Abstract
Even with the advent of laparoscopic techniques for liver tumours, classic resections still represent a major undertaking for numerous liver lesions. The avoidance of surgery using ablative techniques has been the aim for over 20 years. Large volumes can now be rapidly treated with low morbidity with the many technical developments and modifications of the delivery probes. Despite these advances recurrences rates remain high with all of the presently available techniques. The biological and pathophysiological basis underlying may help explain their limitations and are important in understanding where they may be appropriately applied and ways in which they may be improved in the future.
Keywords: Thermal ablation, Radiofrequency, Liver tumors, Microwaves, Cryotherapy, Tumor palliation
INTRODUCTION
Advances in liver surgery and anaesthesia have expanded their potential applications and a fifth of liver tumours are now resectable. Nevertheless, in the majority of patients surgery is not possible and chemotherapy and palliative care remain the mainstays of treatment. In these patients ablative techniques are an attractive option and offer an opportunity to increase survival. Experimental research over the last 20 years has seen the development of a number of ablative modalities and several of these have been applied clinically. This has provided hepatobiliary surgeons with techniques which can be used palliatively but also in combination with resection for multiple and awkwardly positioned lesions. Techniques which achieve local destruction by modification of the tissue temperatures adjacent to the treatment probe have been increasingly favoured over the “chemical” procedures (alcohol injection and tissue chemoembolization)[1]. However ablative techniques which utilise temperature changes are potentially associated with significant complications. In fact, although microwave (MW) and radiofrequency (RF) are used in clinical practice, cryotherapy (CRYO) has been largely abandoned and laser ablation was never widely employed.
Examining the literature regarding thermal ablation (TA) reveal many similarities but also demonstrate significant differences among techniques. These comparisons are valuable, identifying those properties that are essential for the development of a safe and reliable treatment. In addition, the data is important when designing research strategies to facilitate further technical developments. The purpose of this editorial is to examine some of these common critical factors that influence local failures and to propose approaches to overcoming the current limitations.
RECURRENCES RATES AFTER THERMAL ABLATIVE PROCEDURES AND RISK FACTORS
Technical advances have allowed modifications of the equipment used for thermal ablative techniques. Different approaches have been used in an attempt to augment the advantages of the individual techniques and allow wider clinical application. Nevertheless, data from clinical studies does not confirm the superiority of any particular modality especially in respect of recurrence rates. The rate of local recurrences ranged from 0% to 50% after MW ablation[2-6], 2% to 67% after RF[7-10], and 9% to 44% after CRYO[11-13]. Multivariate analysis of the risk factors that could potentially influence local failure after ablation found only a few which were important in determining outcome. The tumor size, number of nodules, and Child-Pugh classification had an influence after MW[14], tumor size and the ablative approach used (surgical versus percutaneous) after RFA[8] and tumor size, the presurgical level of carcinoembryonic antigen and tumor grading after CRYO[15,16].
REASONS FOR LOCAL FAILURE
Tumor size
With all ablative techniques, it is clear that tumor size is crucial and influences the short and long term results. Tumours of 3 cm or larger are most likely to recur and this is related to difficulties with achieving a complete ablation at the initial treatment. The ablation must encompass the lesion with a significant degree of overlap to ensure complete treatment and this can be difficult with large tumours due to the proximity of major vascular structures. One centimeter is considered a safe macroscopic margin and a 3 cm wide lesion therefore requires a 5 cm wide ablation to avoid local recurrence. Treatment zones which can be achieved with MW range between 3.5 and 5 cm[2,17,18], although newer devices have produced ablations with diameters up to 6.5 cm[19-22]. RF ablation is able to create lesions which range from 0.5 to 5 cm[23,24], although ablations of up to 7 cm have been reported[25]. In experimental CRYO studies most of the lesions produced were between 2 and 3 cm[26,27], with only one report described larger lesions with diameters up to 9 cm[28].
Different methods have been used to increase the diameters of ablated lesions and treat tumours greater than 3cm. Modifications of the shape and number of RFA electrodes and MW antennae lead to different shapes of the ablated zones and achieved larger diameters compared to the single straight configuration[18,21,22,29-31]. Another approach employs cooling of the RF-ablated tissue to avoid desiccation. The increase in tissue charring which occurs at the metal electrode–tissue interface during RF ablation results in elevated circuit impedance and markedly reduced RF output, limiting the heat diffusion. RF electrodes with internal cooling, those that release normal saline around the probe (wet electrodes) or where saline is added from an external source (saline-enhanced RFA) have all been investigated with promising results[29]. Finally, the temporary exclusion of vascular inflow (using a Pringle manoeuvre) almost doubled the diameters of the ablated lesions in all TA procedures and could potentially achieve volumes which are up to six-times greater[28,32-38].
Proximity to large vessels
Although tumour size is important both in respect of eligibility for initial surgery and the potential for treatment by an ablative technique some small liver tumours are still inoperable due to their anatomical position. Tumours adjacent to major vessels are often unresectable and at the same time at higher risks of recurrence and complications following TA. Large blood vessels (greater than 3 mm), and to a lesser degree bile ducts, are relatively protected from thermal injuries as the inflowing warm blood conducts the excessive temperature from the vessel wall, but the blood flow also prevents an adequate heating/freezing of perivascular tissues with consequent reduced destruction of tumour cells. The phenomenon is called “heat (or cold)-sink effect” and is the basis for incomplete ablations.
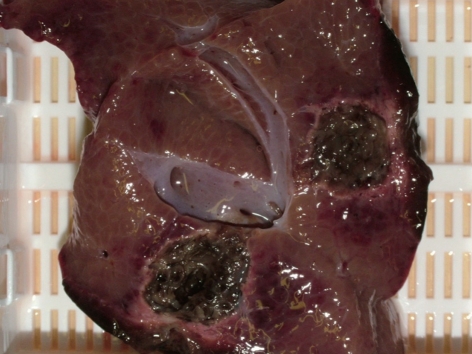
No specific methods have presently been developed to overcome this problem, which still remains a major contraindication to TA. However, there are other modalities which do not rely on temperature changes and are consequently not affected by local blood flow. Electrolytic ablation, also known as low-level direct current therapy, is an alternative method which produces no change in temperature and exerts its effects through electrochemical and pH modifications and the production of free radicals. These changes render the microenvironment close to the probe extremely cytotoxic, produce tissue necrosis and are not influenced to any large degree by the presence of blood vessels or bile ducts[39,40]. Due to the almost complete absence of thermal energy developed, the procedure has proved particularly safe in experimental studies where ablations were conducted close or even within major vessels (Figure 1)[41-43]. This characteristic makes this technique particularly useful for the treatment of lesions positioned close to major vessels[44]. The presence of the “electric-sink” effect, if any, does not modify significantly the shape of the ablated lesion which is able to include the vessel wall without damaging it[45]. If appropriate clinical trials confirm a clear survival advantage in this subgroup of patients with unresectable lesions close to major vessels, this could become a specific indication for electrolytic ablation that would negate the relative disadvantage of long ablation times compared to those of the more common TA procedures.
Figure 1.
Undamaged veins close to the ablated zone. Unpublished personal observations conducted in an ex-vivo normothermic autologous perfused porcine liver model.
Histological zones
Although minor differences exist in descriptions of the pathological changes following different TA procedures, the results are generally very similar and independent of the method employed. The ablated region is composed predominantly of two concentric zones with a central area of complete coagulative necrosis and a peripheral transitional rim of inflammation, congestion, hemorrhage and thrombosis. While the coagulative zone is occupied entirely by dead cells and amorphous material, the transitional rim still contain viable cells that may survive the adverse microconditions produced by the inflammatory environment and ultimately give rise to tumor recurrences[31,46,47]. In this area cells express heat shock proteins and die by apoptosis, the effect of which peaks 6 h following the ablation and expands the zone of definitive necrosis[48-51], and a combination of capillary microthrombosis and vasoconstriction further contributes to the enlargement of the necrotic area[52].
Problems resulting from the production of a transitional zone have to be addressed if recurrences in this rim of tissue are to be eliminated. Experimental studies have provided considerable details about the process and mechanisms, but to date have failed to identify a reliable strategy to overcome the problem. Nevertheless, there are a number of hypotheses that may allow the development of future strategies. Firstly, techniques that selectively enhance the transitional rim may better differentiate tumor recurrences from the physiological repair that occurs normally following ablation. The recent advent of targeted ultrasound (US) contrast media has been achieved by linking specific antibodies against a wide variety of antigens with US echogenic microbubbles[53,54]. In this way, inflammatory molecules (ICAM-1, VCAM-1 and E-selectin) have been successfully targeted by selective microbubbles able to visualise and identify different areas of inflammation[55]. A similar approach has been employed for magnetic resonance imaging contrasts media, in which oxide nanoparticles have been rendered selective for AvB3 Integrin and successfully tested in-vitro[56].
Secondly, the creation of US microbubbles specific for the transitional inflammatory rim and linked with chemotherapeutic agents would guarantee a selective delivery of the anti-tumor therapy to the zone where recurrences are most likely and they would also be able to visualize the zone at the same time to ensure it was effectively treated. Preliminary studies have already been conducted with targeted microbubbles loaded with chemotherapy for the treatment of breast, ovarian and pancreatic tumours[57,58]. In liver tumours a combined therapeutic approach can be envisaged which would consist of a TA procedures for the ablation of the majority of the tumour, and following the ablation selective delivery of chemotherapy to sterilize the remaining cells that may survive in the transitional inflammatory rim. Chemotherapy-loaded contrast agents could target not only inflammatory antigens but also tumor-specific molecules (i.e. CEA), and the simultaneous administering of both (inflammatory and tumor-specific loaded) could further help in the emergence prevention of resistant cells.
CONCLUSION
Although the best treatment for resectable liver tumour remains surgical resection[9], survival rates achieved by TA have been sufficiently promising that some centers have started to advocate trials comparing resection with ablation[10]. Limitations that favour tumour recurrence following TA are common to all techniques, but some of those that did not enter clinical trials due to the disadvantage of long ablation times could help in particular clinical settings. Furthermore, newer techniques like MW have intraoperative advantages over the older RF in terms of faster execution times clearly demonstrated in dose-response studies[19,25,59]. Unfortunately, modification of delivery probes and techniques have failed to reduce recurrence rates and a more integrated approach is required, possibly combining TA techniques with adjuvant therapies tailored to identify and treat specific areas in the transition zone.
Footnotes
Peer reviewer: Salvatore Gruttadauria, MD, PhD, Department of Abdominal Transplant Surgery, ISMETT-UPMC, Via E. Tricomi N. 1, Palermo 90 127, Italy
S- Editor Li LF E- Editor Yang C
References
- 1.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 2.Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, Xu ZF, Zheng YL, Liang JY. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna--experimental and clinical studies. Radiology. 2007;242:914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]
- 3.Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, Kuboki M, Yamamoto S. Radiofrequency ablation versus percutaneous microwave coagulation therapy for small hepatocellular carcinomas: a retrospective comparative study. Hepatogastroenterology. 2007;54:985–989. [PubMed] [Google Scholar]
- 4.Kawamoto C, Ido K, Isoda N, Hozumi M, Nagamine N, Ono K, Sato Y, Kobayashi Y, Nagae G, Sugano K. Long-term outcomes for patients with solitary hepatocellular carcinoma treated by laparoscopic microwave coagulation. Cancer. 2005;103:985–993. doi: 10.1002/cncr.20880. [DOI] [PubMed] [Google Scholar]
- 5.Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. Am J Roentgenol. 2003;180:1547–1555. doi: 10.2214/ajr.180.6.1801547. [DOI] [PubMed] [Google Scholar]
- 6.Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181–190. doi: 10.1001/archsurg.141.2.181. [DOI] [PubMed] [Google Scholar]
- 8.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD 3rd, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, et al. American Society of Clinical Oncology 2009 Clinical Evidence Review on Radiofrequency Ablation of Hepatic Metastases From Colorectal Cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 10.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445–460. doi: 10.1159/000184736. [DOI] [PubMed] [Google Scholar]
- 11.Adam R, Akpinar E, Johann M, Kunstlinger F, Majno P, Bismuth H. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg. 1997;225:39–48; discussion 48-50. doi: 10.1097/00000658-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onik G, Rubinsky B, Zemel R, Weaver L, Diamond D, Cobb C, Porterfield B. Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer. 1991;67:901–907. doi: 10.1002/1097-0142(19910215)67:4<901::aid-cncr2820670408>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Mala T, Edwin B, Mathisen Ø, Tillung T, Fosse E, Bergan A, SØreide O, Gladhaug I. Cryoablation of colorectal liver metastases: minimally invasive tumour control. Scand J Gastroenterol. 2004;39:571–578. doi: 10.1080/00365520410000510. [DOI] [PubMed] [Google Scholar]
- 14.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 15.Seifert JK, Morris DL. Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg. 1998;228:201–208. doi: 10.1097/00000658-199808000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifert JK, Morris DL. Indicators of recurrence following cryotherapy for hepatic metastases from colorectal cancer. Br J Surg. 1999;86:234–240. doi: 10.1046/j.1365-2168.1999.00995.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamashiki N, Kato T, Bejarano PA, Berho M, Montalvo B, Shebert RT, Goodman ZD, Seki T, Schiff ER, Tzakis AG. Histopathological changes after microwave coagulation therapy for patients with hepatocellular carcinoma: review of 15 explanted livers. Am J Gastroenterol. 2003;98:2052–2059. doi: 10.1111/j.1572-0241.2003.07642.x. [DOI] [PubMed] [Google Scholar]
- 18.Shen P, Geisinger KR, Zagoria R, Levine EA. Pathologic correlation study of microwave coagulation therapy for hepatic malignancies using a three-ring probe. J Gastrointest Surg. 2007;11:603–611. doi: 10.1007/s11605-006-0046-2. [DOI] [PubMed] [Google Scholar]
- 19.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, Goldberg SN. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 20.Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford) 2007;9:357–362. doi: 10.1080/13651820701646222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon CJ, Dupuy DE, Iannitti DA, Lu DS, Yu NC, Aswad BI, Busuttil RW, Lassman C. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:W333–W340. doi: 10.2214/AJR.05.0804. [DOI] [PubMed] [Google Scholar]
- 22.Yu NC, Lu DS, Raman SS, Dupuy DE, Simon CJ, Lassman C, Aswad BI, Ianniti D, Busuttil RW. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters--pilot comparison with pathologic findings. Radiology. 2006;239:269–275. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 24.Goldberg SN, Walovitch RC, Straub JA, Shore MT, Gazelle GS. Radio-frequency-induced coagulation necrosis in rabbits: immediate detection at US with a synthetic microsphere contrast agent. Radiology. 1999;213:438–444. doi: 10.1148/radiology.213.2.r99nv17438. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto R, Selig AM, Colucci VM, Jolesz FA. MR monitoring during cryotherapy in the liver: predictability of histologic outcome. J Magn Reson Imaging. 1993;3:770–776. doi: 10.1002/jmri.1880030513. [DOI] [PubMed] [Google Scholar]
- 27.Tacke J, Adam G, Haage P, Sellhaus B, Grosskortenhaus S, Günther RW. MR-guided percutaneous cryotherapy of the liver: in vivo evaluation with histologic correlation in an animal model. J Magn Reson Imaging. 2001;13:50–56. doi: 10.1002/1522-2586(200101)13:1<50::aid-jmri1008>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Seifert JK, Gerharz CD, Mattes F, Nassir F, Fachinger K, Beil C, Junginger T. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology. 2003;47:214–226. doi: 10.1016/j.cryobiol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Mulier S, Miao Y, Mulier P, Dupas B, Pereira P, de Baere T, Lencioni R, Leveillee R, Marchal G, Michel L, et al. Electrodes and multiple electrode systems for radiofrequency ablation: a proposal for updated terminology. Eur Radiol. 2005;15:798–808. doi: 10.1007/s00330-004-2584-x. [DOI] [PubMed] [Google Scholar]
- 30.Mulier S, Ni Y, Frich L, Burdio F, Denys AL, De Wispelaere JF, Dupas B, Habib N, Hoey M, Jansen MC, et al. Experimental and clinical radiofrequency ablation: proposal for standardized description of coagulation size and geometry. Ann Surg Oncol. 2007;14:1381–1396. doi: 10.1245/s10434-006-9033-9. [DOI] [PubMed] [Google Scholar]
- 31.Gravante G, Ong SL, Metcalfe MS, Strickland A, Dennison AR, Lloyd DM. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int. 2008;28:911–921. doi: 10.1111/j.1478-3231.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 32.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC 3rd, Warner TF, Mahvi DM. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. Am J Roentgenol. 2001;176:789–795. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- 34.Chang CK, Hendy MP, Smith JM, Recht MH, Welling RE. Radiofrequency ablation of the porcine liver with complete hepatic vascular occlusion. Ann Surg Oncol. 2002;9:594–598. doi: 10.1007/BF02573897. [DOI] [PubMed] [Google Scholar]
- 35.Shen P, Fleming S, Westcott C, Challa V. Laparoscopic radiofrequency ablation of the liver in proximity to major vasculature: effect of the Pringle maneuver. J Surg Oncol. 2003;83:36–41. doi: 10.1002/jso.10235. [DOI] [PubMed] [Google Scholar]
- 36.Shibata T, Murakami T, Ogata N. Percutaneous microwave coagulation therapy for patients with primary and metastatic hepatic tumors during interruption of hepatic blood flow. Cancer. 2000;88:302–311. [PubMed] [Google Scholar]
- 37.Shibata T, Niinobu T, Ogata N. Comparison of the effects of in-vivo thermal ablation of pig liver by microwave and radiofrequency coagulation. J Hepatobiliary Pancreat Surg. 2000;7:592–598. doi: 10.1007/s005340070009. [DOI] [PubMed] [Google Scholar]
- 38.Mala T, Frich L, Aurdal L, Clausen OP, Edwin B, Søreide O, Gladhaug IP. Hepatic vascular inflow occlusion enhances tissue destruction during cryoablation of porcine liver. J Surg Res. 2003;115:265–271. doi: 10.1016/j.jss.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Hagedorn R, Fuhr G. Steady state electrolysis and isoelectric focusing. Electrophoresis. 1990;11:281–289. doi: 10.1002/elps.1150110402. [DOI] [PubMed] [Google Scholar]
- 40.Baxter PS, Wemyss-Holden SA, Dennison AR, Maddern GJ. Electrochemically induced hepatic necrosis: the next step forward in patients with unresectable liver tumours? Aust N Z J Surg. 1998;68:637–640. doi: 10.1111/j.1445-2197.1998.tb04833.x. [DOI] [PubMed] [Google Scholar]
- 41.Wemyss-Holden SA, Dennison AR, Finch GJ, Hall Pd Pde L, Maddern GJ. Electrolytic ablation as an adjunct to liver resection: experimental studies of predictability and safety. Br J Surg. 2002;89:579–585. doi: 10.1046/j.1365-2168.2002.02064.x. [DOI] [PubMed] [Google Scholar]
- 42.Wemyss-Holden SA, de la M Hall P, Robertson GS, Dennison AR, Vanderzon PS, Maddern GJ. The safety of electrolytically induced hepatic necrosis in a pig model. Aust N Z J Surg. 2000;70:607–612. doi: 10.1046/j.1440-1622.2000.01907.x. [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe MS, Mullin EJ, Texler M, Berry DP, Dennison AR, Maddern GJ. The safety and efficacy of radiofrequency and electrolytic ablation created adjacent to large hepatic veins in a porcine model. Eur J Surg Oncol. 2007;33:662–667. doi: 10.1016/j.ejso.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Wemyss-Holden SA, Dennison AR, Berry DP, Maddern GJ. Local ablation for unresectable liver tumors: is thermal best? J Hepatobiliary Pancreat Surg. 2004;11:97–106. doi: 10.1007/s00534-002-0715-9. [DOI] [PubMed] [Google Scholar]
- 45.Finch JG, Fosh B, Anthony A, Slimani E, Texler M, Berry DP, Dennison AR, Maddern GJ. Liver electrolysis: pH can reliably monitor the extent of hepatic ablation in pigs. Clin Sci (Lond) 2002;102:389–395. [PubMed] [Google Scholar]
- 46.Fraser J, Gill W. Observations on ultra-frozen tissue. Br J Surg. 1967;54:770–776. doi: 10.1002/bjs.1800540907. [DOI] [PubMed] [Google Scholar]
- 47.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41:168–172. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- 48.Ohno T, Kawano K, Sasaki A, Aramaki M, Yoshida T, Kitano S. Expansion of an ablated site and induction of apoptosis after microwave coagulation therapy in rat liver. J Hepatobiliary Pancreat Surg. 2001;8:360–366. doi: 10.1007/s005340170009. [DOI] [PubMed] [Google Scholar]
- 49.Nikfarjam M, Muralidharan V, Su K, Malcontenti-Wilson C, Christophi C. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperthermia. 2005;21:319–332. doi: 10.1080/02656730500133736. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman AL, Wu SS, Obaid AK, French SW, Lois J, McMonigle M, Ramos HC, Sher LS, Lopez RR. Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg. 2002;68:1038–1043. [PubMed] [Google Scholar]
- 51.Rai R, Richardson C, Flecknell P, Robertson H, Burt A, Manas DM. Study of apoptosis and heat shock protein (HSP) expression in hepatocytes following radiofrequency ablation (RFA) J Surg Res. 2005;129:147–151. doi: 10.1016/j.jss.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Strickland AD, Clegg PJ, Cronin NJ, Swift B, Festing M, West KP, Robertson GS, Lloyd DM. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003–1007. doi: 10.1046/j.1365-2168.2002.02155.x. [DOI] [PubMed] [Google Scholar]
- 53.Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 2006;41:354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- 54.Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol. 2007;14:876–884. doi: 10.1016/j.nuclcard.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 56.Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswal S, Wang A, Chen X. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials. 2009;30:6912–6919. doi: 10.1016/j.biomaterials.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48:260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorentzen T, Christensen NE, Nolsłe CP, Torp-Pedersen ST. Radiofrequency tissue ablation with a cooled needle in vitro: ultrasonography, dose response, and lesion temperature. Acad Radiol. 1997;4:292–297. doi: 10.1016/s1076-6332(97)80031-1. [DOI] [PubMed] [Google Scholar]