Abstract
Over the past decades evidence has been accumulating that intestinal barrier integrity loss plays a key role in the development and perpetuation of a variety of disease states including inflammatory bowel disease and celiac disease, and is a key player in the onset of sepsis and multiple organ failure in situations of intestinal hypoperfusion, including trauma and major surgery. Insight into gut barrier integrity and function loss is important to improve our knowledge on disease etiology and pathophysiology and contributes to early detection and/or secondary prevention of disease. A variety of tests have been developed to assess intestinal epithelial cell damage, intestinal tight junction status and consequences of intestinal barrier integrity loss, i.e. increased intestinal permeability. This review discusses currently available methods for evaluating loss of human intestinal barrier integrity and function.
Keywords: Intestinal integrity, Intestinal barrier function, Intestinal permeability, Markers
INTRODUCTION
The gastrointestinal tract is the most extended surface acting as a barrier between external environment and internal milieu. The host integrity is maintained by effective monitoring of the mucosal surface and sealing the host interior against potentially harmful compounds such as bacteria, toxins and antigens. This function of the gastrointestinal tract is referred to as intestinal barrier function. The intestinal epithelial barrier function consists of multiple defense mechanisms which can basically be subdivided into a physical and an immunological barrier[1-3].
The physical intestinal barrier is composed of a lining of epithelial cells, connected by tight junctions (Figure 1A). These adhesion structures serve as a fence sealing the paracellular pathway, thereby preventing exposure of the internal milieu to potentially harmful intraluminal microbiota and microbial products[4]. Tight junctions are anchored in the cell via the filamentous actin (F-actin) cytoskeleton[5]. Zonula occludens proteins (ZO-1, ZO-2 and ZO-3) are important intracellular tight junction proteins, linking the cell cytoskeleton to the transmembrane tight junction proteins: claudins, occluden and junctional adhesion molecules (JAM). Whereas occludin and JAM have a regulatory role, claudins are transmembrane proteins mainly responsible for the intestinal barrier function[6]. The physical barrier is reinforced by the presence of a mucus layer, produced and secreted by goblet cells[7]. The immune barrier is formed by specialized epithelial cells, the Paneth cells, located in the crypts of the small intestine, which can actively sense bacterial presence and prevent colonization of the crypts by releasing antimicrobial proteins including lysozyme and defensins[8,9]. Furthermore, lamina propria immune cells actively participate as immune sensors of microbial pathogens and commensal organisms. Bacterial recognition is dependent on transmembrane and intracellular pattern recognition receptors, including the structurally homologous Toll-like receptor (TLR) and NOD-like receptor (NLR) family. Ligation to these bacterial receptors stimulates central signaling cascades (NF-κB, AKT/phosphatidylinositol-3’-kinase and mitogen-activated protein kinase pathways), resulting in an immunological response[10-13].
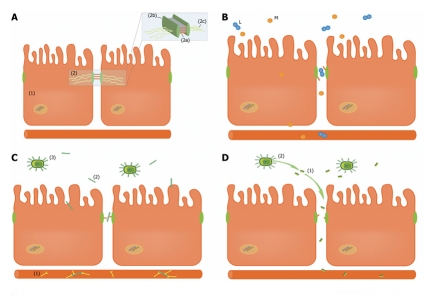
Figure 1.
Translocation of compounds from the gut lumen to the circulation via a defective intestinal barrier. A: The intestinal epithelial barrier is composed of a lining of enterocytes (1) tightly connected by tight junctions (2) to prevent the translocation of intraluminal compounds to the circulation. Claudins (2a), important transmembrane tight junction proteins responsible for sealing the paracellular space, are tightly connected to intracellular protein ZO-1 (2b), which is anchored to the cell cytoskeleton (2c); B: Differential sugar absorption test: Lactulose (L), a disaccharide, is only able to traverse the paracellular pathway in case of compromised intestinal barrier function. Mannitol (M) is a monosaccharide which can cross the intestinal barrier both via the trans- and paracellular pathway, thereby serving as an internal control to correct for confounders as gastric emptying, mucosal perfusion and renal function; C: Endotoxin core antibody (EndoCAb) (1) is consumed when endotoxin (2), derived from intraluminal Gram-negative bacteria (3), translocates from the intestinal lumen to the circulation via the defective intestinal barrier; D: D-Lactate (1) is a fermenting product from intestinal bacteria (2). In case of barrier function loss, D-Lactate can be detected in plasma.
Disturbed intestinal barrier function is considered a key factor in the development and/or progression of intestinal inflammation, and is therefore thought to play a role in both the pathogenesis and the perpetuation of various intestinal diseases including inflammatory bowel disease (IBD) and celiac disease[2,3]. Impaired intestinal barrier function has also been assumed to play a role in the development of sepsis and multiple organ failure (MOF) in patients with decreased gut perfusion following major surgery, trauma or shock[14,15]. Recently the occurrence of splanchnic hypoperfusion during major surgery was reported to result in intestinal ischemia and intestinal barrier integrity loss[16], which could in turn facilitate translocation of bacterial products from the intestinal lumen to the circulation. This phenomenon has been suggested to trigger an excessive inflammatory response, leading to sepsis and MOF in these patients[4,17]. In conclusion, intestinal barrier function loss is associated with a range of diseases; insight in gut barrier integrity and function loss is therefore imperative for clinical practice and important for improving our knowledge on disease etiology and pathophysiology. In this review, the currently available methods aiming to assess either human intestinal barrier integrity or intestinal barrier function will be discussed. In addition, applicability of these tests in different clinical and research situations is described.
ASSESSMENT OF THE EPITHELIAL BARRIER INTEGRITY
The intestinal barrier function is maintained by a lining of enterocytes and tight junctions, sealing the paracellular space between adjacent enterocytes. Intestinal barrier integrity loss can be assessed by evaluation of intestinal epithelial cell damage or tight junction loss.
Intestinal epithelial cell damage: Fatty acid binding proteins
Fatty acid binding proteins (FABP) are small (14-15 kDa) cytosolic water-soluble proteins, present in mature enterocytes of the small and large intestine. Their function is the transport of fatty acids from the apical membrane of the enterocyte to the endoplasmic reticulum where biosynthesis of complex lipids occurs[18]. Three types of FABP are present in the gut; intestinal FABP (I-FABP), liver FABP (L-FABP) and ileal bile acid binding protein (I-BABP). The distribution of these FABP was studied by Pelsers et al and Derikx et al who reported that I-FABP is in particular expressed in jejunum and to a lesser extent in the colon, whereas I-BABP is exclusively present in the ileum[18-21]. In addition, I-FABP and I-BABP are exclusively present in the gut[19,21], whereas L-FABP is also present in the liver and kidney[19]. Since FABP are small, water-soluble cytosolic proteins they are easily released into the circulation upon enterocyte membrane integrity loss and are rapidly renally cleared (half-life of 11 min)[22]. Therefore FABP can be measured sensitively in both plasma and urine using an enzyme-linked immunosorbent assay (ELISA). Basal levels of FABP have been reported to reflect the physiological turnover rate of enterocytes[23]. Several studies showed the usefulness of FABP as markers for intestinal epithelial cell damage. Elevated circulating or urinary FABP levels were reported in patients with intestinal ischemia[24], systemic inflammatory response syndrome and necrotizing enterocolitis[25-27]. High levels of FABP were also detected in patients with intestinal ischemia during major (vascular) surgery and in patients with mesenteric infarction[24,28,29]. Hence, in situations of acute intestinal damage, plasma and urine FABP levels are useful for the assessment of intestinal epithelial damage. In conclusion, measurement of plasma and urinary FABP levels is useful for the early detection of intestinal epithelial cell damage. Since FABP are differentially expressed along the intestinal tract, measurement of specific FABP could be a promising tool to provide information on disease localization.
Intestinal epithelial cell damage: Glutathione S-transferases
The glutathione s-transferases (GSTs) are involved in cell protection, antioxidation and detoxification of a range of toxic and foreign compounds within the cell by conjugating them to glutathione. The GST family consists of four subgroups displaying tissue variation; αGST, μGST, πGST and θGST. Whilst μGST, πGST and θGST are present in cells of various organs, αGST is predominantly present in liver, kidney and intestine and has been proposed as a potential marker for, amongst others, intestinal epithelial cell damage[30,31].
Several studies reported that mesenteric ischemia could reliably be predicted by plasma αGST levels in patients suspected for acute mesenteric ischemia[32-34]. McMonagle et al[31] found that circulating αGST were significantly elevated in patients who displayed signs of intestinal pathology after cardiac surgery with cross clamping of the aorta and consequent intestinal ischemia.
It has to be kept in mind that increased plasma or urine levels of αGST can indicate intestinal damage as well as liver and kidney damage, because αGST is expressed in epithelial cells of all these organs. Therefore, this test might be useful for assessment of intestinal damage when isolated intestinal damage is suspected.
Paracellular barrier integrity loss: Tight junction status
Intestinal epithelial cells are tightly connected by a surrounding system of tight junction strands. Claudins are transmembrane proteins which are mainly held responsible for the intestinal barrier function[6]. These claudins are abundantly present between adjacent healthy intestinal epithelial cells[6,35]. Zeissig et al[1] showed a disturbance of the barrier function which was accompanied by downregulation of several claudins, e.g. claudin-1, 3, 5, 7 and 8 in intestinal biopsies of patients with Crohn’s disease. Claudin-2, a pore-forming claudin was upregulated in these patients. Non-invasive assessment of claudins could provide information on paracellular gut barrier integrity. Claudin-3 seems to be a suitable candidate marker for early non-invasive detection of intestinal tight junction integrity loss due to its small size, abundant endogenous intestinal expression and paracellular localization[35]. Recent studies showed a strong relationship between intestinal tight junction loss and urinary claudin-3 levels in both a rat hemorrhagic shock model and in a clinical setting in patients with IBD, necrotizing enterocolitis and in patients undergoing major surgery, thereby suggesting that measurement of urinary claudin-3 can indeed be used as non-invasive marker for intestinal tight junction loss[16,36,37].
In conclusion, measurement of urine claudin-3 levels offers the opportunity to study paracellular intestinal barrier damage. Detection of tight junction loss offers new opportunities for early diagnosis and follow-up of patients with intestinal diseases and for elucidation of the pathophysiology of gut-related diseases in man. A clear research or clinical question with careful interpretation of results is however imperative, since tight junction distribution is not limited to the intestine. The usefulness of other tight junction proteins for non-invasive evaluation of intestinal paracellular integrity remains to be established.
FUNCTIONAL ASSESSMENT OF INTESTINAL BARRIER LOSS
Methods for the functional assessment of intestinal barrier loss have been studied extensively. Currently available methods are either based on actively measuring either paracellular intestinal leakage of orally administered test substances, or passively measuring the consequences of intestinal barrier function loss, i.e. translocation of luminal content to the circulation.
“Active” assessment of barrier function loss is based on the hypothesis that orally administered large molecular probes cannot cross the paracellular intestinal pathway unless the intestinal barrier function is compromised. In case of barrier function loss such probes cross the intestinal barrier, appear into the circulation and can be detected in urine after renal excretion. “Passive” assessment of barrier function loss is based on the hypothesis that intestinal luminal compounds, such as endotoxins and bacterial fermentation products, translocate to the circulation in case of barrier function loss. Plasma levels of these bacterial components or products are therefore hypothesized to reflect barrier function integrity.
Active measurement of intestinal barrier function loss
In the early 1970s, Menzies introduced oligosaccharides as test probes for the functional assessment of intestinal barrier failure[2,38]. It was hypothesized that large oligosaccharides such as lactulose would not traverse the intestinal membrane in the healthy situation. However, as a result of intestinal barrier integrity loss, these probes cross the intestinal barrier to the circulation and are detectable in urine after being excreted renally. Using this method, increased intestinal permeability was detected in patients with celiac disease[38]. Although test results were promising, the test was prone to various permucosal and postmucosal confounders such as gastric dilution and gastric emptying, bacterial degradation, intestinal transit and renal function. This led to the development of differential sugar absorption tests, where both a di- or oligosaccharide and a monosaccharide, serving as a large- and a small- molecular probe respectively, are administered orally simultaneously, after which their recovery is measured in urine (Figure 1B)[39]. The smaller molecular probe is thought to traverse the intestinal barrier freely, independent of barrier function loss, and is affected in the same way as the large molecular probe by the pre- and postmucosal confounders. The ratio of the urinary concentration of both compounds would therefore more accurately reflect the paracellular passage across the intestinal barrier than isolated measurement of urinary oligosaccharides[2,3].
Currently, the most frequently used sugar probes to assess intestinal permeability are lactulose as oligosaccharide and mannitol or L-rhamnose as monosaccharide. Other macromolecular probes are differently sized polyethylene glycols (PEG: 4000, 1500, 400), and radioactively labeled macromolecules such as chromium labeled EDTA (51Cr-EDTA). These tests will be discussed respectively in the following section.
Differential sugar absorption tests: The differential sugar absorption tests (DST) is based on the oral administration of two sugars that differentially cross the intestinal barrier to the circulation upon barrier integrity loss, after which they are rapidly cleared into urine. The ratio of oligosaccharides and monosaccharides in urine, collected over five to six hours after oral intake, is considered to reflect small intestinal barrier function loss most accurately. Laboratory analysis is usually performed using high pressure liquid chromatography (HPLC) or liquid chromatography in combination with mass spectrometry (LC/MS)[2]. Various oligosaccharides (lactulose, cellobiose) and monosaccharides (mannitol, L-rhamnose) have been used with similar results. Since some of the saccharides, as lactulose, can cause increased intestinal motility, the administered dose should be kept as low as possible[40]. It is important to bear in mind that the classical DST is only useful for assessing small intestinal permeability, since lactulose is degraded by bacteria in the large intestine[2,3]. To evaluate whole intestinal permeability, non-degradable probes as sucralose, which remain unaffected by bacteria in the colon, are added to classical DST, resulting in the so-called triple sugar test. The lactulose excretion over 24 h (likely to represent only small intestinal permeability), subtracted from 24-h sucralose excretion, is considered to give an isolated measure of colonic permeability[41]. Other studies focused on measurement of gastroduodenal permeability, have used sucrose as test substance. Sucrose is rapidly degraded by sucrase, an enzyme secreted in large amounts by mature enterocytes in the duodenum. Therefore, enhanced plasma or urinary levels of sucrose are thought to reflect only permeability of the stomach and proximal duodenum[42,43].
In conclusion, DST are useful to assess small intestinal permeability and additional information on gastroduodenal or colonic permeability can be obtained by adding sucrose or sucralose, respectively, as test probes.
DST in disease: DST have been valuable for evalation of both etiology and disease activity in various intestinal diseases. Increased permeability for saccharides has been reported in patients with Crohn’s disease[44-46], celiac disease[47,48] and food intolerance[3]. However, the test has never gained a place in everyday practice for diagnosis and follow up of such patients groups, mainly because the test is impractical in use and detection methods are complex and not widely available[49]. Apart from intestinal diseases, DST have also been used to assess intestinal permeability in critically ill patients, since the intestinal barrier function has been hypothesized to play a central role in the development of sepsis. Indeed, many studies report increased permeability in critically ill patients and in patients undergoing major (cardiopulmonary) surgery[50,51]. Therefore DST might be useful for early detection of patients at risk of developing severe complications, although the administration of probes and the necessity of urine collection for several hours is impractical and moreover, these patients often have limited urine production. Furthermore, some studies have showed that permeability measurements using DST in intensive care patients with MOF has pitfalls. Firstly, decreased motility and altered clearance of the different sugars as a result of renal dysfunction is a complicating factor in these patients. Secondly, the use of mannitol appeared to be unsuitable in patients receiving red blood cell transfusion, since mannitol is used in the storage solution of bank blood[52].
Polyethylene glycols (PEG): Polyethylene glycols (PEG) with a molecular weight of 400-4000 Da have also been used to assess intestinal barrier function. It is hypothesized that, as saccharides in the DST, large molecular PEG will only cross the intestinal mucosa to the circulation in the case of barrier integrity loss, as measured after renal excretion using gas chromatography (GC) or HPLC[2]. Increased urinary levels of large molecular PEG therefore reflect increased intestinal permeability. Since PEG is biochemically inert and not degraded by bacteria, 24 h urinary levels could provide information on whole intestinal permeability.
PEG measurement in disease: Variously sized PEG probes were used to investigate bowel permeability in a broad range of intestinal diseases. PEGs have the advantage of being inert and can therefore be used to measure both small and large intestinal permeability. They have been used successfully to assess permeability changes in patients with irritable bowel syndrome[53], pancreatitis[54-56], liver cirrhosis[57], and intestinal ischemia reperfusion injury[58].
They have also been used in patients with Crohn’s disease, although both decreased and increased intestinal permeability was found in this patient group. In addition, some studies also reported high inter- and intra-individual variations in test results, even in controls[49,59,60]. Hence, future studies on the permeation pathways of PEG are necessary to improve interpretation of results.
Chromium labeled EDTA: Chromium labeled EDTA (51Cr-EDTA) has similar physiological properties to oligosaccharides with the advantage of being easily detectable. Furthermore, 51Cr-EDTA is not degraded by bacteria in the colon, which makes it a useful marker for both small and large intestinal permeability. Some studies have reported increased colorectal permeability for 51Cr-EDTA in patients with IBD[61]. A disadvantage of 51Cr-EDTA is its radioactivity. It should, therefore, be avoided for research purposes in children and for screening in healthy subjects.
Passive measurement of intestinal barrier function
In the healthy situation the intestinal barrier prevents translocation of intraluminal compounds whilst in situations of impaired intestinal barrier function, bacteria and bacterial products can find their way to the circulation. The presence of such compounds in plasma could therefore provide information on intestinal barrier integrity and function. In the next section, tests for the measurement of bacterial compounds or bacterial fermentation products are summarized. An advantage of these tests is that they can be performed without the need to administer test substances and time consuming urine collection.
Measurement of circulating endotoxin: limulus amebocyte lysate assay: Endotoxin is a lipopolysaccharide (LPS) of the outer membrane of Gram-negative bacteria. It is capable of inducing multiple effects in man, varying from fever and leucocytosis to thrombocytopenia and coagulopathies[62]. The limulus amebocyte lysate assay (LAL assay) allows quantitative determination of plasma endotoxin levels. This assay is based on the fact that endotoxin causes intravascular coagulation in the horseshoe crab (Limulus polyphemus), via the enzymatic conversion of a clottable protein derived from the circulating blood cells (amebocytes) of the crab. The lysate from the amebocytes is also sensitive to the presence of endotoxin in vitro. Several assays have been developed to detect endotoxin using LAL, such as the gel clot LAL assay and the more recent chromogenic LAL assay[62]. In LAL assays, the presence of plasma endotoxin neutralizing factors, detergents, urea and variation in pH influences the test results strongly and can yield false positive or false negative results. To minimize the effect of plasma endotoxin neutralizing factors, Ditter et al[63] reported a modified chromogenic LAL assay. The principle of this assay is that each sample contains a specific amount of factors interfering with the LAL-endotoxin reaction. Therefore, an endotoxin reference curve is established in each sample by spiking it with certain concentrations of endotoxin. The deviation from the standard curve then represents the endogenous unknown endotoxin content. Using this method, each sample has an internal standard, correcting for plasma endotoxin neutralizing factors. Still, the specificity of the LAL assay remains a point of concern since cell wall products of fungi, Gram-positive bacteria and polynucleotides have been reported to account for a (false) positive test. Furthermore, due to its high sensitivity, the LAL assay is prone to false positive results caused by exogenous endotoxin contamination[62,64]. In spite of these complicating factors, several studies have successfully used the LAL assay to show endotoxemia, mostly in patients with sepsis[65,66], which might indicate bacterial translocation from the gut lumen to the circulation as a consequence of intestinal barrier function failure.
INDIRECT MEASUREMENT OF TRANSLOCATION OF BACTERIAL PRODUCTS
Measurement of circulating endotoxin core antibodies
Endotoxin core antibodies (EndoCAb) assay measures the concentration of immunoglobulins (IgG, IgM and IgA) against the inner core of endotoxin. This inner core consists of a hydrophobic part, lipid A, which is attached to a core oligosaccharide. Lipid A is highly conserved across the whole range of Gram-negative microbiota. Moreover, it is this part that is considered most responsible for endotoxin toxicity[67]. In 1989, Barclay et al[68] described the potential value of EndoCAb for diagnostic use in patients with a Gram-negative sepsis. They hypothesized that anti-endotoxin antibodies were consumed by the superabundance of endotoxin in such patients (Figure 1C). In a later stage, IgM EndoCAb levels increase as endotoxin stimulates the synthesis of antibodies to endotoxin[68].
Several studies showed decreased EndoCAb levels postoperatively, accounting for the degree of exposure to endotoxin[69,70]. In addition, successive studies have shown that preoperative low circulating levels of anti-endotoxin antibodies are related to poor outcome in patients after major cardiac[71], and abdominal aortic aneurysm surgery[72,73]. Stable EndoCAb levels have high individual variation and the determining factors for an individual’s stable EndoCAb level have not been fully understood, potentially hampering the interpretation of circulating EndoCAb levels[74].
In summary, EndoCAb assays detect anti-endotoxin immunoglobulins and consumption of these circulating immunoglobulins following translocation of gut-derived endotoxins, can therefore be used to acquire indirect information on the intestinal epithelial barrier function.
Measurement of plasma D-lactate levels
D-lactate is a fermentation product produced by many bacteria present in the human gastrointestinal tract, and was proposed in the 1980s as a marker for diagnosis of bacterial infections[75]. Low circulating levels of D-lactate are found in healthy individuals, but in case of intestinal barrier function loss, these levels will rise as a consequence of increased translocation across the intestinal mucosa. Therefore, plasma D-lactate levels could serve as a measure of impaired barrier function (Figure 1D). Various studies proposed a relationship between plasma D-lactate and intestinal permeability. Sun et al[76] evaluated plasma D-lactate as a marker for increased intestinal permeability in a rat model of severe injuries (intestinal ischemia reperfusion and acute necrotising pancreatitis). Plasma endotoxin levels, measured using the LAL assay, correlated significantly with plasma D-lactate levels at an early stage of intestinal injury.
Few human studies have been performed to evaluate the potential role of plasma D-lactate as a marker for impaired barrier function. In one study, performed in patients undergoing open aortic surgery, a rapid increase of plasma D-lactate levels was observed, which correlated with a histologically proven ischemic colitis[77].
D-lactate measurement has especially been valuable in assessment of ischemic colonic injury and seems to be a reliable marker for colonic barrier function loss in animal models. Results should however be interpreted cautiously where there is bacterial overgrowth since the augmented presence of bacteria could result in increased fermentation of undigested carbohydrates to D-lactate[78]. The usefulness of plasma D-lactate as marker for colonic barrier function in man is a subject for future research.
CONCLUSION
The intestinal epithelial lining should provide an efficient barrier that prevents entry of pathogens and antigens. Over the past decades it has become evident that dysfunction of the intestinal barrier has a significant impact on the health of an individual. The mucosal barrier may become compromised as a consequence of various (intestinal) disorders, and has also been suggested to play a role in the pathogenesis and perpetuation of disease. Although intestinal barrier function tests have been improved over the past decades and new tests have emerged, evaluation of intestinal barrier integrity and barrier function loss remains a challenge to both clinicians and scientists.
In this review, currently available tests that address different aspects of the intestinal epithelial barrier have been evaluated (Table 1). Markers for physical barrier loss as well as methods to assess functional barrier loss have been discussed. A combination of the various tests might provide clinicians and scientists with a more advanced insight in gut wall integrity status. Due to the evident importance of the intestinal barrier in development and perpetuation of disease, further studies should be aimed at validating current available tests for clinical application and development of new tests for accurate assessment of intestinal integrity and barrier function loss. Furthermore, the relationship between physical intestinal barrier damage and functional failure of the barrier function remains subject for future research.
Table 1.
Methods for the assessment of intestinal barrier integrity status and intestinal barrier function loss
| Test | Measured in | Indicative for | Tissue specificity |
| I-FABP | Blood or urine | Intestinal epithelial integrity | Yes (predominantly proximal small gut) |
| Single sample | |||
| I-BABP | Blood or urine | Intestinal epithelial integrity | Yes (predominantly ileum) |
| Single sample | |||
| L-FABP | Blood or urine | Intestinal epithelial integrity | No |
| Single sample | |||
| GST-α | Blood | Intestinal epithelial integrity | No |
| Single sample | |||
| Claudin 3 | Urine | Paracellular integrity | No |
| Single sample | |||
| Dual Sugar Test | Urine | Functional intestinal barrier function | Yes |
| 5 h collection | |||
| PEG | Urine | Functional intestinal barrier function | Yes |
| 6 h collection | |||
| 51Cr-EDTA | Urine | Functional intestinal barrier function | Yes |
| 24 h collection | |||
| LAL-assay | Blood | Intestinal barrier function | N/A |
| Single sample | |||
| EndoCAb | Blood | Intestinal barrier function | N/A |
| Single sample | |||
| D-Lactate | Blood | Intestinal barrier function | Yes |
| Single sample |
I-FABP: Intestinal fatty acid binding protein; I-BABP: Ileal bile acid binding protein; GST: Glutathione S-transferase-α; PEG: Polyethylene glycol;
Cr-EDTA: Chromium labelled EDTA; LAL: Limulus amebocyte lysate; EndoCAb: Endotoxin core antibody; N/A: Not applicable.
Footnotes
Peer reviewer: Francisco José Vizoso, MD, PhD, Unidad de Investigación, Hospital de Jove, Avda/Eduardo Castro s/n, 33290 Gijón, Spain
S- Editor Li LF L- Editor Hughes D E- Editor Lin YP
References
- 1.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 3.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 7.Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 9.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–694. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 12.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derikx JP, Poeze M, van Bijnen AA, Buurman WA, Heineman E. Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock. 2007;28:544–548. doi: 10.1097/shk.0b013e3180644e32. [DOI] [PubMed] [Google Scholar]
- 15.Holland J, Carey M, Hughes N, Sweeney K, Byrne PJ, Healy M, Ravi N, Reynolds JV. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am J Surg. 2005;190:393–400. doi: 10.1016/j.amjsurg.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Derikx JP, van Waardenburg DA, Thuijls G, Willigers HM, Koenraads M, van Bijnen AA, Heineman E, Poeze M, Ambergen T, van Ooij A, et al. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PLoS One. 2008;3:e3954. doi: 10.1371/journal.pone.0003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–535. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 20.Marks WH, Gollin G. Biochemical detection of small intestinal allograft rejection by elevated circulating levels of serum intestinal fatty acid binding protein. Surgery. 1993;114:206–210. [PubMed] [Google Scholar]
- 21.Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, van Heurn LW, Heineman E, Buurman WA. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol. 2009;43:727–733. doi: 10.1097/MCG.0b013e31819194b0. [DOI] [PubMed] [Google Scholar]
- 22.van de Poll MC, Derikx JP, Buurman WA, Peters WH, Roelofs HM, Wigmore SJ, Dejong CH. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31:2033–2038. doi: 10.1007/s00268-007-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derikx JP, Blijlevens NM, Donnelly JP, Fujii H, Kanda T, van Bijnen AA, Heineman E, Buurman WA. Loss of enterocyte mass is accompanied by diminished turnover of enterocytes after myeloablative therapy in haematopoietic stem-cell transplant recipients. Ann Oncol. 2009;20:337–342. doi: 10.1093/annonc/mdn579. [DOI] [PubMed] [Google Scholar]
- 24.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 25.Derikx JP, Evennett NJ, Degraeuwe PL, Mulder TL, van Bijnen AA, van Heurn LW, Buurman WA, Heineman E. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007;56:1473–1475. doi: 10.1136/gut.2007.128934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthmann F, Börchers T, Wolfrum C, Wustrack T, Bartholomäus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. 2002;239:227–234. [PubMed] [Google Scholar]
- 27.Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH, Rozycki HJ. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. 1999;34:1453–1457. doi: 10.1016/s0022-3468(99)90102-1. [DOI] [PubMed] [Google Scholar]
- 28.Holmes JH 4th, Lieberman JM, Probert CB, Marks WH, Hill ME, Paull DL, Guyton SW, Sacchettini J, Hall RA. Elevated intestinal fatty acid binding protein and gastrointestinal complications following cardiopulmonary bypass: a preliminary analysis. J Surg Res. 2001;100:192–196. doi: 10.1006/jsre.2001.6237. [DOI] [PubMed] [Google Scholar]
- 29.Hanssen SJ, Derikx JP, Vermeulen Windsant IC, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117–125. doi: 10.1097/SLA.0b013e3181784cc5. [DOI] [PubMed] [Google Scholar]
- 30.Sundberg AG, Nilsson R, Appelkvist EL, Dallner G. Immunohistochemical localization of alpha and pi class glutathione transferases in normal human tissues. Pharmacol Toxicol. 1993;72:321–331. doi: 10.1111/j.1600-0773.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 31.McMonagle MP, Halpenny M, McCarthy A, Mortell A, Manning F, Kilty C, Mannion D, Wood AE, Corbally MT. Alpha glutathione S-transferase: a potential marker of ischemia-reperfusion injury of the intestine after cardiac surgery? J Pediatr Surg. 2006;41:1526–1531. doi: 10.1016/j.jpedsurg.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Delaney CP, O'Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg. 1999;86:1349–1353. doi: 10.1046/j.1365-2168.1999.01245.x. [DOI] [PubMed] [Google Scholar]
- 33.Khurana S, Corbally MT, Manning F, Armenise T, Kierce B, Kilty C. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37:1543–1548. doi: 10.1053/jpsu.2002.36181. [DOI] [PubMed] [Google Scholar]
- 34.Gearhart SL, Delaney CP, Senagore AJ, Banbury MK, Remzi FH, Kiran RP, Fazio VW. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg. 2003;69:324–329; discussion 329. [PubMed] [Google Scholar]
- 35.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 36.Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–169. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 37.Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruïne A, Masclee AA, Heineman E, Buurman WA. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol. 2010;44:e14–e19. doi: 10.1097/MCG.0b013e31819f5652. [DOI] [PubMed] [Google Scholar]
- 38.Menzies IS. Intestinal permeability in coeliac disease. Gut. 1972;13:847. [PubMed] [Google Scholar]
- 39.Menzies IS, Laker MF, Pounder R, Bull J, Heyer S, Wheeler PG, Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979;2:1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- 40.van Nieuwenhoven MA, de Swart EA, van Eijk HM, Deutz NE, Brouns F, Brummer RJ. Effects of pre- and post-absorptive factors on the lactulose/rhamnose gut permeability test. Clin Sci (Lond) 2000;98:349–353. doi: 10.1042/cs19990274. [DOI] [PubMed] [Google Scholar]
- 41.Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand. 2004;182:171–177. doi: 10.1111/j.1365-201X.2004.01347.x. [DOI] [PubMed] [Google Scholar]
- 42.Meddings JB, Sutherland LR, Byles NI, Wallace JL. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology. 1993;104:1619–1626. doi: 10.1016/0016-5085(93)90637-r. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland LR, Verhoef M, Wallace JL, Van Rosendaal G, Crutcher R, Meddings JB. A simple, non-invasive marker of gastric damage: sucrose permeability. Lancet. 1994;343:998–1000. doi: 10.1016/s0140-6736(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 44.Wyatt J, Oberhuber G, Pongratz S, Püspök A, Moser G, Novacek G, Lochs H, Vogelsang H. Increased gastric and intestinal permeability in patients with Crohn's disease. Am J Gastroenterol. 1997;92:1891–1896. [PubMed] [Google Scholar]
- 45.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 46.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 47.Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Mauriño E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129–1136. doi: 10.1016/s0016-5085(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 48.van Elburg RM, Uil JJ, de Monchy JG, Heymans HS. Intestinal permeability in pediatric gastroenterology. Scand J Gastroenterol Suppl. 1992;194:19–24. doi: 10.3109/00365529209096021. [DOI] [PubMed] [Google Scholar]
- 49.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Harris CE, Griffiths RD, Freestone N, Billington D, Atherton ST, Macmillan RR. Intestinal permeability in the critically ill. Intensive Care Med. 1992;18:38–41. doi: 10.1007/BF01706424. [DOI] [PubMed] [Google Scholar]
- 51.Ohri SK, Somasundaram S, Koak Y, Macpherson A, Keogh BE, Taylor KM, Menzies IS, Bjarnason I. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318–323. doi: 10.1016/0016-5085(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 52.Oudemans-van Straaten HM, van der Voort PJ, Hoek FJ, Bosman RJ, van der Spoel JI, Zandstra DF. Pitfalls in gastrointestinal permeability measurement in ICU patients with multiple organ failure using differential sugar absorption. Intensive Care Med. 2002;28:130–138. doi: 10.1007/s00134-001-1140-2. [DOI] [PubMed] [Google Scholar]
- 53.Kerckhoffs AP, Akkermans LM, de Smet MB, Besselink MG, Hietbrink F, Bartelink IH, Busschers WB, Samsom M, Renooij W. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci. 2010;55:716–723. doi: 10.1007/s10620-009-0765-9. [DOI] [PubMed] [Google Scholar]
- 54.Ryan CM, Schmidt J, Lewandrowski K, Compton CC, Rattner DW, Warshaw AL, Tompkins RG. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993;104:890–895. doi: 10.1016/0016-5085(93)91027-f. [DOI] [PubMed] [Google Scholar]
- 55.Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 56.Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg. 2006;244:959–965; discussion 965-967. doi: 10.1097/01.sla.0000246866.01930.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S, Son SC, Han MJ, Kim WJ, Kim SH, Kim HR, Jeon WK, Park KH, Shin MG. Increased intestinal macromolecular permeability and urine nitrite excretion associated with liver cirrhosis with ascites. World J Gastroenterol. 2008;14:3884–3890. doi: 10.3748/wjg.14.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solligård E, Juel IS, Spigset O, Romundstad P, Grønbech JE, Aadahl P. Gut luminal lactate measured by microdialysis mirrors permeability of the intestinal mucosa after ischemia. Shock. 2008;29:245–251. doi: 10.1097/SHK.0b013e3180cab3ce. [DOI] [PubMed] [Google Scholar]
- 59.Olaison G, Sjödahl R, Tagesson C. Decreased gastrointestinal absorption of peroral polyethyleneglycols (PEG 1000) in Crohn's disease. A sign of jejunal abnormality. Acta Chir Scand. 1987;153:373–377. [PubMed] [Google Scholar]
- 60.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 61.Jenkins RT, Ramage JK, Jones DB, Collins SM, Goodacre RL, Hunt RH. Small bowel and colonic permeability to 51Cr-EDTA in patients with active inflammatory bowel disease. Clin Invest Med. 1988;11:151–155. [PubMed] [Google Scholar]
- 62.Hurley JC. Endotoxemia: methods of detection and clinical correlates. Clin Microbiol Rev. 1995;8:268–292. doi: 10.1128/cmr.8.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ditter B, Becker KP, Urbaschek R, Urbaschek B. [Quantitative endotoxin determination. Automated kinetic Limulus amebocyte lysate microtiter test with measurement of sample-related interferences] Arzneimittelforschung. 1983;33:681–687. [PubMed] [Google Scholar]
- 64.Cohen J. The detection and interpretation of endotoxaemia. Intensive Care Med. 2000;26 Suppl 1:S51–S56. doi: 10.1007/s001340051119. [DOI] [PubMed] [Google Scholar]
- 65.Guidet B, Barakett V, Vassal T, Petit JC, Offenstadt G. Endotoxemia and bacteremia in patients with sepsis syndrome in the intensive care unit. Chest. 1994;106:1194–1201. doi: 10.1378/chest.106.4.1194. [DOI] [PubMed] [Google Scholar]
- 66.Bates DW, Parsonnet J, Ketchum PA, Miller EB, Novitsky TJ, Sands K, Hibberd PL, Graman PS, Lanken PN, Schwartz JS, et al. Limulus amebocyte lysate assay for detection of endotoxin in patients with sepsis syndrome. AMCC Sepsis Project Working Group. Clin Infect Dis. 1998;27:582–591. doi: 10.1086/514713. [DOI] [PubMed] [Google Scholar]
- 67.Strutz F, Heller G, Krasemann K, Krone B, Müller GA. Relationship of antibodies to endotoxin core to mortality in medical patients with sepsis syndrome. Intensive Care Med. 1999;25:435–444. doi: 10.1007/s001340050877. [DOI] [PubMed] [Google Scholar]
- 68.Barclay GR, Scott BB, Wright IH, Rogers PN, Smith DG, Poxton IR. Changes in anti-endotoxin-IgG antibody and endotoxaemia in three cases of gram-negative septic shock. Circ Shock. 1989;29:93–106. [PubMed] [Google Scholar]
- 69.Bennett-Guerrero E, Barclay GR, Weng PL, Bodian CA, Feierman DE, Vela-Cantos F, Mythen MG. Endotoxin-neutralizing capacity of serum from cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15:451–454. doi: 10.1053/jcan.2001.24980. [DOI] [PubMed] [Google Scholar]
- 70.Mythen MG, Barclay GR, Purdy G, Hamilton-Davies C, Mackie IJ, Webb AR, Machin SJ. The role of endotoxin immunity, neutrophil degranulation and contact activation in the pathogenesis of post-operative organ dysfunction. Blood Coagul Fibrinolysis. 1993;4:999–1005. [PubMed] [Google Scholar]
- 71.Bennett-Guerrero E, Ayuso L, Hamilton-Davies C, White WD, Barclay GR, Smith PK, King SA, Muhlbaier LH, Newman MF, Mythen MG. Relationship of preoperative antiendotoxin core antibodies and adverse outcomes following cardiac surgery. JAMA. 1997;277:646–650. [PubMed] [Google Scholar]
- 72.Bennett-Guerrero E, Panah MH, Barclay GR, Bodian CA, Winfree WJ, Andres LA, Reich DL, Mythen MG. Decreased endotoxin immunity is associated with greater mortality and/or prolonged hospitalization after surgery. Anesthesiology. 2001;94:992–998. doi: 10.1097/00000542-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Braun JP, Buhner S, Kastrup M, Dietz E, Langer K, Dohmen PM, Lochs H, Spies C. Barrier function of the gut and multiple organ dysfunction after cardiac surgery. J Int Med Res. 2007;35:72–83. doi: 10.1177/147323000703500107. [DOI] [PubMed] [Google Scholar]
- 74.Barclay GR. Endotoxin-core antibodies: time for a reappraisal? Intensive Care Med. 1999;25:427–429. doi: 10.1007/s001340050874. [DOI] [PubMed] [Google Scholar]
- 75.Smith SM, Eng RH, Buccini F. Use of D-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis. 1986;154:658–664. doi: 10.1093/infdis/154.4.658. [DOI] [PubMed] [Google Scholar]
- 76.Sun XQ, Fu XB, Zhang R, Lu Y, Deng Q, Jiang XG, Sheng ZY. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J Gastroenterol. 2001;7:555–558. doi: 10.3748/wjg.v7.i4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Assadian A, Assadian O, Senekowitsch C, Rotter R, Bahrami S, Fürst W, Jaksch W, Hagmüller GW, Hübl W. Plasma D-lactate as a potential early marker for colon ischaemia after open aortic reconstruction. Eur J Vasc Endovasc Surg. 2006;31:470–474. doi: 10.1016/j.ejvs.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 78.Herrera DJ, Morris K, Johnston C, Griffiths P. Automated assay for plasma D-lactate by enzymatic spectrophotometric analysis with sample blank correction. Ann Clin Biochem. 2008;45:177–183. doi: 10.1258/acb.2007.007088. [DOI] [PubMed] [Google Scholar]