Abstract
Intraductal papillary mucinous neoplasms (IPMN) can be difficult to distinguish from other cystic lesions of the pancreas. To understand better and discuss the current knowledge on this topic, the literature and the institutional experience at a large pancreatic disease center have been reviewed. A combination of preoperative demographic, historical, radiographic, laboratory data, as well as postoperative pathologic analyses can often distinguish IPMN from other lesions in the differential diagnosis.
Keywords: Intraductal papillary mucinous neoplasms, Pancreatic cyst, Differential diagnosis, Pancreas cancer
INTRODUCTION
Intraductal papillary mucinous neoplasm (IPMN) is defined as an intraductal grossly visible (typically ≥ 1.0 cm) epithelial neoplasm of mucin-producing cells, arising in the main pancreatic duct or its branches[1,2]. This relatively new term, IPMN, has replaced such terms as “mucin-producing tumor” and “mucinous ductal ectasia.” Distinguishing IPMN from other cystic lesions of the pancreas can often be accomplished on clinical, endoscopic, cytological and radiographic grounds. The diagnostic entities that must be considered in patients with cystic lesions of the pancreas are IPMN, mucinous cystic neoplasm, serous cystadenoma, pancreatic pseudocyst, solid-pseudopapillary neoplasm, lymphoepithelial cyst, cystic neuroendocrine tumor, cystic degeneration of invasive pancreatic carcinoma, and other rare entities such as acinar cell cystadenocarcinoma. Here we briefly review the highlights in the literature, and then present our approach to differentiating IPMN from of other cystic lesions of the pancreas.
LITERATURE REVIEW
The literature prior to the 1996 World Health Organization (WHO) definition of IPMN is difficult to interpret owing to lack of consensus definition and inconsistent recognition of these lesions. The rising incidence of IPMNs since the 1990s may therefore be attributed to increased recognition and detection. Major advances in the literature since the 1996 WHO definition have included the publication of several large series, the Sendai guidelines, and nomograms to aid clinical decision-making (vide infra).
One of the largest single-institution series of IMPNs in the literature, from Johns Hopkins Hospital, was recently updated to include a total of 136 resections for IPMN[3,4]. These patients had a mean age of 67 years, and underwent either pancreaticoduodenectomy (71%), total pancreatectomy (15%), distal pancreatectomy (12%), or central pancreatectomy (2%). Patients were stratified into those who had an IPMN associated with an invasive carcinoma (38%) and those who had an IPMN without an associated invasive carcinoma (62%). Based on histological features, noninvasive lesions were categorized as having low-grade dysplasia (17%), moderate dysplasia (28%), or high-grade dysplasia (55%). Interestingly, those patients with an IPMN and an invasive carcinoma were older than those with a noninvasive IPMN with low-grade dysplasia (63 years vs 68 years; P = 0.08), with high-grade dysplasia patients having an intermediate age of 67 years, suggesting the possibility of a progression over years, akin to that observed in the progression from colon adenomas to invasive colon carcinomas. The overall 5-year survival of patients with noninvasive IPMNs was 77% while only 43% of patients with an IPMN with an associated invasive carcinoma survived 5 years. Other series[5-9] have found similar results regarding the demographics, the proportion associated with an invasive carcinoma, and 5-year survival. The largest collaborative series, from Massachusetts General Hospital and the University of Verona[5], was also recently updated. When branch-duct IPMN was compared to either main-duct or combined IPMN, there were significantly more low-grade dysplasias in the branch-duct group and significantly more IPMNs with an associated invasive carcinoma in the main-duct/combined group, an observation that is part of the foundation for the now widely recognized importance of recommending resection to patients with main-duct lesions, as expressed in consensus statements[10,11]. IPMNs that do progress to invasive cancer, however, have a significantly longer 5-year survival (42%) than do invasive ductal adenocarcinoma not associated with IPMN (19%; P < 0.001 )[12].
The first adequate - and currently the most commonly employed - set of consensus guidelines regarding the clinical management of IPMNs was the Sendai International Consensus Guidelines, first published online in 2005 by the International Association of Pancreatology[10,11]. These guidelines addressed not only to the accurate diagnosis of IPMNs (viz. differentiating IPMN from mucinous cystic neoplasm), but the determination of which lesions warrant resection and which can be safely observed. Although the best choice of diagnostic imaging modality is largely institution-dependent, Tanaka et al[10,11] recommend magnetic resonance imaging (MRI) as the best modality to outline the gross appearance of the lesion and endoscopic retrograde cholangiopancreatography (ERCP) as the best method to identify ductal communication. The Sendai guidelines identify the presence of symptoms, a main-duct component, diameter > 3 cm, and any solid component as relative indications for resection in appropriately selected patients. We would add to this list rapid rate of growth and young age.
One very recent study has evaluated the use of Markov modeling and nomograms to assist with clinical decision-making in patients with small asymptomatic branch-duct IPMNs who are balancing the risks and benefits of resection versus observation: Weinberg et al[13] found that the decision to resect or observe depended on patient age and comorbidities, cyst size, and patients’ valuing of overall survival versus quality-adjusted survival. For those valuing overall survival primarily, irrespective of quality of life, resection was optimal for lesions > 2 cm. Patients focused on quality of life however, required a 3-cm threshold for resection except for the extreme elderly.
DIFFERENTIATING INTRADUCTAL PAPILLARY MUCINOUS NEOPLASM FROM OTHER LESIONS
Our approach for differentiating IPMN from other lesions is based on the distinguishing characteristics of these tumors and is presented in Tables 1 and 2. These characteristics have been identified from our experience at the Johns Hopkins Hospital and from the expanding body of literature on pancreatic cystic lesions.
Table 1.
| Typical characteristics | IPMN | MCN | SC | PSEUDO | SPN | LEC | cNET | cPDAC |
| Age Group | Elderly | Middle | Middle-Elderly | Any | Young | Elderly | Middle-Elderly | Elderly |
| Gender | 70% male | 95% female | > 50% female | > 50% male | 80%-90% female | 80% male | 50% each | > 50% male |
| History | Asx; Pain; ± jaundice | Asx; Pain; nausea | Asx; VHL | Pancreatitis | Asx; Pain; nausea | Asx | Asx; Fxnl; MEN | Asx; Pain; ± jaundice |
| Location in pancreas | Head in 70%; Multi-focal | Body/Tail in 95% | Anywhere | Anywhere | Anywhere | Peripheral | Anywhere | Anywhere |
| Shape | Ovoid | Spheroid | Ovoid | Spheroid | Ovoid | Ovoid | Spheroid | Variable |
| Locularity | Any | Uni or Oligo | Oligo or Multi | Uni | Oligo or Multi | Oligo | Uni | Any |
| Duct Com-munication | Common | No | No | Common | No | No | No | Some |
| Calcification | No | No | Central sunburst | No | Some | No | Some | No |
| Cyst fluid appearance | Viscous, clear, muc | Viscous, clear, muc | Thin, clear, nonmuc | Opaque, bloody/necrotic debris | Opaque, bloody/necrotic debris | Nonmuc, crystalline debris | Nonmuc | Thin |
| High CEA/Mucina | + | + | - | - | - | - | - | ± |
| High Ca 19-9 | ± | ± | - | - | - | - | - | ± |
| High amylase | + | - | - | + | - | - | - | ± |
| Epithelium | Columnar, Papillary | Columnar | Cuboidal | No epithelium | Poorly cohesive cells with nuclear grooves | Squamoid | Uniform | Gland-forming |
| Stroma | Fibrotic | Ovarian | Fibrotic | Fibrotic | Sometimes hyalinized | Lymphoid | Sometimes hyalinized | Fibrotic |
aMay be positive in cases of luminal contamination of endoscopic needle aspirate. IPMN: Intraductal papillary mucinous neoplasm; MCN: Mucinous cystic neoplasm; SC: Serous cystadenoma; PSEUDO: Pancreatic pseudocyst; SPN: Solid-pseudopapillary neoplasm; LE: Lymphoepithelial cyst; cNET: Cystic neuroendocrine tumor; cPDAC: Pancreatic ductal adenocarcinoma with cystic degeneration; VHL: Von hippel-lindau disease; Muc: Mucinous; Nonmuc: Nonmucinous; Asx: Asymptomatic; Fxnl: Functional. These data are derived generalizations of the literature, with the understanding that there is significant overlap among cyst types and there are inherent sampling errors associated with various tests; diagnostic and treatment decisions should not rely solely on the information presented in this review. An electronic worksheet version of this table is available at http://pathology.jhu.edu/pancreas/professionals/ipmn.php
Table 2.
Key questions to aid in making likely diagnoses[19]
| Key question | Likely diagnoses to consider | |
| Demographics and history | Male? | MCN unlikely |
| No history of pancreatitis? | PSEUDO unlikely | |
| Young female? | SPN | |
| History of MEN? | cNET | |
| History VHL? | SC | |
| Imaging | Spheroid? | PSEUDO or MCN |
| Central sunburst calcification? | SC | |
| Location in head? | MCN unlikely | |
| Cyst fluid | No CEA/mucin? | IPMN or MCN unlikely |
| High CEA, high amylase? | IPMN | |
| High CEA, low amylase | MCN | |
| Low CEA, high amylase? | PSUEDO | |
| High amylase? | IPMN or PSEUDO | |
| Histology | Epithelial lining? | PSEUDO unlikely |
| Ovarian stroma? | MCN |
IPMN: Intraductal papillary mucinous neoplasm; MCN: Mucinous cystic neoplasm; SC: Serous cystadenoma; PSEUDO: Pancreatic pseudocyst; SPN: Solid-pseudopapillary neoplasm; VHL: Von hippel-lindau disease; MEN: Multiple endocrine neoplasia. These data are derived generalizations of the literature, with the understanding that there is significant overlap among cyst types and there are inherent sampling errors associated with various tests; diagnostic and treatment decisions should not rely solely on the information presented in this review. An electronic worksheet version of this table is available at http://pathology.jhu.edu/pancreas/professionals/ipmn.php
When presented with a patient harboring a cystic lesion of unknown identity in the pancreas, one can often eliminate immediately several entities from the list of likely diagnoses, depending on the patient’s demographics and history. For example, a helpful starting point is simply the question, What is the patient’s gender? If the patient is male, then at least one diagnosis, mucinous cystic neoplasm, is very unlikely, as 95% of mucinous cystic neoplasms occur in women (Figure 1). Similarly if the patient does not have a history of pancreatitis then a diagnosis of pancreatic pseudocyst is virtually excluded (Figure 2). We then consider the patient’s age, which is helpful if the patient is very young, since one of the diagnoses in the differential - solid-pseudopapillary neoplasm - tends to occur in young (and female) patients(Figure 3). Next we evaluate the patient’s family medical history. Although uncommon, some patients with cystic lesions of the pancreas have a familial or personal history of von Hippel-Lindau (VHL) disease or multiple endocrine neoplasia (MEN), and VHL and MEN are associated with serous cystadenoma (Figure 4) and cystic neuroendocrine neoplasms (Figure 5), respectively.
Figure 1.
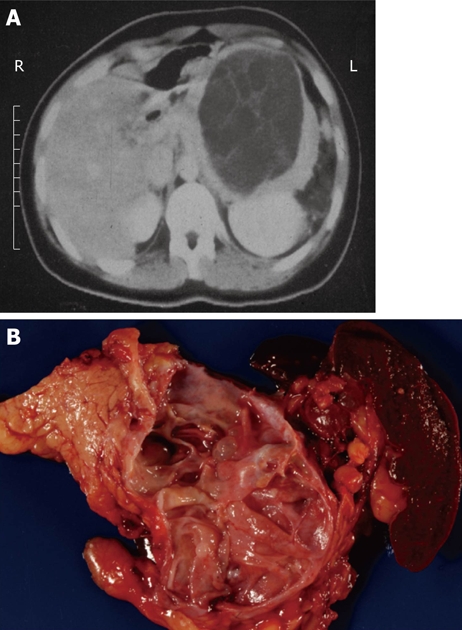
Typical computed tomography (A) and gross (B) appearance of a mucinous cystic neoplasm showing the distal location and the lack of communication with the duct, respectively.
Figure 2.
Typical computed tomography (A) and gross (B) appearance of a small pancreatic pseudocyst showing the typical spheroid shape, unilocularity, and necrotic debris contents.
Figure 3.
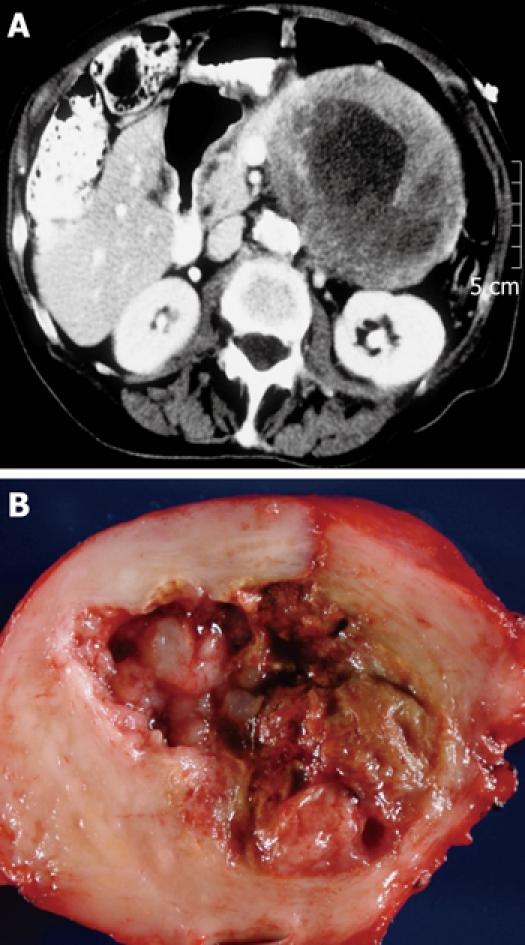
Typical computed tomography (A) and gross (B) appearance of a solid pseudopapillary neoplasm showing the typical ovoid shape and necrotic debris contents.
Figure 4.
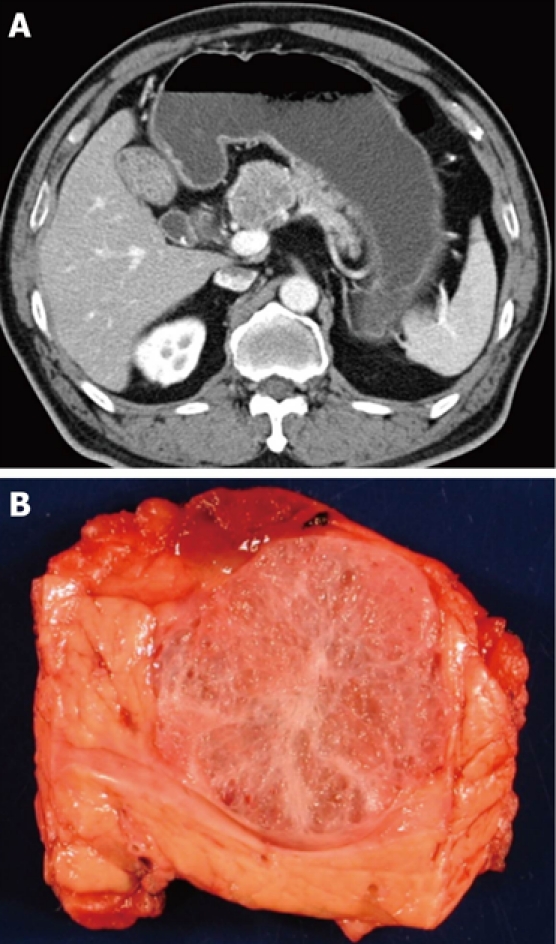
Typical computed tomography (A) and gross (B) appearance of a serous cystadenoma showing the honeycomb appearance.
Figure 5.
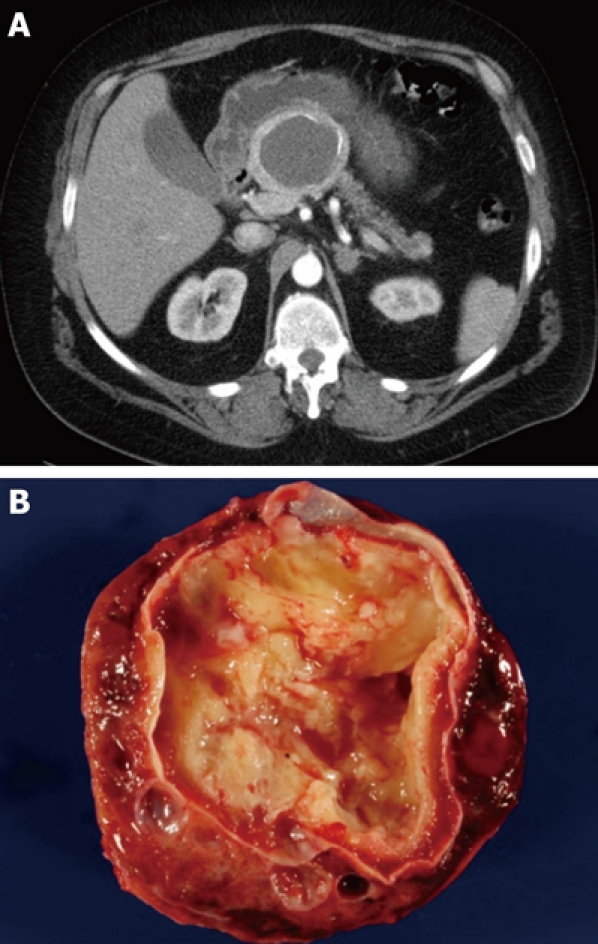
Typical computed tomography (A) and gross (B) appearance of a cystic neuroendocrine tumor showing the spherical shape and the occasionally seen calcification.
The next most available information after demographics and history is typically imaging data. The baseline imaging modality of choice is largely institution-dependent. Some centers rely heavily on endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI). At our institution, computed tomography (CT) imaging of the pancreas and its interpretation are exceptionally good, so we tend to use it very often, especially as an initial screening tool. We also use EUS to look for nodules and to obtain tissue or fluid when indicated. MRI, especially in combination secretin stimulation, is used selectively and can be quite sensitive in following smaller cysts in the pancreas. As with male gender, location of the cyst in the head of the pancreas significantly reduces the likelihood that the lesion is a mucinous cystic neoplasm, as most mucinous cystic neoplasms arise in the body or tail of the gland. Simply assessing the shape of the lesion may also help, as many mucinous cystic neoplasms and serous cystadenomas are often spherical. Using ERCP, MRCP, or (as discussed below) determination of the amylase content of fluid obtained by fine needle aspiration, one may next answer the key question, Does the cyst communicate with the pancreatic duct (Figure 1)? We have found ERCP and fluid amylase concentration to be more sensitive and more reliable than MRCP. An affirmative answer here, in the absence of a history of pancreatitis, weighs heavily in favor of a diagnosis of IPMN since the vast majority of the other cystic lesions do not communicate with the duct system (Figure 6). Finally, the identification of a typical sunburst pattern of central calcification or honeycomb appearance is virtually pathognomonic for serous cystadenoma (Figure 4).
Figure 6.
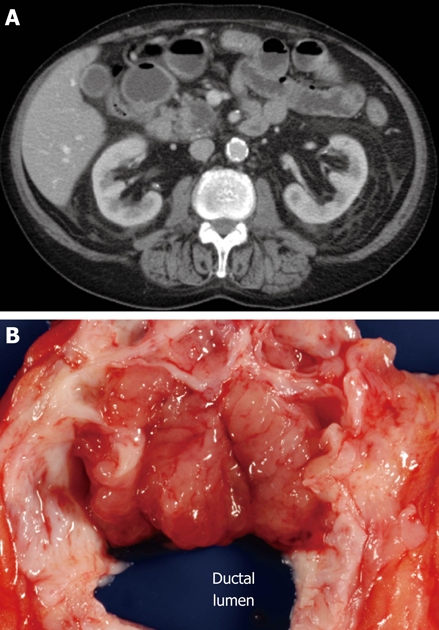
Typical computed tomography (A) and gross (B) appearance of an intraductal papillary mucinous neoplasm showing the ovoid shape and communication with the duct, respectively.
The character of the cyst fluid, which is often ascertained at the time of EUS and fine needle biopsy, can also help in the differential diagnosis. The first and easiest characteristic to assess is the gross appearance of the cystic fluid: viscous, mucinous fluid is consistent with IPMN or mucinous cystic neoplasm, while opaque fluid with necrotic or hemorrhagic debris is typical of pancreatic pseudocyst or solid-pseudopapillary neoplasm, and fluid that is thin (nonmucinous) and clear (may be straw-colored or blood-stained) is usually seen with serous cystadenoma and the less common lymphoepithelial cyst (Figure 7), cystic neuroendocrine neoplasm, and invasive carcinoma with cystic degeneration (Figure 8).
Figure 7.
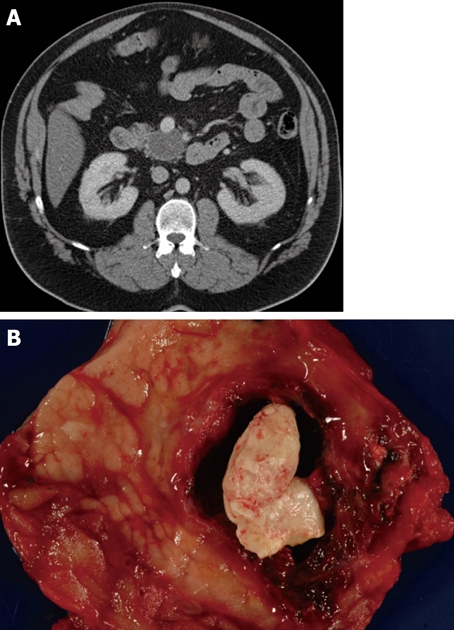
Typical computed tomography (A) and gross (B) appearance of a lymphoepithelial cyst showing the typical ovoid shape, peripheral location, and proteinaceous concretions (not always present on computed tomography imaging).
Figure 8.
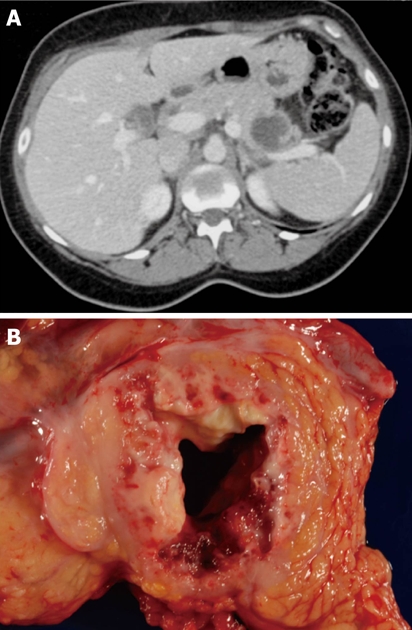
Typical computed tomography (A) and gross (B) appearance of an invasive carcinoma with cystic degeneration.
Laboratory evaluation of the cyst fluid can also help focus the differential diagnosis. Most commonly, positive mucin staining or high levels of CEA, while sometimes the result of gastrointestinal luminal contamination, supports a diagnosis of either IPMN or mucinous cystic neoplasm, which can be distinguished from each other by the cyst fluid amylase level (high in IPMNs communicating with the duct and low in mucinous cystic neoplasm, which do not communicate with the duct). The absence of mucin or low levels of CEA make IPMN and mucinous cystic neoplasm less likely diagnoses, pushing higher on list of possible diagnoses serous cystadenoma, pancreatic pseudocyst, and solid-pseudopapillary neoplasm. While pancreatic pseudocyst can be eliminated if the cyst amylase levels are low, serous cystadenoma and solid-pseudopapillary neoplasm have similar cyst fluid laboratory profiles.
Of course the goal is to be able to make the diagnosis prior to resection, but diagnostic uncertainty can persist until the final pathologic examination of the resected specimen. Pseudocysts lack an epithelial lining, IPMNs are composed of columnar mucin-producing cells that involve the pancreatic duct system, mucinous cystic neoplasms have ovarian-type stroma, and solid-pseudopapillary neoplasms are composed of loosely cohesive cells and delicate branching blood vessels.
Footnotes
Peer reviewer: Giuseppe R Nigri, Professor, Department of Surgery, Sapienza University, Via di Grottarossa 1035, Rome 00189, Italy
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
References
- 1.Klöppel G, Heitz PU, Capella C, Solcia E. Pathology and nomenclature of human gastrointestinal neuroendocrine (carcinoid) tumors and related lesions. World J Surg. 1996;20:132–141. doi: 10.1007/s002689900021. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797; discussion 797-799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321; discussion 321-322. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raut CP, Cleary KR, Staerkel GA, Abbruzzese JL, Wolff RA, Lee JH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 8.Azar C, Van de Stadt J, Rickaert F, Devière M, Baize M, Klöppel G, Gelin M, Cremer M. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called "mucinous ductal ectasia". Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576–589. doi: 10.1097/00000478-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M. International consensus guidelines for the management of IPMN and MCN of the pancreas. Nippon Shokakibyo Gakkai Zasshi. 2007;104:1338–1343. [PubMed] [Google Scholar]
- 11.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 12.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg BM, Spiegel BM, Tomlinson JS, Farrell JJ. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology. 2010;138:531–540. doi: 10.1053/j.gastro.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 15.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Fasanella KE, McGrath K. Cystic lesions and intraductal neoplasms of the pancreas. Best Pract Res Clin Gastroenterol. 2009;23:35–48. doi: 10.1016/j.bpg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Adsay NV, Hasteh F, Cheng JD, Bejarano PA, Lauwers GY, Batts KP, Klöppel G, Klimstra DS. Lymphoepithelial cysts of the pancreas: a report of 12 cases and a review of the literature. Mod Pathol. 2002;15:492–501. doi: 10.1038/modpathol.3880553. [DOI] [PubMed] [Google Scholar]
- 18.Hruban RH. Johns Hopkins Pancreas Cancer Web, Johns Hopkins Medical Institutions. Available from: http://pathology2.jhu.edu/pancreascyst.
- 19.Cunningham SC, Hruban RH, Schulick RD. The Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins: Pancreas Cyst Worksheet; Johns Hopkins Medical Institutions. Available from: http://pathology.jhu.edu/pancreas/professionals/ipmn.php.



