Abstract
Loco-regional treatments for hepatocellular carcinoma (HCC) are important alternatives to curative transplantation or resection. Among them, radiofrequency ablation (RFA) is accepted as the most popular technique showing excellent local tumor control and acceptable morbidity. The current role of RFA is well documented in the evidence-based practice guidelines of European Association of Study of Liver, American Association of Study of the Liver Disease and Japanese academic societies. Several randomized controlled trials have confirmed that RFA is superior to percutaneous ethanol injections in terms of local tumor control and survival. The overall survival after RFA is comparable to after surgical resection in a selected group of patients with smaller (< 3 cm) tumors. Currently, the clinical benefits of combined RFA with transarterial chemoembolization for intermediate stage HCC are increasingly being explored. Here we review the ongoing technical advancements of RFA and future potential.
Keywords: Image-guided tumor ablation, Radiofrequency ablation, Hepatocellular carcinoma, Thermal ablation, Loco-regional therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most frequent cause of death from cancer. Chronic hepatitis B and C viral infections are the predominant factors predisposing patients to HCC in Southeast Asia, Africa, Western countries and Japan. The incidence of HCC is increasing in Western countries and is expected to equal that currently reported in Asian countries[1-3].
Liver transplantation is the best curative option with good survival rates, although its use is restricted by the shortage of donor organs. Surgical resection was accepted as a treatment of choice before the era of transplantation. However, the tumors in most patients are unresectable because of a variety of factors including: poor hepatic reserve, multifocal disease or inability to obtain an optimal tumor free margin[4,5]. Therefore, for the majority of patients with HCC, loco-regional treatment is the only alternative treatment option[6-12].
The image-guided loco-regional treatment for patients with unresectable HCC includes chemical or thermal ablative techniques and catheter-based approaches. Among the ablative techniques, radiofrequency ablation (RFA) has been used as the most popular method for treating early stage HCC (single or 3 nodules less than 3 cm in diameter). During the past two decades, many clinical studies have confirmed the safety and therapeutic efficacy of RF[13-19]. The purpose of this article is to review and summarize the current status of RFA for HCC. The current and potential roles of RFA in treating HCC will be presented with a review of the evidence of its safety and therapeutic efficacy.
CURRENT ROLE OF RFA IN THE TREATMENT OF HCC
It is difficult to define the current role of RFA in the treatment of HCC because it is still an evolving technique. However, consensus meetings of major scientific societies have presented guidelines for its use. The evidence-based practice guidelines for management of HCC have been proposed by the European Association of Study of Liver (EASL) and the American Association of Study of the Liver Disease (AASLD)[4,20]. In both guidelines, RFA is recommended as a non-surgical technique for the treatment of early stage (Child A or B, solitary HCC or up to 3 nodules < 3 cm in size) HCC.
According to the EASL and AASLD guidelines, local ablation using RFA and percutaneous ethanol injections (PEI), is accepted as a safe and effective therapy for patients that cannot undergo resection or as a bridge to transplantation based on level II (nonrandomized controlled trials, cohort or case-control analytic studies, multiple time series, dramatic uncontrolled experiments) evidence. In addition, RFA is as effective as PEI for smaller (< 2 cm) tumors but clearly superior to PEI for larger tumors based on level I (randomized controlled trial) evidence[4,20].
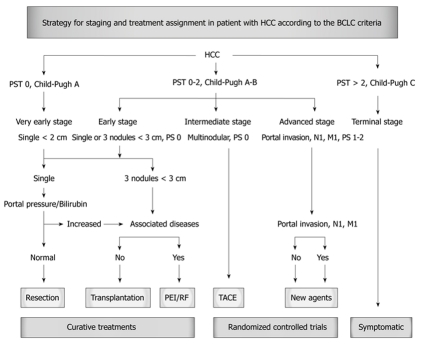
The barcelona clinic liver cancer staging and treatment assessment system is widely used worldwide. Using this system, RFA is classified as a treatment option for early stage HCC. Patients with early stage disease can be effectively treated by resection, transplantation or percutaneous ablation with the possibility of long-term cure and a 5-year survival rate ranging from 50% to 75%. However, many issues regarding the treatment of choice remain to be resolved by further investigations; currently there are no studies available that have compared treatments considered to be effective for early stage disease (surgical resection, transplantation and percutaneous ablation) or comparing these methods of treatment to no treatment (Figure 1)[4,20,21].
Figure 1.
Strategy for staging and treatment assignment in patient with hepatocellular carcinoma (HCC) according to the barcelona clinic liver cancer (BCLC) criteria. BCLC staging system was developed based on the collection of data from several independent studies representing different disease stages and/or treatment modalities. It includes variables related to tumor stage, liver functional status, physical status and cancer related symptoms. The main advantage of the BCLC criteria staging system is that it links staging with treatment modalities and with an estimation of life expectancy that is based on published response rates to the various treatments. Early stage disease includes patients with preserved liver function (Child-Pugh Class A and B) with solitary HCC or up to 3 nodules < 3 cm in diameter. These patients can be effectively treated by resection, transplantation or percutaneous ablation with the possibility for long-term survival ranging from 50% to 75%.
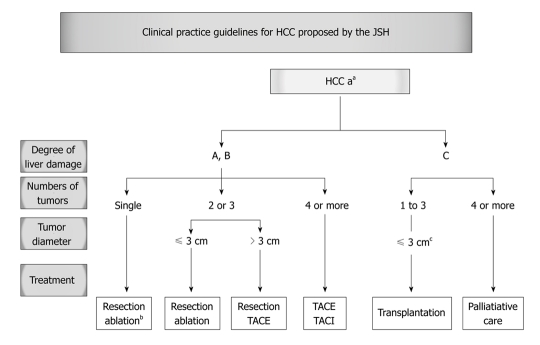
According to the Japanese evidence-based guidelines, if there is only one tumor in a patient with Child A or B disease, hepatectomy is recommended regardless of the diameter of the tumor. However, percutaneous local ablation may also be selected if the severity of liver damage is class B and the diameter of the tumor is not more than 2 cm. If there are 2 or more tumors and their diameters are no more than 3 cm, hepatectomy or ablation is recommended. If there are 2 or 3 tumors and their diameters are 3 cm more, hepatectomy or hepatic artery embolization is recommended. If there are more than 4 tumors, transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy is recommended (Figure 2)[22]. Recently, the expert panel of the Japanese Society of Hepatology established a consensus-based treatment algorithm based on therapeutic protocols used in Japan. This algorithm essentially follows the evidence-based algorithm; however, the treatments widely performed in Japan were included by consensus, even though the evidence was not always present[23].
Figure 2.
Clinical Practice Guidelines for HCC proposed by the Japan Society of Hepatology. aPresence of vascular invasion or extrahepatic metastasis to be indicated separately; bSelected when the severity of damage is class B and the tumor diameter is no greater than 3 cm; cTumor diameter should be no greater than 5 cm when there is only one tumor; JSH: Japan society of hepatology.
SAFETY OF RFA
One of the most attractive features of local ablation therapy, including RFA, is that the procedure is minimally invasive compared to curative surgical resection or transplantation. Although RFA is considered to be much safer than surgical treatment, it is not a complication-free procedure. Thus, an operator should be aware of all major complications with the potential morbidity and mortality and should be ready to detect complications as early as possible and manage them appropriately[24-26].
There have been several multicenter studies on the complications in patients after RFA procedures for hepatic tumors. In 2002, the collaborative Italian Group, using the Cool-tip electrode, reported the results of a multicenter study of the complications that occurred in patients after RFA procedures. The mortality, major and minor complication rates were 0.3%, 2.2% and 5% respectively[27]. Another Italian group, using the multi-tined expandable electrodes, reported the complications in 872 patients. The mortality, major and minor complication rates were 0.1%, 3.1% and 6.3% respectively[28]. A Korean multicenter study on complications was performed on 1139 patients treated by RFA. The mortality and major complication rates were 0.1% and 2.4%[29]. A French study with 312 patients reported that the mortality, major and minor complication rates were 1.4%, 10.6% and 6.3% respectively[30].
An extensive meta-analysis of 82 independent reports including 3670 patients, reported by Mulier et al[24], revealed that the overall mortality rate was 0.5%, and the major/minor complication rate was 8.9%. The most common complications were abdominal hemorrhage, abdominal infection (abscess), biliary tract damage, liver failure, pulmonary complications and ground pad burns. The broad spectrum and incidence of major complications are similar to the findings of many single center studies.
There have been many investigations that have focused on methods to minimize the complications associated with RFA procedures[31-38]. The most useful method to prevent collateral thermal injury of abutting organs is the use of artificial fluid or air injected into the peritoneal or pleural spaces. Song et al[38] recently reported the feasibility and efficacy of artificial ascites in 143 patients with HCC abutting the diaphragm or bowel. Artificial ascites separates the organs at risk for damage from the RF ablation zone and improves the sonic window by downward displacement of the liver[31,34,36,38].
THERAPEUTIC EFFICACY OF RFA
It is difficult to objectively review and compare the therapeutic efficacy data of treatment modalities. This is because there are significant variations among studies in terms of study design and the technical details of treatment. In addition, patient demographics, including etiology and extent of liver disease, as well as tumor features (number, size, location), vary considerably from one study to another[39-41]. Currently, the international working group of image-guided tumor ablation has proposed a “Proposal for Standardization for Terms and Reporting Criteria”, which was acknowledged by the society of interventional radiology. The aim of the proposal is to facilitate the effective communication of ideas and appropriate comparisons among treatments[42]. Currently, there are so many non-surgical ablation techniques including radiofrequency, ethanol, microwave, laser, high intensity focused ultrasound, radioembolization and TACE with novel drug eluting beads. However, only RFA and PEI are being widely performed worldwide and accepted as a standard treatment in all the guidelines supported by considerable evidence with many investigations including a randomized controlled study.
Below, we summarize the current therapeutic efficacy of RFA for treating HCC according to the following categories of treatment: (1) RFA alone; (2) Comparison between RFA and PEI; (3) Comparison between RFA and surgery; and (4) RFA combined with surgery or TACE.
RFA alone
Since 2005, six clinical cohort studies with large series of patients (more than 200 patients) have been reported in the medical literature. The survival results are summarized in Table 1.
Table 1.
Summary of therapeutic results of 6 large series cohort studies with percutaneous RFA alone
| Year | Author | PatientNo. | Size (cm)1 | FU (mo)2 | LTP (%)3 | New recur (%)4 | Major Cx (%)5 |
Overall survival (%) |
Median survival(mo) | Evidence6 | ||
| 1 yr | 3 yr | 5 yr | ||||||||||
| 2005 | Lencioni et al[13] | 206 | < 5 | 24 | 10 | 49 | 2.0 | 97 | 67 | 41 | 57 | 2 |
| 2005 | Tateishi et al[14] | 319 | < 5 | 28 | 8.7 | 60 | 4.0 | 95 | 78 | 54 | NA | 2 |
| 2005 | Chen et al[15] | 256 | < 8 | 2-69 | NA | NA | 2.4 | 83 | 67 | 41 | NA | 2 |
| 2007 | Choi et al[16] | 570 | < 5 | 30 | 11.8 | 52 | 1.9 | 95 | 70 | 58 | 77 | 2 |
| 2008 | Livraghi et al[17] | 216 | < 2 | 31 | 0.9 | NA | 1.8 | NA | 76 | 55 | NA | 2 |
| 2009 | N'Kontchou et al[18] | 235 | < 5 | 27 | 11.5 | 42 | 0.9 | NA | 60 | 40 | 48 | |
RFA: Radiofrequency ablation;
Maximum diameter of tumor;
Mean follow-up period;
Rate of local tumor progression;
Rate of new recurrence including intrahepatic remote and extrahepatic metastasis;
Rate of major complications requiring additional hospitalization or therapeutic procedure;
Level of evidence.
Lencioni et al[13] performed a prospective, intention-to-treat clinical trial with 206 patients with early stage unresectable HCC (mean size 2.8 cm). No procedure-related death was observed. Major complications were observed in three (2%) of 187 patients, including two cases of intraperitoneal bleeding and one tumor seeding along the needle track. Overall survival rates were 97%, 67% and 41% at 1, 3 and 5 years respectively. The prognostic factors related to overall survival were Child Class and tumor multiplicity. The 1-, 3- and 5-year local tumor progression rates were 4%, 10% and 10%.
Tateishi et al[14] reported therapeutic results of 1000 RFA procedures used to treat 2140 HCC nodules (mean size, 2.6 cm) in 664 patients. Major complications occurred in 4% per treatment and 1.9% per session. The most common complications were tumor seeding along the needle track, hepatic abscess formation requiring drainage and intraperitoneal hemorrhage, in order of decreasing frequency. There were no deaths related to the RFA procedure. The 1-, 3- and 5-year overall survival rates for 319 patients treated, as the first line treatment were 95%, 78% and 54%. Child-Pugh Class, tumor size, and AFP levels were prognostic factors for overall survival.
Chen et al[15] reported on the long term outcome of RFA for HCC (mean size 3.8 cm) in 256 patients. Major complications had an incidence of 2.4% and included track tumor seeding, intraperitoneal hemorrhage and bowel perforation. The overall survival rates were 83% at 1 year, 67% at 3 years and 41% at 5 years.
Choi et al[16] evaluated the long-term results and prognostic factors in 570 patients with 674 early stage HCCs. There were no procedure-related deaths. The incidence of major complications was 1.9% per treatment. The cumulative survival rates at 1, 3 and 5 years were 95%, 70% and 58% respectively. The local tumor progression rates at 1, 2 and 3 years were 8%, 11% and 12% respectively. The prognostic factors for survival were Child-Pugh Class, age and pre-treatment AFP levels.
Livraghi et al[17] reported on the therapeutic results after RFA procedures for very early HCC in 218 patients. They assessed two primary end points that could be easily compared to surgical resections: (1) the rate of sustained, local and complete response; and (2) the rate of treatment-related complications. The secondary end point was the 5-year survival in 100 patients that had tumors that were considered potentially operable. The sustained complete response rate was 97.2%. The perioperative mortality, major complication rate and 5-year survival were 0%, 1.8% and 69% respectively. They concluded that RFA could be considered the treatment of choice for patients with a single HCC less than 2 cm in diameter, even when surgical resection was possible.
Recently, N’Kontchou et al[18] evaluated the long-term results and prognostic factors in 235 consecutive patients with HCC (mean size 2.9 cm). Major complications occurred in three patients (0.9%), including one treatment-related death. The overall 5-year and recurrence-free survival rates were 40% and 17% respectively. However, the overall 5-year survival rate was 76% for operable patients. The prognostic factors associated with overall survival were prothrombin time and serum AFP levels. The tumor size was associated with local tumor progression but not with overall and tumor-free survival.
RFA vs PEI
In addition to the many studies on RFA alone, there have been many comparative studies performed to confirm the therapeutic efficacy of RFA by comparing other ablative techniques (especially PEI). During the past few years, five randomized clinical trials and three meta-analysis studies on the therapeutic efficacy of RFA vs PEI have been published[43-50]. The data of seven studies are summarized in Table 2.
Table 2.
Summary of 5 randomized controlled studies on comparison between RFA and PEI
| Year | Author | Treatment | Patient No. | FU (mo)1 | Initial CR2/Tumor (%) | Initial CR2/Patient (%) |
Overall survival (%) |
Evidence4 | ||
| 1 yr | 2 yr | 3 yr | ||||||||
| 2003 | Lencioni et al[43] | RFA | 42 | 23 | 91 | NA3 | 100 | 98 | NA | 2 |
| PEI | 44 | 22 | 82 | NA | 96 | 88 | NA | |||
| 2004 | Lin et al[44] | RFA | 52 | 24 | 96 | 96 | 90 | 82 | 74 | 1 |
| PEI | 105 | 24 | 91 | 91 | 87 | 62 | 48 | |||
| 2005 | Lin et al[45] | RFA | 62 | 28 | 97 | 97 | 93 | 81 | 74 | 1 |
| PEI | 62 | 26 | 88 | 89 | 81 | 66 | 51 | |||
| 2005 | Shiina et al[46] | RFA | 118 | ~4.3 yr | 100 | 97 | 97 | 91 | 81 | 1 |
| PEI | 187 | ~4.2 yr | 100 | 91 | 91 | 81 | 67 | |||
| 2008 | Brunello et al[47] | RFA | 70 | 26 | NA | 96 | NA | NA | 63 | 1 |
| PEI | 69 | 25 | NA | 66 | NA | NA | 59 | |||
PEI: Percutaneous ethanol injections;
Mean follow-up period;
Complete response rate;
Not available;
Level of evidence.
The key points from the five randomized controlled trials and three meta-analysis studies are that PEI and RFA are equally effective for tumors less than 2 cm. However, the necrotic effect of RFA is more predictable for all tumor sizes and its efficacy is clearly superior to that of PEI in larger tumors (level I)[4,20,43-50]. Overall, RFA demonstrated superior efficacy in regard to lower local tumor progression and a longer disease-free survival. The local tumor control rate was reported to range between 91% and 96% for RFA and between 65% and 88% for PEI. Both treatment groups presented similar adverse events; only one study found RFA associated with more major complications[43].
RFA vs surgical resection
After the introduction of percutaneous ablation therapy, the efficacy compared with curative treatment, namely surgical resection, for the treatment of small HCC has been debated[51-57]. The therapeutic efficacy reported by these comparative studies of RFA and surgical resection are summarized in Table 3.
Table 3.
Summary of 6 clinical studies on comparison between RFA and surgical resection
| Year | Author | Study | Treatment | Patient No. | FU (mo)1 | Tumor size (cm) |
Overall survival (%) |
P-value | Evidence3 | ||||
| 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | |||||||||
| 2004 | Vivarelli et al[51] | NR4 | RFA | 79 | 29 | < 5 | 78 | NA | 33 | NA | NA | 0.020 | 2 |
| Resection | 79 | 88 | NA | 65 | NA | NA | |||||||
| 2005 | Montorsi et al[52] | NR | RFA | 58 | NA2 | < 5 | 85 | 75 | 61 | 45 | NA | 0.139 | 2 |
| Resection | 48 | 84 | 79 | 73 | 61 | NA | |||||||
| 2005 | Hong et al[53] | NR | RFA | 55 | 35 | < 5 | 100 | NA | 74 | NA | NA | 0.240 | 2 |
| Resection | 93 | 98 | NA | 84 | NA | NA | |||||||
| 2005 | Chen et al[54] | R5 | RFA | 47 | 36 | < 5 | 93 | 82 | 64 | NA | NA | 0.753 | 1 |
| Resection | 65 | 93 | 86 | 67 | NA | NA | |||||||
| 2006 | Lü et al[55] | R | RFA | 51 | NA | < 5 | 94 | 87 | 87 | NA | NA | 0.808 | 1 |
| Resection | 54 | 91 | 86 | 86 | NA | NA | |||||||
| 2009 | Ueno et al[56] | NR | RFA | 110 | 36 | < 5 | 98 | NA | 92 | NA | 63 | 0.060 | 2 |
| Resection | 123 | 99 | NA | 92 | NA | 80 | |||||||
Mean follow-up period;
Not available;
Level of evidence;
Non-randomized study;
Randomized controlled study.
Several non-randomized studies have demonstrated equivalent outcomes for RFA and surgery. Montorsi et al[52] performed a prospective nonrandomized trial comparing RFA (58 patients) with surgery (40 patients) in 98 patients with a single HCC less than 5 cm in diameter. While long-term (up to 4 years) survival was equivalent in both treatment groups, RFA resulted in significantly higher rates of intrahepatic recurrence compared to the surgical resection group. Another nonrandomized comparative study reported by Hong et al[53] demonstrated that RFA was as effective as surgical resection for single small (< 5 cm) HCC in patients with Child A disease, similar to the results reported by Montorsi et al[52]. A large Japanese prospective study with 7185 patients with small HCC demonstrated no significant difference in overall survival for hepatic resection vs RFA vs PEI group, although the time-to-recurrence rates were better for the hepatic resection group[57]. Ueno et al[56] performed a retrospective study on 278 consecutive patients with HCC classified by the Milan criteria that were treated by surgical resection (123 patients) and RFA (155 patients). The overall survival and disease-free survival was significantly better in the surgical resection group than in the RFA group, although differences in liver function reserve existed. A recent study by Livraghi et al[17] focused on early stage disease and demonstrated a sustained local complete response after RFA comparable with that of hepatic resection.
Two randomized controlled trials compared RFA to hepatic resection in patients with early HCC. Chen et al[54] reported a randomized controlled trial in 112 patients with a single HCC less than 5 cm that received either resection (65 patients) or percutaneous RFA (47 patients). No significant differences in local recurrence, overall survival or disease-free survival were detected between the two groups. Most clinical trials, including randomized controlled trials, have shown that RFA is comparable to surgical resection in terms of overall survival; in addition, it is less invasive and associated with lower complication rates and lower costs[51-57].
Direct comparison by a well designed randomized controlled trial is the only way to assess whether RFA might replace surgical resection for treating early stage, resectable HCC. The difference in survival between the two treatments appears to be fairly small, based on the currently available data. The sample size required to ensure meaningful conclusions should be quite large. Thus, this kind of randomized controlled study may be not feasible[17].
RFA combined with other treatments (surgery or TACE)
RFA combined with surgery: RFA can be used as one complimentary method for multifocal or larger tumors. In patients with multifocal HCCs that are not feasible for hepatic resection, resection of the dominant tumors can be performed first and then the remaining small tumors can be simultaneously ablated by RFA. Using this approach, more patients previously considered inoperable become eligible for a curative resection[58-61]. Choi et al[62] reported acceptable perioperative morbidity and long-term survival in a series of 53 patients that had combined hepatectomy and RFA for multifocal HCCs. They confirmed an important role for RFA in increasing the chance of curative treatment for patients with multifocal tumors that might be traditionally considered unresectable. However, further investigation is needed to compare the outcome of hepatectomy plus RFA with that of hepatectomy alone to assess whether the survival results are truly comparable[58,59].
RFA combined with TACE: Another promising role of RFA is combined treatment with TACE for intermediate to large tumors. Although RFA shows excellent local tumor control for small tumors less than 3 cm, the limited size of the ablation zone usually fails to achieve complete ablation of large HCC greater than 5 cm[12-18,63]. To obtain a large coagulation area, various techniques including multiple overlapping ablations[64-67], saline-enhanced ablation to reduce the tissue impedance[68,69] and temporary occlusion of tumor blood supply have been attempted[64,70-73]. The combination of TACE with RFA has two theoretical merits: (1) Occlusion of hepatic arterial flow by means of embolization may contribute to the decrease in the heat-sink effects during RFA and increase the ablation volume by RFA; and (2) Combined treatment may have the effect of anticancer agents on cancer cells, which is enhanced by the hyperthermia.
Yamakado et al[74] compared the therapeutic efficacy of combined TACE plus RFA and Surgical resection in 142 patients with HCC (< 5 cm, up to 3 in number). The 1-, 3- and 5-year overall survival rate after TACE followed by RFA (98%, 94% and 75%) were similar to surgical resection (97%, 93% and 81%)[75]. In addition, they reported another study with 20 patients with HCC larger than 5 cm. The overall and recurrence-free survival rates were 100% and 71% at 1 year, 62% and 28% at 3 years and 41% and 14% at 5 years. Recently, Shibata et al[76] reported a prospective study comparing the therapeutic efficacy of a combined TACE and RFA group (46 patients) with a RFA alone group (43 patients). They concluded that combined TACE with RFA had equivalent effectiveness for the treatment of small (< 3 cm) HCCs; therefore, combined treatment may not be necessary for small tumors.
PERSPECTIVE ON RFA
Based on current evidence, RFA will remain the mainstay of local treatment for early stage HCC because of its excellent local tumor control and minimal morbidity. The therapeutic efficacy of RFA will continue to be refined with advancements in technology in terms of planning, targeting, monitoring, controlling and assessment of therapeutic efficacy. The technical advancements will include novel guiding modalities (CE-US, fusion imaging or robotic guidance)[77-81], more powerful ablation strategies (multiple applicators) and combined treatment with adjuvant therapy such as thermo-sensitive drugs or targeted agents such as sorafenib[22,82-84]. However, RFA technology will be challenged by other ablative techniques including novel microwave or cryosurgery technologies as well as non-invasive emerging techniques such as high intensity focused ultrasound treatment and irreversible electroporation in the near future[85-90].
CONCLUSION
RFA is the most popular non-surgical technique for treating early stage unresectable HCC because of its excellent local tumor control and acceptable morbidity. RFA is superior to PEI in terms of local tumor control and survival. Overall survival of RFA is comparable to surgical resection in a selected group of patients with smaller tumors. Currently, combined RFA with TACE is increasingly being investigated for the treatment of intermediate stage HCC. Considering the ongoing technical advances, RFA remains an attractive technique with additional potential to be explored by further investigations.
Footnotes
Peer reviewer: Yong-Song Guan, Professor, Oncology and Radiology; State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
References
- 1.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Hong K, Georgiades CS, Geschwind JF. Technology insight: Image-guided therapies for hepatocellular carcinoma--intra-arterial and ablative techniques. Nat Clin Pract Oncol. 2006;3:315–324. doi: 10.1038/ncponc0512. [DOI] [PubMed] [Google Scholar]
- 6.Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331–347. doi: 10.1016/j.ejso.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd GD 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, Gillams AR, Karahan OI, Rhim H. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 9.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72 Suppl 1:30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 10.Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, Yin SS, Zhang H, Poon RT. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17:671–683. doi: 10.1097/01.RVI.0000201985.61501.9E. [DOI] [PubMed] [Google Scholar]
- 11.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R, Della Pina C, Bartolozzi C. Percutaneous image-guided radiofrequency ablation in the therapeutic management of hepatocellular carcinoma. Abdom Imaging. 2005;30:401–408. doi: 10.1007/s00261-004-0254-8. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 15.Chen MH, Yan K, Yang W, Gao W, Dai Y, Huo L, Zhang H, Huang XF. [Long term (5 years) outcome of radiofrequency ablation for hepatocellular carcinoma in 256 cases] Beijing Da Xue Xue Bao. 2005;37:671–672. [PubMed] [Google Scholar]
- 16.Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 17.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 18.N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 19.Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol. 2006;12:828–829. doi: 10.3748/wjg.v12.i5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72 Suppl 1:2–15. doi: 10.1159/000111702. [DOI] [PubMed] [Google Scholar]
- 24.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 25.Rhim H, Dodd GD 3rd, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, Sewell PE, Goldberg SN. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24:41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 26.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 27.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 28.de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 29.Lencioni R, Veltri A, Guglielmi A, Bianchini M, Filauro M, Bartolozzi C. Complications of percutaneous radiofre¬quency ablation of liver malignancies with expandable multiprobe needles: results of a multicenter study. The 87th RSNA Annual Meeting Abstract, 2003 [Google Scholar]
- 30.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134; discussion 134-136. doi: 10.1148/rg.231025054. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Rhim H, Paik SS. Radiofrequency ablation of the liver in a rabbit model: creation of artificial ascites to minimize collateral thermal injury to the diaphragm and stomach. J Vasc Interv Radiol. 2006;17:541–547. doi: 10.1097/01.rvi.0000208305.65202.84. [DOI] [PubMed] [Google Scholar]
- 32.Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006;93:1277–1282. doi: 10.1002/bjs.5374. [DOI] [PubMed] [Google Scholar]
- 33.Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, Zhang H, Wu W. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428–436. doi: 10.1007/s00261-007-9283-4. [DOI] [PubMed] [Google Scholar]
- 34.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91–98. doi: 10.2214/AJR.07.2384. [DOI] [PubMed] [Google Scholar]
- 35.Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14:1183–1186. doi: 10.1097/01.rvi.0000086530.86489.05. [DOI] [PubMed] [Google Scholar]
- 36.Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, Hiasa Y, Onji M. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007;42:306–311. doi: 10.1007/s00535-006-1949-0. [DOI] [PubMed] [Google Scholar]
- 37.Shibata T, Iimuro Y, Ikai I, Hatano E, Yamaoka Y, Konishi J. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002;13:313–315. doi: 10.1016/s1051-0443(07)61725-4. [DOI] [PubMed] [Google Scholar]
- 38.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 39.Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg. 2008;195:508–520. doi: 10.1016/j.amjsurg.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 41.Mendizabal M, Reddy KR. Current management of hepatocellular carcinoma. Med Clin North Am. 2009;93:885–900, viii. doi: 10.1016/j.mcna.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT Jr, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 44.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–17123. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 48.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 49.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–524. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 50.Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, Costa M. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–67; discussion 67-68. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 54.Chen MS, Li JQ, Liang HH, Lin XJ, Guo RP, Zheng Y, Zhang YQ. [Comparison of effects of percutaneous radiofrequency ablation and surgical resection on small hepatocellular carcinoma] Zhonghua Yi Xue Za Zhi. 2005;85:80–83. [PubMed] [Google Scholar]
- 55.Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, Li DM, Lai JM, Li SQ. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial] Zhonghua Yi Xue Za Zhi. 2006;86:801–805. [PubMed] [Google Scholar]
- 56.Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589–594. doi: 10.1016/j.jhep.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Poon RT. Radiofrequency ablation combined with resection enhances chance for curative treatment of hepatocellular carcinoma. Ann Surg Oncol. 2007;14:3299–3300. doi: 10.1245/s10434-007-9567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825; discussion 825-827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elias D, Goharin A, El Otmany A, Taieb J, Duvillard P, Lasser P, de Baere T. Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763–769. doi: 10.1053/ejso.2000.1000. [DOI] [PubMed] [Google Scholar]
- 61.Kim YS, Rhim H, Lim HK, Choi D, Lee WJ, Jeon TY, Joh JW, Kim SJ. Intraoperative radiofrequency ablation for hepatocellular carcinoma: long-term results in a large series. Ann Surg Oncol. 2008;15:1862–1870. doi: 10.1245/s10434-008-9941-y. [DOI] [PubMed] [Google Scholar]
- 62.Choi D, Lim HK, Joh JW, Kim SJ, Kim MJ, Rhim H, Kim YS, Yoo BC, Paik SW, Park CK. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14:3510–3518. doi: 10.1245/s10434-007-9492-7. [DOI] [PubMed] [Google Scholar]
- 63.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 64.Rhim H, Goldberg SN, Dodd GB 3rd, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21:S17–S35. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 65.Dodd GB 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analyses of the size of the thermal injury by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 66.Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, Coderc E, Trinchet JC, Beaugrand M, Sellier N. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology. 2008;248:288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- 67.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, Choi BI. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 68.Hänsler J, Frieser M, Tietz V, Uhlke D, Wissniowski TT, Bernatik T, Hahn EG, Strobel D. Percutaneous ultrasound-guided radiofrequency ablation (RFA) using saline-perfused (wet) needle electrodes for the treatment of hepatocellular carcinoma--long term experience. Ultraschall Med. 2007;28:604–611. doi: 10.1055/s-2007-963581. [DOI] [PubMed] [Google Scholar]
- 69.Lee JM, Han JK, Kim SH, Sohn KL, Lee KH, Ah SK, Choi BI. A comparative experimental study of the in-vivo efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol. 2003;4:13–19. doi: 10.3348/kjr.2003.4.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhim H, Dodd GD 3rd. Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound. 1999;27:221–229. doi: 10.1002/(sici)1097-0096(199906)27:5<221::aid-jcu1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 71.Kim SK, Lim HK, Ryu JA, Choi D, Lee WJ, Lee JY, Lee JH, Sung YM, Cho EY, Hong SM, et al. Radiofrequency ablation of rabbit liver in vivo: effect of the Pringles maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol. 2004;5:240–249. doi: 10.3348/kjr.2004.5.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Baere T, Deschamps F, Briggs P, Dromain C, Boige V, Hechelhammer L, Abdel-Rehim M, Aupérin A, Goere D, Elias D. Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology. 2008;248:1056–1066. doi: 10.1148/radiol.2483070222. [DOI] [PubMed] [Google Scholar]
- 73.Sudheendra D, Neeman Z, Kam A, Locklin J, Libutti SK, Wood BJ. Intermittent hepatic vein balloon occlusion during radiofrequency ablation in the liver. Cardiovasc Intervent Radiol. 2006;29:1088–1092. doi: 10.1007/s00270-006-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, Isaji S, Shiraki K, Fuke H, Uemoto S, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247:260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 75.Takaki H, Yamakado K, Uraki J, Nakatsuka A, Fuke H, Yamamoto N, Shiraki K, Yamada T, Takeda K. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009;20:217–224. doi: 10.1016/j.jvir.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 76.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: Is radiofrequency ablation combined with transarterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 77.Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y, Terae S, Shirato H. Sonazoid-enhanced sonography for guiding radiofrequency ablation for hepatocellular carcinoma: better tumor visualization by Kupffer-phase imaging and vascular-phase imaging after reinjection. Jpn J Radiol. 2009;27:185–193. doi: 10.1007/s11604-009-0317-4. [DOI] [PubMed] [Google Scholar]
- 78.Chen MH, Yang W, Yan K, Dai Y, Wu W, Fan ZH, Callstrom MR, Charboneau JW. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol. 2007;62:752–760. doi: 10.1016/j.crad.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 79.Giesel FL, Mehndiratta A, Locklin J, McAuliffe MJ, White S, Choyke PL, Knopp MV, Wood BJ, Haberkorn U, von Tengg-Kobligk H. Image fusion using CT, MRI and OET for treatment planning, navigation and follow up in percutaneous RFA. Exp Oncol. 2009;31:106–114. [PMC free article] [PubMed] [Google Scholar]
- 80.Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, Ueshima K, Shiozaki H. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol. 2008;190:W335–W341. doi: 10.2214/AJR.07.3092. [DOI] [PubMed] [Google Scholar]
- 81.Wood BJ, Locklin JK, Viswanathan A, Kruecker J, Haemmerich D, Cebral J, Sofer A, Cheng R, McCreedy E, Cleary K, McAuliffe MJ, Glossop N, Yanof J. Technologies for guidance of radio¬frequency ablation in the multimodality interventional suite of the future. J Vasc Interv Radiol. 2007;18:9–24. doi: 10.1016/j.jvir.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spangenberg HC, Thimme R, Blum HE. Evolving therapies in the management of hepatocellular carcinoma. Biologics. 2008;2:453–462. doi: 10.2147/btt.s3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hakimé A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, Regan M, Goldberg SN. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology. 2007;244:464–470. doi: 10.1148/radiol.2442061005. [DOI] [PubMed] [Google Scholar]
- 84.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 85.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72 Suppl 1:124–131. doi: 10.1159/000111718. [DOI] [PubMed] [Google Scholar]
- 87.Li YY, Sha WH, Zhou YJ, Nie YQ. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:2148–2154. doi: 10.1111/j.1440-1746.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 88.Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659–667. doi: 10.1148/radiol.2352030916. [DOI] [PubMed] [Google Scholar]
- 89.Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, Mir LM. Tumor ablation with irreversible electroporation. PLoS One. 2007;2:e1135. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6:287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]