 and IL-15 signaling (69). The T cell leukemia developing in these two SCID-X1 patients after ex vivo transduction of the γc chain gene into CD34+ bone marrow cells may be partially a result of increased cytokine signaling and an enhanced activation state in the lymphocytes, the status of which renders the lymphocytes leukemia-prone and susceptible to a “second hit” caused by an insertional mutagenic event. We have seen a similar picture in the leukemia-prone Eμ-ret transgenic mouse model (J.F., unpublished data). Further experimentation is necessary to evaluate the ability of IL-7 to alter the response to rapamycin in this leukemia mouse model.
Our observation that IL-7 reversed the apoptotic response of rapamycin on these ALL cells is supported by data reported by others. Karawajew et al. (25) have shown that IL-7 inhibits spontaneous apoptosis in T cell ALL cells. They found also that IL-7 induced inhibition of apoptosis correlated to a better early cytoreduction in patients with ALL. Yada et al. (70) have shown that IL-7 inhibits the spontaneous apoptosis of intestinal intraepithelial lymphocytes by inhibiting caspase-dependent and caspase-independent pathways. We have found that the inhibition of cell growth mediated by rapamycin was partially due to apoptosis. Other mechanisms must also be present to cause the profound growth arrest seen with rapamycin.
Also, we have observed that IL-7 was capable of reversing the inhibitory effect of rapamycin in pre-B-ALL cells, but the effect was dependent on the dose of both IL-7 and rapamycin. These data suggest that the two pathways intersect at some point, with p70 S6 kinase being one likely candidate. By inhibiting mTOR, rapamycin inhibits the phosphorylation of p70 S6 kinase at the Thr-389 site, resulting in the inhibition of protein synthesis (51, 52, 71). We have observed that the addition of IL-7 to rapamycin-treated cells reverses the rapamycin-induced dephosphorylation of p70 S6 kinase, suggesting that p70 S6 kinase is a downstream target of both IL-7 and mTOR pathways. An alternative explanation is that the mTOR pathways and IL-7-mediated signaling pathways function in parallel within progenitor B cells. Rapamycin-induced inhibition of the mTOR pathway then allows for the IL-7R signaling pathway to play a more dominant role within a cell. There is a balance of proliferation and growth-inhibitory signals. When rapamycin is present, the cell growth-arrest signals dominate, whereas, when IL-7 is present, the survival signals predominate and the growth-arrest signals are attenuated. Further experiments are needed to determine if IL-7 bypasses the effect of rapamycin on other mTOR pathway intermediates, such as p27kip1 and 4EBP1.
In summary, rapamycin is a well studied mTOR inhibitor with immunosuppressive and antitumorigenic properties. In other preclinical studies, rapamycin inhibits IL-7-mediated cell cycle progression and cellular proliferation of T cell ALL cells (28). Rapamycin has been shown also to inhibit metastatic solid-tumor growth and angiogenesis in preclinical studies (72). Our data show that the mTOR inhibitor rapamycin demonstrates activity against pre-B-ALL both in vitro and in vivo in a leukemic mouse model. Furthermore, IL-7-mediated signaling appears to play a role in sustaining cell survival and thus contributing to the transforming process of early precursor B cells. Further characterization of the molecular mechanisms by which B cell precursors undergo malignant transformation may help us evaluate IL-7 signaling as a therapeutic target and its interactions with the mTOR pathway. The data presented here have resulted in two Phase I clinical trials (CHP-753 and CHP-755), which are underway, using rapamycin in patients with relapsed or refractory leukemia.
Acknowledgments
We thank Dr. Jane Minturn, Dr. Martin Carroll, and Ms. Xueyuan Liu for assistance with preparation of this manuscript. This work was supported by National Institutes of Health Grants 5-T32-CA-09615 (to J.M.K.), T32-HL-07150 (to V.I.B.), K-K12-CA-76931 (to J.M.K. and V.I.B.), and R01 CA82156 (to S.A.G.); an American Society of Clinical Oncology Young Investigator Award (to J.M.K.); and a Children's Cancer Research Fund Young Investigator Award (to V.I.B.).
Abbreviations: ALL, acute lymphoblastic leukemia; pre-B-ALL, B-precursor ALL; Eμ, μ enhancer; IL-7R, IL-7 receptor; mTOR, mammalian target of rapamycin; MTT, methylthiazoletetrazolium; WBC, white blood cell count.
References
- 1.Vogler, L. B., Crist, W. M., Bockman, D. E., Pearl, E. R., Lawton, A. R. & Cooper, M. D. (1978) N. Engl. J. Med. 298, 872-878. [DOI] [PubMed] [Google Scholar]
- 2.LeBien, T. W. (2000) Blood 96, 9-23. [PubMed] [Google Scholar]
- 3.Loken, M. R., Shah, V. O., Dattilio, K. L. & Civin, C. I. (1987) Blood 70, 1316-1324. [PubMed] [Google Scholar]
- 4.Cronin, F. E., Jiang, M., Abbas, A. K. & Grupp, S. A. (1998) J. Immunol. 161, 252-259. [PubMed] [Google Scholar]
- 5.Stoddart, A., Flemming, H. E. & Paige, C. J. (2000) Immunol. Rev. 175, 47-58. [PubMed] [Google Scholar]
- 6.Ichihara, M., Iwamoto, T., Isobe, K., Takahashi, M., Nakayama, A., Pu, M., Dai, Y., Parashar, A., Ohkus, K. & Kato, M. (1995) Br. J. Cancer 71, 808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserman, R., Li, Y. S. & Hardy, R. R. (1995) J. Immunol. 155, 644. [PubMed] [Google Scholar]
- 8.Goodfellow, P. J. & Wells, S. A. J. (1995) J. Natl. Cancer Inst. 87, 1515-1523. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto, T., Pu, M., Ito, M., Takahashi, M., Isobe, K., Nagase, F., Kawashima, K., Ichihara, M. & Nakashima, I. (1991) Eur. J. Immunol. 21, 1809-1814. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman, R., Zeng, X. X. & Hardy, R. R. (1998) Blood 92, 273-282. [PubMed] [Google Scholar]
- 11.Zeng, X.-X., Zhang, H., Hardy, R. R. & Wasserman, R. (1998) Blood 92, 3529-3536. [PubMed] [Google Scholar]
- 12.Hayakawa, K., Li, Y. S., Wasserman, R., Sauder, S., Shinton, S. & Hardy, R. R. (1997) Ann. N.Y. Acad. Sci. 815, 15-29. [DOI] [PubMed] [Google Scholar]
- 13.Appasamy, P. M. (1999) Cytokines Cell. Mol. Ther. 5, 25-39. [PubMed] [Google Scholar]
- 14.Fry, T. J. & Mackall, C. L. (2001) Trends Immunol. 22, 564-571. [DOI] [PubMed] [Google Scholar]
- 15.Page, T. H., Lali, F. V. & Foxwell, B. M. (1995) Eur. J. Immunol. 25, 2956-2960. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeister, R., Khaled, A. R., Benbernou, N., Rajnavolgyi, E., Muegge, K. & Durum, S. K. (1999) Cytokine Growth Factor Rev. 10, 41-60. [DOI] [PubMed] [Google Scholar]
- 17.Pribyl, J. A. & LeBien, T. W. (1996) Proc. Natl. Acad. Sci. USA 93, 10348-10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dittel, B. N. & LeBien, T. W. (1995) J. Immunol. 155, 58-67. [PubMed] [Google Scholar]
- 19.Smart, F. M. & Venkitaraman, A. R. (2000) J. Exp. Med. 191, 737-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato, A. K., Yanai, N., Okubo, T., Mori, K. J. & Obinata, M. (2001) Cell Struct. Funct. 26, 95-101. [DOI] [PubMed] [Google Scholar]
- 21.van der Plas, D. C., Smiers, F., Pouwels, K., Hoefsloot, L. H., Lowenberg, B. & Touw, I. P. (1996) Leukemia 10, 1317-1325. [PubMed] [Google Scholar]
- 22.Dadi, H. K., Ke, S. & Roifman, C. M. (1993) Biochem. Biophys. Res. Commun. 192, 459-464. [DOI] [PubMed] [Google Scholar]
- 23.Dadi, H. & Roifman, C. M. (1994) Blood 84, 1579-1586. [PubMed] [Google Scholar]
- 24.Seckinger, P. & Fougereau, M. (1994) J. Immunol. 153, 97-109. [PubMed] [Google Scholar]
- 25.Karawajew, L., Wuchter, C., Kosser, A., Schrappe, M., Dorken, B. & Ludwig, W.-D. (2000) Blood 96, 297-306. [PubMed] [Google Scholar]
- 26.Wuchter, C., Ruppert, V., Schrappe, M., Dorken, B., Ludwig, W. D. & Karawajew, L. (2002) Blood 99, 4109-4115. [DOI] [PubMed] [Google Scholar]
- 27.Touw, I., Pouwels, K., van Agthoven, T., van Gurp, R., Budel, L., Hoogerbrugge, H., Delwel, R., Goodwin, R. G., Namen, A. E. & Lowenberg, B. (1990) Blood 75, 2097-2101. [PubMed] [Google Scholar]
- 28.Barata, J. T., Cardoso, A. A., Nadler, L. M. & Boussiotis, V. A. (2001) Blood 98, 1524-1531. [DOI] [PubMed] [Google Scholar]
- 29.Foss, H. D., Hummel, M., Gottstein, S., Ziemann, K., Falini, B., Herbst, H. & Stein, H. (1995) Am. J. Pathol. 146, 33-39. [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin, D., Sharma, V., Knobloch, T. J., Armitage, R. J., Dayton, M. A. & Goodwin, R. G. (1994) J. Immunol. 152, 4749-4757. [PubMed] [Google Scholar]
- 31.Renard, N., Duvert, V., Matthews, D. J., Pages, M.-P., Magaud, J.-P., Manel, A.-M., Pandrau-Garcia, D., Philippe, N., Banchereau, J. & Saeland, S. (1995) Leukemia 9, 1219-1226. [PubMed] [Google Scholar]
- 32.Bierer, E. A. (1990) Proc. Natl. Acad. Sci. USA 87, 9231-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wicker, L. S., Boltz, R. C., Matt, V., Nichols, E. A., Peterson, L. B. & Sigal, N. H. (1990) Eur. J. Immunol. 20, 2277-2283. [DOI] [PubMed] [Google Scholar]
- 34.Kay, J. E., Kromwel, L., Doe, S. E. A. & Denyer, M. (1991) Immunology 72, 544-549. [PMC free article] [PubMed] [Google Scholar]
- 35.Sakata, A., Kuwahara, K., Ohmura, T., Inui, S. & Sakaguchi, N. (1999) Immunol. Lett. 68, 301-309. [DOI] [PubMed] [Google Scholar]
- 36.Morris, R. E. (1991) Immunol. Today 12, 137-140. [DOI] [PubMed] [Google Scholar]
- 37.Ettenger, R. B. & Grimm, E. M. (2001) Am. J. Kidney Dis. 38, S22-28. [DOI] [PubMed] [Google Scholar]
- 38.Saunders, R. N., Metcalfe, M. S. & Nicholson, M. L. (2001) Kidney Int. 59, 3-16. [DOI] [PubMed] [Google Scholar]
- 39.Calne, R. Y., Collier, D. S., Lim, S., Pollard, S. G., Samaan, A., White, D. J. & Thiru, S. (1989) Lancet ii, 227 (lett.). [DOI] [PubMed] [Google Scholar]
- 40.Schreiber, S. L. (1991) Science 251, 283-287. [DOI] [PubMed] [Google Scholar]
- 41.Huang, S. & Houghton, P. J. (2002) Curr. Opin. Investig. Drugs 3, 295-304. [PubMed] [Google Scholar]
- 42.Huang, S. & Houghton, P. J. (2001) Drug Resist. Updat. 4, 378-391. [DOI] [PubMed] [Google Scholar]
- 43.Elit, L. (2002) Curr. Opin. Investig. Drugs 3, 1249-1253. [PubMed] [Google Scholar]
- 44.Hidalgo, M. & Rowinsky, E. K. (2000) Oncogene 19, 6680-6686. [DOI] [PubMed] [Google Scholar]
- 45.Garber, K. (2001) J. Natl. Cancer Inst. 93, 1517-1519. [DOI] [PubMed] [Google Scholar]
- 46.Castedo, M., Ferri, K. F. & Kroemer, G. (2002) Cell Death Differ. 9, 99-100. [DOI] [PubMed] [Google Scholar]
- 47.Brunn, G. J., Hudson, C. C., Sekulic, A., Williams, J. M., Hosoi, H., Houghton, P. J., Lawrence, J. C. & Abraham, R. T. (1997) Science 277, 99-101. [DOI] [PubMed] [Google Scholar]
- 48.Burnett, P. E., Barrow, R. K., Cohen, N. A., Snyder, S. H. & Sabatini, D. M. (1998) Proc. Natl. Acad. Sci. USA 95, 1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West, M. J., Stoneley, M. & Willis, A. E. (1998) Oncogene 17, 769-780. [DOI] [PubMed] [Google Scholar]
- 50.Raught, B., Gingras, A. C. & Sonenberg, N. (2001) Proc. Natl. Acad. Sci. USA 98, 7037-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumont, F. J., Altmeyer, A., Kastner, C., Fischer, P. A., Lemon, K. P., Chung, J., Blenis, J. & Staruch, M. J. (1994) J. Immunol. 152, 992-1003. [PubMed] [Google Scholar]
- 52.Kuo, C. J., Chung, J., Fiorentino, D. F., Flanagan, W. M., Blenis, J. & Crabtree, G. R. (1992) Nature 358, 70-73. [DOI] [PubMed] [Google Scholar]
- 53.Chen, Y., Chen, H., Rhoad, A. E., Warner, L., Caggiano, T. J., Failli, A., Zhag, H., Hsiao, C.-L., Nakanishi, K. & Molnar-Kimber, K. L. (1994) Biochem. Biophys. Res. Commun. 203, 1-7. [DOI] [PubMed] [Google Scholar]
- 54.Brown, E. J., Albers, M. W., Shin, T. B., Ichikawa, K., Keith, C. T., Lane, W. S. & Schreiber, S. L. (1994) Nature 369, 756-758. [DOI] [PubMed] [Google Scholar]
- 55.Degiannis, D. & Hornung, N. (1995) Transplantation 59, 1076-1079. [PubMed] [Google Scholar]
- 56.Li, H.-L., Davis, W. & Pure, E. (1999) J. Biol. Chem. 274, 9812-9820. [DOI] [PubMed] [Google Scholar]
- 57.Mosmann, T. (1983) J. Immunol. Methods 65, 55-63. [DOI] [PubMed] [Google Scholar]
- 58.Kim, J. M., Fang, J., Rheingold, S., Aplenc, R., Wasserman, R. & Grupp, S. A. (2002) Cancer Res. 62, 4212-4216. [PubMed] [Google Scholar]
- 59.MacDonald, A., Scarola, J., Burke, J. T. & Zimmerman, J. J. (2000) Clin. Ther. 22, B101-B121. [DOI] [PubMed] [Google Scholar]
- 60.Hacein-Bey-Abina, S., von Kalle, C., Schmidt, M., Le Deist, F., Wulffraat, N., McIntyre, E., Radford, I., Villeval, J. L., Fraser, C. C., Cavazzana-Calvo, M., et al. (2003) N. Engl. J. Med. 348, 255-256. [DOI] [PubMed] [Google Scholar]
- 61.Gaynon, P. S., Qu, R. P., Chappell, R. J., Willoughby, M. L., Tubergen, D. G., Steinherz, P. G. & Trigg, M. E. (1998) Cancer (Philadelphia) 82, 1387-1395. [DOI] [PubMed] [Google Scholar]
- 62.Chessells, J. M. (1998) Br. J. Haematol. 102, 423-438. [DOI] [PubMed] [Google Scholar]
- 63.Wei, C., Zeff, R. & Goldschneider, I. (2000) J. Immunol. 164, 1961-1970. [DOI] [PubMed] [Google Scholar]
- 64.Yehuda, A. B., Wirtheim, E., Abdulhai, A., Or, R., Slavin, S., Babaey, S. & Friedman, G. (1999) J. Gerontol. 54, B143-B148. [DOI] [PubMed] [Google Scholar]
- 65.Hacein-Bey-Abina, S., Le Deist, F., Carlier, F., Bouneaud, C., Hue, C., De Villartay, J. P., Thrasher, A. J., Wulffraat, N., Sorensen, R., Dupuis-Girod, S., et al. (2002) N. Engl. J. Med. 346, 1185-1193. [DOI] [PubMed] [Google Scholar]
- 66.Cavazzana-Calvo, M., Hacein-Bey, S., de Saint Basile, G., Gross, F., Yvon, E., Nusbaum, P., Selz, F., Hue, C., Certain, S., Casanova, J. L., et al. (2000) Science 288, 669-672. [DOI] [PubMed] [Google Scholar]
- 67.Fischer, A. (2002) Scand. J. Immunol. 55, 238-241. [DOI] [PubMed] [Google Scholar]
- 68.Fischer, A. (2000) Clin. Exp. Immunol. 122, 143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavazzana-Calvo, M., Hacein-Bey, S., Yates, F., de Villartay, J. P., Le Deist, F. & Fischer, A. (2001) J. Gene Med. 3, 201-206. [DOI] [PubMed] [Google Scholar]
- 70.Yada, S., Nukina, H., Kishihara, K., Takamura, N., Yoshida, H., Inagaki-Ohara, K., Nomoto, K. & Lin, T. (2001) Cell. Immunol. 208, 88-95. [DOI] [PubMed] [Google Scholar]
- 71.Weng, Q. P., Kozlowski, M., Belham, C., Zhang, A., Comb, M. J. & Avruch, J. (1998) J. Biol. Chem. 273, 16621-16629. [DOI] [PubMed] [Google Scholar]
- 72.Guba, M., von Breitenbuch, P., Steinbauer, M., Koehl, G., Flegel, S., Hornung, M., Bruns, C. J., Zuelke, C., Farkas, S., Anthuber, M., et al. (2002) Nat. Med. 8, 128-135. [DOI] [PubMed] [Google Scholar]
- 73.(2000) Schalm's Veterinary Hematology, eds. Feldman, B., Zinkl, J. & Jain, N. (Lippincott, Williams & Wilkins, Philadelphia), 5th Ed.