Abstract
Cholangiocarcinoma (CCA) is a devastating cancer arising from the neoplastic transformation of the biliary epithelium. It is characterized by a progressive increase in incidence and prevalence. The only curative therapy is radical surgery or liver transplantation but, unfortunately, the majority of patients present with advanced stage disease, which is not amenable to surgical therapies. Recently, proposed serum and bile biomarkers could help in the screening and surveillance of categories at risk and in diagnosing CCA at an early stage. The molecular mechanisms triggering neoplastic transformation and growth of biliary epithelium are still undefined, but significant progress has been achieved in the last few years. This review deals with the most recent advances on epidemiology, biology, and clinical management of CCA.
Keywords: Cholangiocarcinoma, Cholangiocytes, Growth factors, Proliferation, Apoptosis
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor arising from the malignant transformation of cholangiocytes, the epithelial cells lining the biliary tree. CCA is the second most common primary hepatic malignancy, with recent epidemiologic studies suggesting a progressive increasing incidence in Western countries[1]. CCA is characterized by a bad prognosis, with a median survival of less than 24 mo[2-4] and a scarce response to chemotherapy[5-9]. From the anatomical point of view, CCA is classified as intrahepatic (IH-CCA) or extra-hepatic (EH-CCA), the latter being further divided into proximal or perihilar and distal, depending on the location of the cancer within the extra-hepatic biliary system. Perihilar CCA is also known as Klatskin tumor. Three different growth patterns of EH-CCA can be observed: (1) periductal infiltrating; (2) papillary or intraductal; and (3) mass forming[10]. Intrahepatic CCA typically presents as an intrahepatic mass. The only curative therapy is surgical resection or liver transplantation but, unfortunately, the majority of patients are diagnosed at advanced stage, when surgical therapies are excluded. This should stimulate research on the identification of effective surveillance strategies that would permit detection of early CCA or, better yet, premalignant lesions in patients at increased risk, particularly patients with primary sclerosing cholangitis (PSC). Serum and bile tumor markers, non invasive and endoscopic-based imaging modalities, and histology and cytology have been attempted with varying success[11].
The manuscript deals with the most recent advances on the epidemiology, biology and clinical management of CCA.
EPIDEMIOLOGY
Epidemiological studies in different geographical areas give information on the incidence, prevalence, and mortality time trends and, therefore, are important as they might yield clues to potential etiological factors[12-16]. Hepatobiliary malignancies account for 13% of the 7.6 million annual cancer-related deaths worldwide and for 3% of the 560000 annual cancer-related deaths in the United States. CCA accounts for 10% to 20% of the deaths from hepatobiliary malignancies. The prevalence of CCA shows a wide geographical variability, with the highest rates in Asia and the lowest in Australia[14]. In the United States, the incidence of CCA has been reported to be 0.95/100000 for IH-CCA and 0.82/100000 for EH-CCA[14,15]. CCA prevalence in different racial and ethnic groups is heterogeneously distributed, with the highest age-adjusted prevalence in Hispanics (1.22/100000) and the lowest in African Americans (0.17-0.50/100000)[14-18]. A number of recent studies have highlighted a progressive increase, in the past three decades, in the mortality for IH-CCA[15] , while EH-CCA mortality is stable or slightly decreasing[12,15]. With the exception of Denmark[19,20], this scenario has been reported worldwide[1,15-18]. The significant increase in age-adjusted incidence of IH-CCA was confirmed even after correction for a prior misclassification of hilar CCA as IH-CCA[1]. In Europe, the increase in the IH-CCA mortality was higher in Western Europe than in Central or Northern Europe. In contrast, mortality rates for EH-CCA showed a decreasing trend in most countries[1,15-17]. Very recently, data on the mortality and incidence trend for CCA have also been reported in Italy, where a 40-fold increase in mortality for IH-CCA has been documented from 1980 to 2003. For EH-CCA, in contrast, mortality rates were stable or slightly decreasing in the last 10 years. Thus, as described in most countries, in Italy the increased mortality for CCA mainly involves the intrahepatic form, suggesting different etiology and risk factors for IH- and EH-CCA[14-16]. Interestingly, in all epidemiological studies concerning primary liver malignancies, a high percentage (about 40%) of primitive liver cancers are classified as adenocarcinoma and are therefore excluded from the group of either CCA or hepatocellular carcinoma. This probably accounts for a significant underscoring of IH-CCA incidence and mortality because it is a common clinical opinion that most primary liver adenocarcinomas are indeed CCA[21]. Biological, immunohistochemical, or genetic markers[4,21] could definitively permit an exact diagnosis and classification of primary liver cancers and avoid these classification biases.
RISK FACTORS
A number of different risk factors have been definitively identified. PSC is the most commonly recognized risk factor[22-25]. The prevalence of CCA in PSC is 5%-15%, and the annual incidence rate is 0.6% to 1.5%[22-25]. The majority of PSC patients will develop CCA within the first 2.5 years after the diagnosis of PSC[22-25]. Thus, the symptomatic patient who presents with their first diagnosis of PSC should be carefully screened for CCA. Infectious etiologies, such as parasitic[26,27] and bacterial infections (i.e. Opisthorchis Viverrini, Clonorchis Sinensis, Schistosomiasis Japonica and Salmonella Tiphi), lead to an increased risk of CCA in endemic regions of Asia.
Exposures to certain xenobiotics might lead to an increased risk of CCA. Multiple case-control studies have reported an association between CCA and alcohol use[26-29]. Iatrogenic exposure to thorotrast (thorium dioxide), a radiocontrast agent used in the 1950s and 1960s, first led to reports of CCA in the 1970s[30,31]. Since that time, hundreds of cases of CCA (as well as other primary hepatic malignancies) attributed to thorotrast exposure have been described. Caroli disease, congenital choledocal cist[32-35], bilio-enteric surgical drainage, abnormal biliary-pancreatic junction, and intra-hepatic lithiasis[36-38] are other risk factors for CCA. In contrast to patients submitted to bilio-enteric surgical drainage for benign diseases that represent a well recognized category at risk, patients submitted to endoscopic sphincterotomy during endoscopic retrograde cholangiopancreatography (ERCP) failed to express increased risk of CCA, and this has been definitively demonstrated in three different studies performed in a large series of patients with long follow-up[39-41]. Several case-control studies have described an increased risk of CCA in patients with chronic hepatitis C virus (HCV) infection[42,43], and HCV-RNA has been detected in some cases of resected CCA[44].
A prospective study of 600 HCV-infected individuals in Japan between 1980 and 1997 (median follow-up 7.2 years), detected a 2.3% incidence of CCA, which is well above the baseline population incidence[45]. Although hepatitis B virus nucleic acids have been detected in selected cases of CCA[46,47], an association between hepatitis B virus infection and CCA is less well established[45-48]. More recently, obesity, diabetes[37,49] and smoking have been taken into consideration, especially for IH-CCA, but further confirmation is need. In PSC patients[22-25], smoking and alcohol further increases the risk of CCA development. Whether concomitant ulcerative colitis and its duration could increase CCA risk remains to be definitively established[22-25].
MOLECULAR AND CELLULAR PATHOGENESIS
The molecular mechanisms underlying the development, growth, and metastatic diffusion of biliary tract cancers are still undefined. Recent attention has been paid to the origin of CCA from the neoplastic transformation of resident hepatic stem cells[50,51]. However, evidence for a role of resident stem cells exists, so far, only for primitive hepatic cancers characterized by mixed hepatocellular carcinoma/CCA phenotypes[50,51], which are currently classified as hepato-CCA.
The recognized risk factors for CCA share, as a common basis, a condition of chronic inflammation of the biliary epithelium together with a partial biliary obstruction[52,53]. As a consequence, most studies deal with pathways linking inflammation and carcinogenesis[54-57]. In general, chronic inflammation is thought to promote carcinogenesis by causing damage in DNA mismatch repair genes/proteins, protooncogenes, and tumor suppressor genes and, by creating a local environment enriched with cytokines and other growth factors capable to accelerating the cell cycle, to favor accumulation of somatic mutations[58,59].
Current evidence supports a primary role played by nitric oxide (NO) induced by pro-inflammatory cytokines (TNF-α, IL-6 etc.)[54-57]. These cytokines are able to activate inducible nitric oxyde synthase (iNOS), which, at the immunohistochemical level, is overexpressed in more than 70% of CCA. Increased iNOS activity results in the generation of NO and reactive oxygen species known to interact with cellular DNA and to inhibit DNA repair mechanisms, thus triggering oncogenetic mutations[55,56]. Dysregulation of the proto-oncogene k-ras[60], nuclear accumulation of p53[61-63], and up-regulation of the related proteins mdm-2 and WAF-1 have been observed in CCA[64-66]. Other inactivated suppressor genes include p16INK4a[67], DPC4/Smad4, and APC[68-70]. The majority of these genetic changes were described in IH-CCA. NO, together with different cytokines, can also inhibit cholangiocytes apoptosis by nytrosilation of caspase 9 and might induce proliferation, thus favouring accumulation of somatic mutations.
Interleukin-6 (IL-6) also plays a key role in the pathogenesis of CCA and, especially, in cholangiocyte evasion from apoptosis. IL-6 is produced at high levels by CCA cells, and elevated IL-6 serum concentrations have been reported in CCA patients[71,72]. Autocrine and paracrine IL-6 stimulation activates the prosurvival p38 mitogen activated protein kinase[73,74]. In addition, IL-6 upregulated the expression of myeloid cell leukemia-1 (Mcl-1), through STAT3 and AKT related signaling pathways[75,76]. Mcl-1 is an anti-apoptotic protein of the Bcl-2 family of apoptotic proteins.
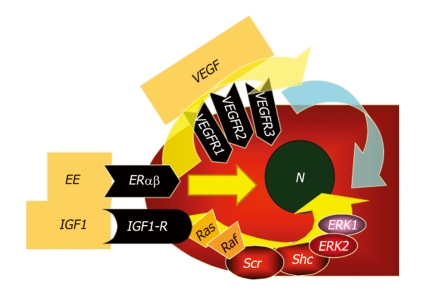
Cycloxygenase 2 (COX-2), the rate-limiting enzyme in prostaglandins biosynthesis from arachidonic acid, which is activated by inflammatory cytokines and NO, further accelerates cell cycle via PgE2 and inhibits different apoptotic cascades[77]. Indeed, increased COX-2 immunohistochemical expression has been documented in more than 70% of CCA. Oxysterols, oxygenated cholesterol derivatives formed in bile of patients with inflammatory diseases of biliary tree, together with bile acids[78-80], are also able to activate COX-2[81-85]. Other COX-2–inducing molecules include the tyrosine kinase ErbB-2[86], which is overexpressed in CCA and involved in CCA carcinogenesis and progression[87,88]. ErbB-2 is an epidermal growth factor receptor (EGFR) homolog and is able to homodimerize or heterodimerize with other members of the EGF superfamily, resulting in activation of the Raf/MAPK-pathway[89,90]. Hydrophobic bile salts, such as deoxycholate, might play a carcinogenetic role through transactivation of EGFR and impairment of Mcl-1 functions[78]. Constitutive overexpression of ErbB2 and/or ErbB1 in malignant cholangiocytes has been documented in more than 50% of IH-CCA. In addition, rodent models of intrahepatic cholangiocarcinogenes have been developed and are associated with constitutive ErbB2 overexpression[87]. ErbB2 and ErbB1 interact with different molecular signalling pathways associated with IH-CCA development and progression, including bile acids, IL-6/gp130, transmembrane mucins, hepatocyte growth factor/Met[91], and vascular endothelial growth factor (VEGF) signalling. The relevance of ErbB2 or ErbB1 related pathways in CCA have raised interest in the possibility that agents that selectively target these receptors could potentially be effective in CCA therapy[86,89]. However, current experience with such ErbB targeted therapies produced only modest responses in patients with biliary tract cancers. Another recently proposed mechanism linking chronic inflammation with CCA development is related to activation-induced cytidine deaminase (AID), a member of the DNA/RNA editing enzyme family, implicated in human carcinogenesis via its mutagenic activity[92]. AID was found to be increased in biopsies from patients with PSC or CCA, whereas only trace amounts of AID were detected in the normal liver. Very recently, a relevant role in modulating CCA growth and proliferation has been attributed to estrogens, insulin like growth factor 1 (IGF1), leptin, opioid receptor modulators, endothelin, and serotonin[54,93-96]. As far as estrogens are concerned, recent studies suggest their synergistic action with growth factors (IGF1, VEGF) in sustaining the cholangiocyte proliferative machinery and in depressing apoptosis (Figure 1). Indeed, cross-talk between IGF1 and estrogens has been demonstrated to modulate CCA proliferation, where estrogens act at several points of the IGF1 signal transduction pathway (Figure 1)[93,94]. In addition, it has been shown that the estrogen proliferative effect on CCA cells is also due to the stimulation of VEGF synthesis and secretion (Figure 1)[93,94].
Figure 1.
Proposed mechanisms of estrogen-induced proliferation of cholangiocarcinoma (CCA) cells. Cross-talk between IGF1 and estrogens has been recently demonstrated to modulate cholangiocarcinoma proliferation, where estrogens act at several points of the IGF1 signal transduction pathway (see ref.# 93,94). In addition, it has been shown that the estrogen proliferative effect on CCA cells is also due to the stimulation of vascular endothelial growth factor (VEGF) synthesis and secretion (see ref.# 93,94).
In this experimental background, a number of different genetic/epigenetic abnormalities involving inflammation-related genes, as well as genes involved in the control of cell cycle or DNA repair, have been documented with implications in terms of genetic susceptibility of individual/categories at risk[79,80]. These genetic studies, however, carry a number of biases caused by the anatomical and morphological CCA heterogeneity, geographic/racial differences, and different concurrent risk factors. In addition, most studies come from oriental countries that are not necessarily applicable in western populations[79]. In spite of all these difficulties, genetic variants of the Natural Killer Cell Receptor G2D receptor[80] , which drives the clearance of damaged cholangiocytes by natural killer lymphocytes, have been recently associated with the development of CCA in PSC patients, suggesting the possibility to identify and submit to strict surveillance a subgroup of PSC patients. Among other inflammation-related genes, specific haplotypes of COX2-coding gene (PTGS2) or IL8RB have been recently associated with a significant risk of CCA development in oriental populations[79].
SERUM AND BILE BIOMARKERS AIDING CCA DIAGNOSIS
In the majority of cases, CCA is clinically silent, with symptoms only developing at advanced stages. Surgical resection is the only effective therapeutic option for CCA, but this is applicable in less than 50% of cases, because this cancer is mostly diagnosed at late stages. In early-cancer, the five-year survival after radical surgery is higher than 80% and therefore, any attempt to facilitate early diagnosis is to be welcomed. For many years, efforts have been made to identify, in serum or biological fluid[97-99], biomarkers with adequate diagnostic accuracy for CCA, which could also be useful for population screening or for the surveillance of pathologies at risk, including PSC[98-101]. Serum tumor markers are attractive because of the ease of obtaining samples and their relatively low cost. Therefore, they have been the objects of extensive investigation to aid CCA diagnosis but, unfortunately, none of these markers has reached adequate specificity for CCA (Table 1)[102-105].
Table 1.
Recently proposed serum and bile biomarkers for the diagnosis of Cholangiocarcinoma
| Biomarker | Sensitivity (%) | Specificity (%) |
| Serum | ||
| CA19-9 | 53-92 | 50-98 |
| CEA | 33-68 | 79-100 |
| IL-6 | 73 | 92 |
| Trypsinogen-2 | AUC = 0.804 | |
| MUC5AC | 71.01 | 90 |
| CYFRA 21-1 | 74.7 | 92.2 |
| Bile | ||
| CA19-9 | 46-61 | 60-70 |
| CEA | 67-84 | 33-80 |
| IGF1 | 100 | 100 |
| Pancreatic elastase/amylase ratio | 82 | 89 |
| Mcm5 | 62 | 67 |
CEA: Carcinoembryonic antigen; IL-6: Interleukin-6; IGF1: Insulin like growth factor 1
Carcinoembryonic antigen (CEA), which is mainly used for colorectal cancers, is of scarce utility being increased only in approximately 30% of patients with CCA[102-104]. Carbohydrate antigen (CA 19-9) is the most widely used serum marker for CCA but it is also elevated in pancreatic cancer, gastric cancer, and primary biliary cirrhosis. In smokers, it might be transiently increased during cholangitis or cholestasis. The sensitivity and specificity for detection of CCA in PSC are 79% and 98%, respectively, at a cutoff value of 129 U/mL. Other investigators have documented that only a higher cutoff (> 180 U/mL) could achieve this degree of specificity[106]. According to Levy et al[107] a change from baseline of > 63 U/L has a sensitivity of 90% and specificity of 98% for CCA detection. In patients without PSC, CA 19-9 sensitivity is 53% at a cutoff of > 100 U/L and its negative predictive value is 76%-92%[108]. CA 19-9 can also be elevated in bacterial cholangitis and other gastrointestinal and gynecologic neoplasias; patients lacking the blood type Lewis antigen (10% of individuals) do not produce this tumor marker[109-112]. In general, as extensively discussed in recent reviews[102-104], high sensitivity and specificity have been reported for CA 19-9 in patients with CCA, depending on the study population and cutoff values, and this is also true for CCA complicating PSC. In addition, CA 19-9 usually allows CCA diagnosis in advanced stages when radical treatments are not allowed. Therefore, current efforts aim to identify novel serum markers that can be substituted for CA19-9 or can improve, when measured together, the diagnostic accuracy of CA 19-9. The serum level of interleukin 6[71], at a 25.8 pg/mL cutoff, provides a diagnostic sensitivity of 73% and a specificity of 92%. Serum levels of IL-6 have been correlated with tumor burden in CCA patients and, interestingly, one month after treatment with photodynamic therapy, the mean IL-6 level decreased significantly. Although these findings are encouraging, it should be considered that serum IL-6 is also elevated in many patients with hepatocellular carcinoma, benign biliary disease, and metastatic lesions[105]. Other biomarkers such as trypsinogen-2[113], platelet–lymphocyte ratio (PLR), mucin-5AC[114,115], soluble fragment of cytokeratin 19 (CYFRA21-1)[116] have been recently shown to help in the diagnosis of CCA with, in some cases, a prognostic value[117,118]. In particular mucin 1 (MUC1) and MUC5AC are not expressed by hepatocellular carcinoma, suggesting a possible role in the differential diagnosis[105].
As far as bile is concerned, the ratio of pancreatic elastase/amylase, mucin-4, Mcm5 (minichromosome maintenance replication protein) and IGF1 have been explored with the IGF1 biliary concentration capable of completely discriminating CCA from benign biliary pathologies and pancreatic cancer (Table 1). Specifically, we measured IGF1 and VEGF in the bile of patients undergoing ERCP for biliary obstruction and evaluated the performance of these markers in differentiating EH-CCA from pancreatic cancer or benign biliary abnormalities[99]. The biliary IGF1 concentration was 15-20 fold higher in EH-CCA than in the other two groups. In contrast, biliary VEGF concentration was similar in the three groups. In substance, this study indicated a marker (bile IGF1), which could be used, with absolute diagnostic accuracy, to differentiate EH-CCA from either pancreatic cancer or benign biliary disorders, in patients undergoing ERCP for biliary obstruction.
Proteomic analysis of serum and bile are under investigation but definitive findings are currently unavailable. Another strategy is to evaluate the diagnostic performance of serum CA 19-9 together with imaging techniques[119-121]. It has been demonstrated that the combination of serum CA 19-9 with CT, MRI, MRCP or ERCP shows the best sensitivity (about 100%) but a low specificity (about 40%) in diagnosing CCA occurring in PSC patients. In contrast, the combination of serum CA19-9 and US had intermediate specificity (62%) with a good sensitivity (91%) for detecting CCA. On the basis of test properties, cost and availability, combination of serum CA 19-9 (cut-off value of 20 U/mL) and abdominal US at 12-mo intervals was proposed as a useful strategy for the screening/surveillance of CCA in PSC[10,100].
TREATMENT
There is no current effective medical therapy for CCA, and the median survival after treatment of unresectable disease is 9-12 mo[122,123]. Complete operative resection might be curative, but local extension of the disease often precludes complete resection[124,125].
Accurate preoperative staging will determine the treatment approach in these patients. Although a pathological staging system has been developed for ductal CCA, it is of limited value in EH-CCA. TNM classification does not correlate with resectability in patients with EH-CCA. Conversely, the Memorial Sloan-Kettering staging system can evaluate the biliary and vascular involvement of these tumors, which clearly correlates with resectability and survival. In addition, clinical staging is fundamental in pre-surgical evaluation, and this is based on evaluation of the proximal and distal extent of the disease, vascular involvement, and presence of metastasis, which can be done by Doppler ultrasound, MRI, CT, or EUS examinations. FDG-PET scanning changes the surgical management in a third of patients, with an overall sensitivity for metastasis detection of approximately 65%. Solitary IH-CCAs are managed by segmentectomy or lobectomy. Five-year survival rates are 22%-44% and correlate with R0 (negative margin) resection, absence of lymph node metastases, and vascular invasion[126-130]. Even with complete resection, local recurrence is common, with most series reporting five-year survival of 25%-35%. In PSC, outcomes of surgical resection are complicated by advanced liver disease in the majority of these patients, recurrent cholangitis with a biliary–enteric anastomosis, the multi-focal nature of the cancer, and the increased risk for further CCA[131]. Biliary-enteric anastomosis is a risk factor for de novo CCA[132,133]; therefore, creating a biliary-enteric anastomosis in a PSC patient should be viewed with caution, and informed consent regarding the potential development of additional CCA should be considered. Patients with PSC plus CCA might be better evaluated as potential liver transplant candidates. In the past, CCA was considered as a contraindication for liver transplantation. Recently, however, the development of new liver transplantation protocols at the Mayo Clinic and the University of Nebraska yielded promising results for EH-CCA[134,135]. Strict selection criteria have been developed, and this protocol includes neoadjuvant therapy with external beam radiation concurrent with 5-fluorouracil (5-FU) chemotherapy, followed by brachytherapy and chemotherapy with capecitabine. The patients, prior to transplantation, underwent explorative laparotomy for restaging. Survival analysis of patients treated according to the Mayo Clinic protocol has yielded one-and five-year survival rates of 91% and 76%, respectively[136,137].
For the majority of CCA patients who are not candidates for surgical resection or transplantation, recent proposals deal with the use of photodynamic therapy (PDT)[138-140]. In these studies, PDT alone, or plus stenting, improved cholestasis and quality of life considerably, and had a favourable side-effect profile. In the light of these findings, recent review articles recommended PDT for patients with not resectable disease[140]. The role of PDT adjuvant treatment before or after surgical resection needs to be assessed. Finally, it is important to reiterate that PDT requires carefully patient management. Radiotherapy (external, intraluminal, or brachytherapy) has received recent attention and positive findings have been reported by different centers for perihilar-CCA[140].
As far as pharmacological therapy is concerned, CCA is characterized by a remarkable resistance to common chemotherapy[122,123]. Several drugs have been tested alone in unresectable CCA and in restricted phase II studies (5-FU, methanesulfon-m-anisidide, cisplatin, rifampicin, mitomycin C, paclitaxel, and gemcitabine) with partial response of 0%-9% and average survival of 2-12 mo. Certainly, 5-FU was the drug most used, but with the same disappointing results of the other drugs used in monotherapy[141]. Recently, the Italian Health Care System approved the use of gemcitabine. In 2005, a review went back over 13 single arm phase II studies suggesting the role of gemcitabine as an alternative supportive care[142]. However, randomized controlled trials are necessary to assert the real and potential increase of survival determined by the drug which, nevertheless, shows a low toxicity. With regard to combination therapies, one of the most used therapeutic plans is ECF (epirubicin + cisplatin + 5-FU), but with disappointing results[143]. Several phase Ib and II studies evaluated the addition to gemcitabine[144], cetuximab[145,146], oxaliplatin/cisplatin[147-149], erlotinib[150], or capecitabine[151]. Among these studies, the effect seems to be more consistent with the combination of capecitabine and addition of oxaliplatin/cisplatin. With regard to biological therapy, there are ongoing pilot studies, not yet published, considering the use of sorafenib, lapatinib, or bevacizumab in the treatment of unresectable CCA. Studies of cellular and molecular biology have clearly demonstrated, in a high percentage of CCA, the activation of signaling pathways such as PI3-kinase and MEK/ERK, multiple receptor pathways (epidermal growth factor EGF; IGF1; estrogen receptors; VEGF) and COX2/PgE2. On the basis of this evidence, there is a rationale for pilot studies testing biological drugs acting on these targets. Paucity of data makes it impossible to use adjuvant chemotherapy after surgical resection[152]. In particular, chemotherapy with 5-FU and mitomycin C failed to improve the survival of patients undergoing surgical resection, as recently observed in a phase III study[153].
The benefit of adjuvant radiotherapy after surgical resection has been recently reviewed[152]. Overall, there are retrospective data which, taken together, show a benefit, especially with regard to dose-scaling radiation[151]. However, the only prospective study[153] denies a real benefit of radiotherapy after surgical resection of peri-hilar CCA in terms of survival. Further prospective studies are needed to reach a definitive conclusion. Although data are poor, there are some studies[152] demonstrating a slight but significant improvement in survival for distal common bile duct cancers undergoing surgical resection and adjuvant radio-chemotherapy[154].
Footnotes
Peer reviewers: Dae Hwan Kang, MD, PhD, Associate Professor, Gastroenterology and Hepatology Section, Department of Internal Medicine, College of Medicine Pusan National University, Pusan National University Hospital, Mulgeum-Gigu, 3-3, South Korea; Lars Mueller, MD, Department of General and Thoracic Surgery, University Hospital Schleswig-Holstein, Campus Kiel, Arnold-Heller-Str. 3, 24105 Kiel, Germany
S- Editor Li LF L- Editor Stewart G E- Editor Yang C
References
- 1.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 2.Farley DR, Weaver AL, Nagorney DM. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 4.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Il Colangiocarcinoma. Commissione aisf 2009; Available from: http://www.webaisf.com.
- 6.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Fava G, Marzioni M, Benedetti A, Glaser S, DeMorrow S, Francis H, Alpini G. Molecular pathology of biliary tract cancers. Cancer Lett. 2007;250:155–167. doi: 10.1016/j.canlet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 10.Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging. 2004;29:540–547. doi: 10.1007/s00261-004-0187-2. [DOI] [PubMed] [Google Scholar]
- 11.Yachimski P, Pratt DS. Cholangiocarcinoma: natural history, treatment, and strategies for surveillance in high-risk patients. J Clin Gastroenterol. 2008;42:178–190. doi: 10.1097/MCG.0b013e31806daf89. [DOI] [PubMed] [Google Scholar]
- 12.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Blendis L, Halpern Z. An increasing incidence of cholangiocarcinoma: why? Gastroenterology. 2004;127:1008–1009. doi: 10.1053/j.gastro.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 15.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 16.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006;26:1047–1053. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen P, Vilstrup H, Tarone RE, Friis S, Sørensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–897. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 21.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150, ix. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 23.Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology. 1999;30:325–332. doi: 10.1002/hep.510300101. [DOI] [PubMed] [Google Scholar]
- 24.Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–1211. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 25.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 26.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 27.Andoh H, Yasui O, Kurokawa T, Sato T. Cholangiocarcinoma coincident with schistosomiasis japonica. J Gastroenterol. 2004;39:64–68. doi: 10.1007/s00535-003-1249-x. [DOI] [PubMed] [Google Scholar]
- 28.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, Todoroki T, Jedpiyawongse A, Kittiwatanachot P, Sripa B, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 29.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Rota AN, Weindling HK, Goodman PG. Cholangiocarcinoma associated with thorium dioxide (thorotrast): report of a case. Mich Med. 1971;70:911–915. [PubMed] [Google Scholar]
- 31.Ito Y, Kojiro M, Nakashima T, Mori T. Pathomorphologic characteristics of 102 cases of thorotrast-related hepatocellular carcinoma, cholangiocarcinoma, and hepatic angiosarcoma. Cancer. 1988;62:1153–1162. doi: 10.1002/1097-0142(19880915)62:6<1153::aid-cncr2820620619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Robertson JF, Raine PA. Choledochal cyst: a 33-year review. Br J Surg. 1988;75:799–801. doi: 10.1002/bjs.1800750826. [DOI] [PubMed] [Google Scholar]
- 33.de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, Bosma A, Heij HA, Gouma DJ, van Gulik TM. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002;37:1568–1573. doi: 10.1053/jpsu.2002.36186. [DOI] [PubMed] [Google Scholar]
- 34.Pisano G, Donlon JB, Platell C, Hall JC. Cholangiocarcinoma in a type III choledochal cyst. Aust N Z J Surg. 1991;61:855–857. doi: 10.1111/j.1445-2197.1991.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 35.Voyles CR, Smadja C, Shands WC, Blumgart LH. Carcinoma in choledochal cysts. Age-related incidence. Arch Surg. 1983;118:986–988. doi: 10.1001/archsurg.1983.01390080088022. [DOI] [PubMed] [Google Scholar]
- 36.Su CH, Shyr YM, Lui WY, P'Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969–973. doi: 10.1002/bjs.1800840717. [DOI] [PubMed] [Google Scholar]
- 37.Ishiguro S, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Risk factors of biliary tract cancer in a large-scale population-based cohort study in Japan (JPHC study); with special focus on cholelithiasis, body mass index, and their effect modification. Cancer Causes Control. 2008;19:33–41. doi: 10.1007/s10552-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 38.Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577–1582. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccalà G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy. 2002;34:273–279. doi: 10.1055/s-2002-23632. [DOI] [PubMed] [Google Scholar]
- 40.Strömberg C, Luo J, Enochsson L, Arnelo U, Nilsson M. Endoscopic sphincterotomy and risk of malignancy in the bile ducts, liver, and pancreas. Clin Gastroenterol Hepatol. 2008;6:1049–1053. doi: 10.1016/j.cgh.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen FV, Jepsen P, Tarone RE, Funch-Jensen P, Jensen LS, Sørensen HT. Endoscopic sphincterotomy and long-term risk of cholangiocarcinoma: a population-based follow-up study. J Natl Cancer Inst. 2008;100:745–750. doi: 10.1093/jnci/djn102. [DOI] [PubMed] [Google Scholar]
- 42.Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Ye MQ, Thung SN, Dash S, Gerber MA. Detection of hepatitis C virus RNA sequences in cholangiocarcinomas in Chinese and American patients. Chin Med J (Engl) 2000;113:1138–1141. [PubMed] [Google Scholar]
- 45.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 46.Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211–1216. doi: 10.1016/j.humpath.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 47.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of a Mixture of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) (CAS No. 1746-01-6), 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4), and 3,3',4,4',5-Pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in Female Harlan Sprague-Dawley Rats (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 2006:1–180. [PubMed] [Google Scholar]
- 48.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahrens W, Timmer A, Vyberg M, Fletcher T, Guénel P, Merler E, Merletti F, Morales M, Olsson H, Olsen J, et al. Risk factors for extrahepatic biliary tract carcinoma in men: medical conditions and lifestyle: results from a European multicentre case-control study. Eur J Gastroenterol Hepatol. 2007;19:623–630. doi: 10.1097/01.meg.0000243876.79325.a1. [DOI] [PubMed] [Google Scholar]
- 50.Nomoto K, Tsuneyama K, Cheng C, Takahashi H, Hori R, Murai Y, Takano Y. Intrahepatic cholangiocarcinoma arising in cirrhotic liver frequently expressed p63-positive basal/stem-cell phenotype. Pathol Res Pract. 2006;202:71–76. doi: 10.1016/j.prp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Sell S, Dunsford HA. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 52.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 53.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 54.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 55.Prawan A, Buranrat B, Kukongviriyapan U, Sripa B, Kukongviriyapan V. Inflammatory cytokines suppress NAD(P)H:quinone oxidoreductase-1 and induce oxidative stress in cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2009;135:515–522. doi: 10.1007/s00432-008-0483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinlaor S, Sripa B, Ma N, Hiraku Y, Yongvanit P, Wongkham S, Pairojkul C, Bhudhisawasdi V, Oikawa S, Murata M, et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J Gastroenterol. 2005;11:4644–4649. doi: 10.3748/wjg.v11.i30.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mon NN, Kokuryo T, Hamaguchi M. Inflammation and tumor progression: a lesson from TNF-alpha-dependent FAK signaling in cholangiocarcinoma. Methods Mol Biol. 2009;512:279–293. doi: 10.1007/978-1-60327-530-9_15. [DOI] [PubMed] [Google Scholar]
- 58.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 59.Wise C, Pilanthananond M, Perry BF, Alpini G, McNeal M, Glaser SS. Mechanisms of biliary carcinogenesis and growth. World J Gastroenterol. 2008;14:2986–2989. doi: 10.3748/wjg.14.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boberg KM, Schrumpf E, Bergquist A, Broomé U, Pares A, Remotti H, Schjölberg A, Spurkland A, Clausen OP. Cholangiocarcinoma in primary sclerosing cholangitis: K-ras mutations and Tp53 dysfunction are implicated in the neoplastic development. J Hepatol. 2000;32:374–380. doi: 10.1016/s0168-8278(00)80386-4. [DOI] [PubMed] [Google Scholar]
- 61.Furubo S, Harada K, Shimonishi T, Katayanagi K, Tsui W, Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35:230–240. doi: 10.1046/j.1365-2559.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe M, Asaka M, Tanaka J, Kurosawa M, Kasai M, Miyazaki T. Point mutation of K-ras gene codon 12 in biliary tract tumors. Gastroenterology. 1994;107:1147–1153. doi: 10.1016/0016-5085(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 63.Tada M, Omata M, Ohto M. High incidence of ras gene mutation in intrahepatic cholangiocarcinoma. Cancer. 1992;69:1115–1118. doi: 10.1002/cncr.2820690509. [DOI] [PubMed] [Google Scholar]
- 64.Ahrendt SA, Rashid A, Chow JT, Eisenberger CF, Pitt HA, Sidransky D. p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J Hepatobiliary Pancreat Surg. 2000;7:426–431. doi: 10.1007/s005340070039. [DOI] [PubMed] [Google Scholar]
- 65.Isa T, Tomita S, Nakachi A, Miyazato H, Shimoji H, Kusano T, Muto Y, Furukawa M. Analysis of microsatellite instability, K-ras gene mutation and p53 protein overexpression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2002;49:604–608. [PubMed] [Google Scholar]
- 66.Levi S, Urbano-Ispizua A, Gill R, Thomas DM, Gilbertson J, Foster C, Marshall CJ. Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991;51:3497–3502. [PubMed] [Google Scholar]
- 67.Tannapfel A, Benicke M, Katalinic A, Uhlmann D, Köckerling F, Hauss J, Wittekind C. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–727. doi: 10.1136/gut.47.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang YK, Kim WH, Lee HW, Lee HK, Kim YI. Mutation of p53 and K-ras, and loss of heterozygosity of APC in intrahepatic cholangiocarcinoma. Lab Invest. 1999;79:477–483. [PubMed] [Google Scholar]
- 69.Hahn SA, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W. Mutations of the DPC4/Smad4 gene in biliary tract carcinoma. Cancer Res. 1998;58:1124–1126. [PubMed] [Google Scholar]
- 70.Taniai M, Higuchi H, Burgart LJ, Gores GJ. p16INK4a promoter mutations are frequent in primary sclerosing cholangitis (PSC) and PSC-associated cholangiocarcinoma. Gastroenterology. 2002;123:1090–1098. doi: 10.1053/gast.2002.36021. [DOI] [PubMed] [Google Scholar]
- 71.Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokomuro S, Tsuji H, Lunz JG 3rd, Sakamoto T, Ezure T, Murase N, Demetris AJ. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000;32:26–35. doi: 10.1053/jhep.2000.8535. [DOI] [PubMed] [Google Scholar]
- 73.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 74.Tadlock L, Patel T. Involvement of p38 mitogen-activated protein kinase signaling in transformed growth of a cholangiocarcinoma cell line. Hepatology. 2001;33:43–51. doi: 10.1053/jhep.2001.20676. [DOI] [PubMed] [Google Scholar]
- 75.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–1338. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, Sato Y, Harada K, Zen Y, Sato H, et al. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829–841. doi: 10.2353/ajpath.2009.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 79.Sakoda LC, Gao YT, Chen BE, Chen J, Rosenberg PS, Rashid A, Deng J, Shen MC, Wang BS, Han TQ, et al. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene polymorphisms and risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Carcinogenesis. 2006;27:1251–1256. doi: 10.1093/carcin/bgi314. [DOI] [PubMed] [Google Scholar]
- 80.Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E, Boberg KM, Lie BA. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology. 2008;47:90–96. doi: 10.1002/hep.21964. [DOI] [PubMed] [Google Scholar]
- 81.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 82.Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res. 2004;64:1369–1376. doi: 10.1158/0008-5472.can-03-1086. [DOI] [PubMed] [Google Scholar]
- 83.Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology. 2002;35:552–559. doi: 10.1053/jhep.2002.31774. [DOI] [PubMed] [Google Scholar]
- 84.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase upregulates cyclooxygenase-2 in mouse cholangiocytes promoting cell growth. Am J Physiol Gastrointest Liver Physiol. 2004;287:G88–G95. doi: 10.1152/ajpgi.00539.2003. [DOI] [PubMed] [Google Scholar]
- 85.Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–24063. doi: 10.1074/jbc.M500562200. [DOI] [PubMed] [Google Scholar]
- 86.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, Rozich RA, Hixson DC, Sirica AE. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–2057. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269–278. doi: 10.1046/j.1365-2559.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 89.Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41:808–814. doi: 10.1016/j.jhep.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 90.Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, Rozich RA, Hixson DC, Sirica AE. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–2057. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Socoteanu MP, Mott F, Alpini G, Frankel AE. c-Met targeted therapy of cholangiocarcinoma. World J Gastroenterol. 2008;14:2990–2994. doi: 10.3748/wjg.14.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Komori J, Marusawa H, Machimoto T, Endo Y, Kinoshita K, Kou T, Haga H, Ikai I, Uemoto S, Chiba T. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888–896. doi: 10.1002/hep.22125. [DOI] [PubMed] [Google Scholar]
- 93.Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–888. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156–163. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A, Marzioni M, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–6761. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–154. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 98.Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865–869. doi: 10.1016/0016-5085(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 99.Alvaro D, Macarri G, Mancino MG, Marzioni M, Bragazzi M, Onori P, Corradini SG, Invernizzi P, Franchitto A, Attili AF, et al. Serum and biliary insulin-like growth factor I and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Intern Med. 2007;147:451–459. doi: 10.7326/0003-4819-147-7-200710020-00003. [DOI] [PubMed] [Google Scholar]
- 100.Björnsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999;19:501–508. doi: 10.1111/j.1478-3231.1999.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 101.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 102.Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317–323. doi: 10.1097/MOG.0b013e3280495451. [DOI] [PubMed] [Google Scholar]
- 103.Morris-Stiff G, Bhati C, Olliff S, Hübscher S, Gunson B, Mayer D, Mirza D, Buckels J, Bramhall SR. Cholangiocarcinoma complicating primary sclerosing cholangitis: a 24-year experience. Dig Surg. 2008;25:126–132. doi: 10.1159/000128169. [DOI] [PubMed] [Google Scholar]
- 104.Schulick RD. Primary sclerosing cholangitis: detection of cancer in strictures. J Gastrointest Surg. 2008;12:420–422. doi: 10.1007/s11605-007-0345-2. [DOI] [PubMed] [Google Scholar]
- 105.Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279–284. doi: 10.1097/mog.0b013e328325a894. [DOI] [PubMed] [Google Scholar]
- 106.Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, Abu-Elmaagd K, Madariaga JR, Slivka A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002;56:40–47. doi: 10.1067/mge.2002.125105. [DOI] [PubMed] [Google Scholar]
- 107.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 108.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 109.Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, Nishihara S, Sugano K, Okura H, Fujita S, Hirohashi S. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58:512–518. [PubMed] [Google Scholar]
- 110.Vestergaard EM, Hein HO, Meyer H, Grunnet N, Jørgensen J, Wolf H, Orntoft TF. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999;45:54–61. [PubMed] [Google Scholar]
- 111.Akdoğan M, Saşmaz N, Kayhan B, Biyikoğlu I, Dişibeyaz S, Sahin B. Extraordinarily elevated CA19-9 in benign conditions: a case report and review of the literature. Tumori. 2001;87:337–339. doi: 10.1177/030089160108700513. [DOI] [PubMed] [Google Scholar]
- 112.Albert MB, Steinberg WM, Henry JP. Elevated serum levels of tumor marker CA19-9 in acute cholangitis. Dig Dis Sci. 1988;33:1223–1225. doi: 10.1007/BF01536670. [DOI] [PubMed] [Google Scholar]
- 113.Lempinen M, Isoniemi H, Mäkisalo H, Nordin A, Halme L, Arola J, Höckerstedt K, Stenman UH. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47:677–683. doi: 10.1016/j.jhep.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 114.Bamrungphon W, Prempracha N, Bunchu N, Rangdaeng S, Sandhu T, Srisukho S, Boonla C, Wongkham S. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301–308. doi: 10.1016/j.canlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 115.Boonla C, Sripa B, Thuwajit P, Cha-On U, Puapairoj A, Miwa M, Wongkham S. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:4939–4946. doi: 10.3748/wjg.v11.i32.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uenishi T, Yamazaki O, Tanaka H, Takemura S, Yamamoto T, Tanaka S, Nishiguchi S, Kubo S. Serum cytokeratin 19 fragment (CYFRA21-1) as a prognostic factor in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:583–589. doi: 10.1245/s10434-007-9650-y. [DOI] [PubMed] [Google Scholar]
- 117.Klump B, Hsieh CJ, Dette S, Holzmann K, Kiebetalich R, Jung M, Sinn U, Ortner M, Porschen R, Gregor M. Promoter methylation of INK4a/ARF as detected in bile-significance for the differential diagnosis in biliary disease. Clin Cancer Res. 2003;9:1773–1778. [PubMed] [Google Scholar]
- 118.Su WC, Shiesh SC, Liu HS, Chen CY, Chow NH, Lin XZ. Expression of oncogene products HER2/Neu and Ras and fibrosis-related growth factors bFGF, TGF-beta, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig Dis Sci. 2001;46:1387–1392. doi: 10.1023/a:1010619316436. [DOI] [PubMed] [Google Scholar]
- 119.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155–164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 120.Abu-Hamda EM, Baron TH. Endoscopic management of cholangiocarcinoma. Semin Liver Dis. 2004;24:165–175. doi: 10.1055/s-2004-828893. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe H, Enjoji M, Nakashima M, Noguchi K, Kinukawa N, Sugimoto R, Kotoh K, Nakamuta M, Nawata H, Watanabe T. Clinical significance of serum RCAS1 levels detected by monoclonal antibody 22-1-1 in patients with cholangiocellular carcinoma. J Hepatol. 2003;39:559–563. doi: 10.1016/s0168-8278(03)00363-5. [DOI] [PubMed] [Google Scholar]
- 122.Seehofer D, Kamphues C, Neuhaus P. Management of bile duct tumors. Expert Opin Pharmacother. 2008;9:2843–2856. doi: 10.1517/14656566.9.16.2843. [DOI] [PubMed] [Google Scholar]
- 123.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 124.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, Cappellani A, Malfermoni G, Iacono C. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 125.Casavilla FA, Marsh JW, Iwatsuki S, Todo S, Lee RG, Madariaga JR, Pinna A, Dvorchik I, Fung JJ, Starzl TE. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429–436. doi: 10.1016/s1072-7515(97)00088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lieser MJ, Barry MK, Rowland C, Ilstrup DM, Nagorney DM. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998;5:41–47. doi: 10.1007/pl00009949. [DOI] [PubMed] [Google Scholar]
- 127.Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70–79. doi: 10.1097/00000658-199801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–1531. doi: 10.1046/j.1365-2168.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 129.Isaji S, Kawarada Y, Taoka H, Tabata M, Suzuki H, Yokoi H. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6:108–116. doi: 10.1007/s005340050092. [DOI] [PubMed] [Google Scholar]
- 130.Valverde A, Bonhomme N, Farges O, Sauvanet A, Flejou JF, Belghiti J. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122–127. doi: 10.1007/s005340050094. [DOI] [PubMed] [Google Scholar]
- 131.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473; discussion 473-475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg. 2001;234:210–214. doi: 10.1097/00000658-200108000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bettschart V, Clayton RA, Parks RW, Garden OJ, Bellamy CO. Cholangiocarcinoma arising after biliary-enteric drainage procedures for benign disease. Gut. 2002;51:128–129. doi: 10.1136/gut.51.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 135.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 136.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, Nyberg SL, Ishitani MB, Rosen CB. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 137.Gores GJ, Nagorney DM, Rosen CB. Cholangiocarcinoma: is transplantation an option? For whom? J Hepatol. 2007;47:455–459. doi: 10.1016/j.jhep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 138.Zoepf T. Photodynamic therapy of cholangiocarcinoma. HPB (Oxford) 2008;10:161–163. doi: 10.1080/13651820801992625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baisden JM, Kahaleh M, Weiss GR, Sanfey H, Moskaluk CA, Yeaton P, de Lange EE, Rich TA. Multimodality Treatment With Helical Tomotherapy Intensity Modulated Radiotherapy, Capecitabine, and Photodynamic Therapy is Feasible and Well Tolerated in Patients With Hilar Cholangiocarcinoma. Gastrointest Cancer Res. 2008;2:219–224. [PMC free article] [PubMed] [Google Scholar]
- 140.Kiesslich T, Wolkersdörfer G, Neureiter D, Salmhofer H, Berr F. Photodynamic therapy for non-resectable perihilar cholangiocarcinoma. Photochem Photobiol Sci. 2009;8:23–30. doi: 10.1039/b813183j. [DOI] [PubMed] [Google Scholar]
- 141.Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965–969. doi: 10.1002/1097-0142(19840915)54:6<965::aid-cncr2820540603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 142.Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005;19:711–716. doi: 10.1155/2005/565479. [DOI] [PubMed] [Google Scholar]
- 143.Lee MA, Woo IS, Kang JH, Hong YS, Lee KS. Epirubicin, cisplatin, and protracted infusion of 5-FU (ECF) in advanced intrahepatic cholangiocarcinoma. J Cancer Res Clin Oncol. 2004;130:346–350. doi: 10.1007/s00432-003-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamashita Y, Taketomi A, Fukuzawa K, Yoshizumi T, Uchiyama H, Simada M, Shirabe K, Wakasugi K, Maehara Y. Gemcitabine combined with 5-fluorouracil and cisplatin (GFP) in patients with advanced biliary tree cancers: a pilot study. Anticancer Res. 2006;26:771–775. [PubMed] [Google Scholar]
- 145.Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C, Bralet MP. Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology. 2007;72:105–110. doi: 10.1159/000111117. [DOI] [PubMed] [Google Scholar]
- 146.Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 147.Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, Earle CC, Kulke MH, Bhargava P, Fuchs CS. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53:564–570. doi: 10.1007/s10620-007-9885-2. [DOI] [PubMed] [Google Scholar]
- 148.Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S. Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol. 2007;13:2852–2854. doi: 10.3748/wjg.v13.i20.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, Lee NS, Park BJ, Kim JS. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008;61:47–52. doi: 10.1007/s00280-007-0444-5. [DOI] [PubMed] [Google Scholar]
- 150.Dragovich T, Huberman M, Von Hoff DD, Rowinsky EK, Nadler P, Wood D, Hamilton M, Hage G, Wolf J, Patnaik A. Erlotinib plus gemcitabine in patients with unresectable pancreatic cancer and other solid tumors: phase IB trial. Cancer Chemother Pharmacol. 2007;60:295–303. doi: 10.1007/s00280-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 151.Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, Koppenhöfer U, Hochhaus A, Stieler J, Trojan J, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev. 2009;35:322–327. doi: 10.1016/j.ctrv.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 153.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 154.Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA, Hruban RH, Abrams RA. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68:178–182. doi: 10.1016/j.ijrobp.2006.11.048. [DOI] [PubMed] [Google Scholar]