Abstract
After the first report by Kalloo et al on transgastric peritoneoscopy in pigs, it rapidly became apparent that there was no room for an under-evaluated concept and blind adoption of an appealing (r)evolution in minimal access surgery. Systematic experimental work became mandatory before any translation to the clinical setting. Choice and management of the access site, techniques of dissection, exposure, retraction and tissue approximation-sealing were the basics that needed to be evaluated before considering any surgical procedure or study of the relevance of natural orifice transluminal endoscopic surgery (NOTES). After several years of testing in experimental labs, the revolutionary concept of NOTES, is now progressively being experimented on in clinical settings. In this paper the authors analyse the challenges, limitations and solutions to assess how to move from the lab to clinical implementation of transgastric endoscopic cholecystectomy.
Keywords: Flexible surgery, Cholecystectomy, Natural orifice transluminal endoscopic surgery, Minimal invasive surgery, Endoscopic surgery
INTRODUCTION
Breaching the lumen of a healthy organ to perform an operation without surgical incision raises several scientific and ethical concerns. Preservation of the abdominal wall is the single obvious inherent advantage. Complications related to the breach in organs can be disastrous. After the first report by Kalloo et al[1] on transgastric peritoneoscopy in pigs, it rapidly became apparent that there was no room for an under-evaluated concept and blind adoption of an appealing (r)evolution in minimal access surgery. Systematic experimental work became mandatory before any translation to the clinical setting. Choice and management of the access site, techniques of dissection, exposure, retraction and tissue approximation-sealing were the basics that needed to be evaluated before considering any surgical procedure or study of the relevance of natural orifice transluminal endoscopic surgery (NOTES). The second step was to identify the surgical procedures that would benefit the most from this new approach in terms of outcome and acceptance by the surgical community. The third step was to analyze the physiological consequences of NOTES and compare it to open and laparoscopic similar operations. Then, once all these issues were overcome, translation to the clinical setting was considered.
In parallel, each of these challenges stimulated technological innovation to overcome the inherent difficulties associated with the utilization of instruments that were originally developed to work inside and not outside the lumen of hollow organs.
In 2005, IRCAD strasbourg established an intensive research program on NOTES. A step by step analysis of the challenges of NOTES was performed. The value and adequacy of the different natural orifices and various surgical procedures were studied. This experimental work provided essential information about the feasibility and potentialities of NOTES and extensive surgical training.
Between 2005 and 2008, over 400 experimental procedures were performed on inanimate models, ex-vivo tissues, animal models and human cadavers. A systematic analysis of the steps of the transgastric route was carried out from the introduction of the endoscope into the oro-pharynx to the closure of the stomach after various surgical procedures. Transgastric cholecystectomy was identified as the procedure that would initiate the translation to humans. Translation from the laboratory to human application is reported.
TRANSGASTRIC ACCESS AND CLOSURE TECHNIQUES
Although most believe that the transgastric route will dominate NOTES in the future, a factor limiting the transgastric route is the lack of a secure and reliable method for creating and closing the gastrotomy required by the procedure. Indeed, creating a gastrotomy from within the stomach requires a blind entry to the peritoneal cavity making it hard to avoid damage to neighbouring structures[2] and to ensure the gastrotomy is sited in the best possible position.
Several gastrotomy techniques were evaluated and the most used method is based on the established safety of percutaneous endoscopic gastrotomy placement with balloon dilatation[3]. A flexible wire is passed through the anterior abdominal wall and guides the stomach incision at the level of the antrum and dilatation of the gastrotomy. This method was used extensively in the experimental setting and provided efficient, reliable and reproducible access to the peritoneal cavity.
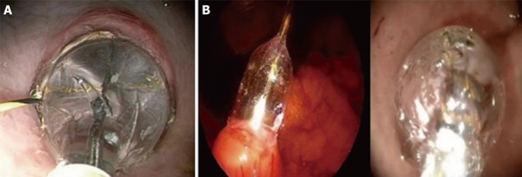
In the clinical setting, this method of small gastrotomy with guided balloon dilatation was elected. At this point, gastric incision was still a blind manoeuvre and the clinical protocol imposed a visual control of this step. Therefore, transgastric access was obtained under laparoscopic visual control by means of a 5-mm rigid laparoscope introduced in an umbilical trocar[4]. An endoscopic monopolar needle-knife was used to create a 0.5-cm gastrotomy on the anterior gastric wall in the antrum of the stomach. A guide-wire was passed through the gastrotomy to guide a 18 mm balloon dilator which expanded the gastrotomy and allowed for the passage of the 12 mm gastroscope (KARL STORZ® Endoskope, Germany) (Figure 1). This method was successfully utilized in a series of 11 transgastric cholecystectomies. No bleeding or injuries to adjacent organs were observed.
Figure 1.
Balloon dilatation of the gastrotomy in the animal model (A) and in a patient (B).
Closure of the gastrotomy is crucial. There is general agreement that there must be near-zero tolerance for leaks. The ideal closure should be rapid, reproducible and safe, ideally performed under vision to avoid any injury to the adjacent organs and should grant a full thickness bite.
Different methods have been tested in the laboratory[5-14]. The simple application of current endoclips enables only a single-layer mucosal approximation. In addition, their application might sometimes be difficult due to tangential orientation of the tissue or because of tissue edema. Using two endoscopes to provide layer-by-layer endoscopic clip closure was another alternative that uses current endoscopic instruments[15]. An original technique using a cardiac septal occluder has demonstrated a zero leak rate[16]. The system was widely used for survival studies on animal models but it was not transferable to the clinical setting for cost issues and concerns about the long term outcome of the intraperitoneal, non absorbable part of the mechanism made with nitinol.
In patients, the gastrotomy was closed with extracorporeal interrupted 3/0 Vicryl stitches by means of a 2-mm laparoscope and a 3-mm needle holder that were inserted side by side into the 5 mm umbilical port. Gastroscopy was carried out in order to inspect the closure and to confirm an airtight seal by the attainment of a satisfactory pneumogastrium.
This method of creation and closure of the gastrotomy was successfully used in a series of transgastric cholecystectomies. Neither bleeding nor injuries to adjacent organs were observed. No gastric or biliary leaks occurred.
EXPOSURE AND RETRACTION
Dissection of the critical view of safety is a basic rule for laparoscopic cholecystectomy. This requires retraction of the gallbladder and exposure of the triangle of Calot which cannot be offered by current flexible endoscopes and instrumentation. The different methods experimented with in the laboratory such as retracting needle, suspension thread and T-tags provided adequate exposure thanks to a transparietal element. Magnetic retraction eliminates this necessity but poses several problems in bringing the internal part of the system in the peritoneal cavity and grabbing the gallbladder[17,18]. The transoral dual scope technique brings more instruments in the peritoneal cavity. A single channel endoscope serves as a surgical assistant and provides retraction of the gallbladder. Another scope performs the dissection and clipping of the pedicle. This method raises several problems in terms of manoeuvrability and tolerance of the oesophagus to the movements of the two endoscopes.
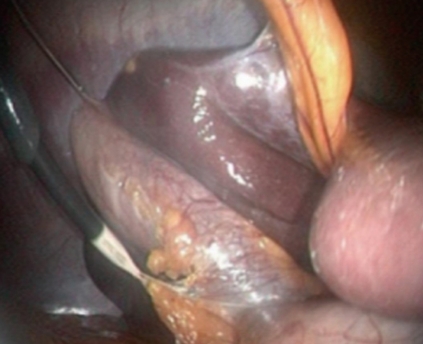
Whilst a transvaginal grasper can be used in a transvaginal cholecystectomy to provide retraction[19], this is not possible in the transgastric route. Transparietal assistance is obligatory and a micro-laparoscopic grasper was inserted alternatively through the umbilicus or the right hypochondrium in patients (Figure 2).
Figure 2.
Retraction of the gallbladder with a laparoscopic micro-instrument introduced in the right hypochondrium.
DISSECTION
Once exposure of the gallbladder is obtained, the dissection can be carried out using exclusively flexible instruments inserted via the two working channels of the endoscope (Figure 3). Feasibility of this method has been extensively studied in the animal model[17] (Figure 4). However, these instruments are obviously not designed or adapted to perform such tasks and, as a result, the dissection becomes time consuming.
Figure 3.
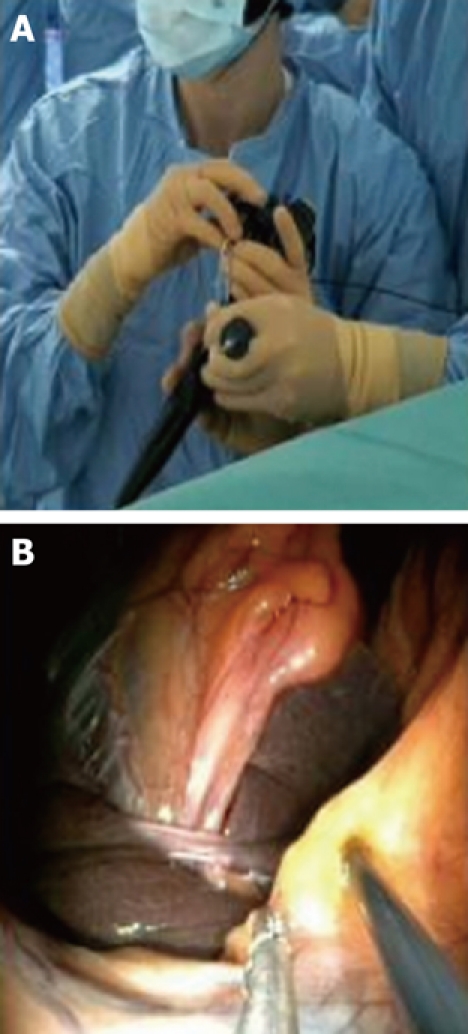
Dissection of the cystic pedicle with a flexible endoscope: “four hands” technique. Extrernal view (A) and endoscopic view (B).
Figure 4.
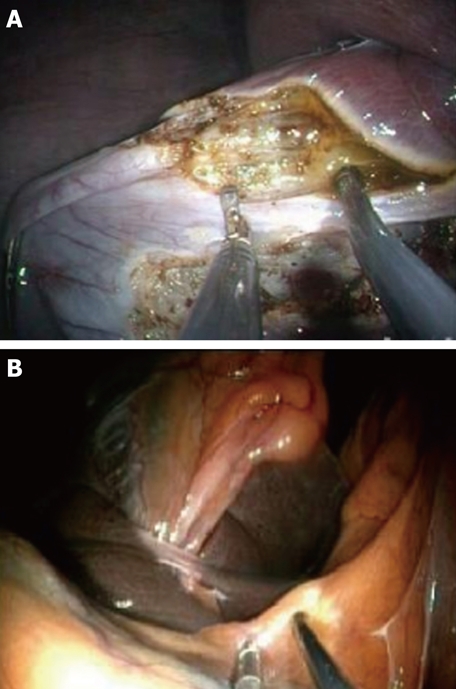
Dissection of the cystic pedicle with a flexible endoscope in the animal model (A) and in a patient (B).
As a laparoscopic trocar was inserted systematically at the umbilicus to monitor the creation of the gastrotomy, the operating time improved dramatically by using standard laparoscopic instruments such as a hook inserted through this trocar which was also necessary for the clip applier.
TISSUE SEALING
Currently there is no flexible device that can be introduced orally to seal vessels or biliary structures. This is one of the major current limitations of NOTES. Although flexible endoclips have been used successfully in the laboratory, postoperative bleeding was observed in one patient after the description of transvaginal cholecystectomy[20]. At this point, sealing the cystic duct and artery necessitates a laparoscopic clip applier introduced through a 5-mm laparoscopic trocar. An alternative is the flexible endoloop whose development was not intended to sealing these elements.
CONTROLLING CONTAMINATION
Controlling contamination is a rather contentious issue. One of the biggest concerns associated with NOTES surgery is the risk of intra-abdominal infection due to intraoperative spillage or via inadequate closure of the gastrointestinal tract. Perforation may occur inadvertently during abdominal surgery as well as endoscopic procedures. This issue has been addressed in studies that investigate the bacterial load and contamination in patients during laparoscopic Roux-en-Y gastric bypass and transgastric staging peritoneoscopy[21,22]. These findings demonstrated that while transgastric access does contaminate the abdominal cavity, introduced pathogens are clinically insignificant due to species or bacterial load. A systematic study of the bacteria content of peritoneal fluid samples before and after transgastric cholecystectomy confirmed these findings.
EXTRACTION OF SPECIMEN
Extraction of a specimen such as the gallbladder is a minor concern in the animal model. The gallbladder is normal and thin and is passed easily through the gastrotomy and esophagus. In humans, an unexpected limitation of the transgastric access was the size of the gallbladder stones. Indeed, if passage inside the stomach is not a real problem, large stones may cause esophageal laceration when the gallbladder is extracted through the mouth. A maximum size of 20 mm seems to be the upper limit and fragmentation of large stones may be challenging in a NOTES setting.
TRANSGASTRIC CHOLECYSTECTOMY IN HUMAN BEINGS
From September 2007 to June 2009, 11 patients (7 men and 4 women) with a mean age of 48.5 years (range 28-65 years) and a mean BMI of 23.3 (range: 21-31) were enrolled in the study. Three patients had a past surgical history of appendectomy and one of hysterectomy.
All procedures were completed using a hybrid approach with a 5-mm umbilical trocar. In one patient, there was a need to switch to a laparoscopic procedure (conversion) because of lack of exposure of the triangle of calot.
Transgastric peritoneal access was achieved without complication or injury to adjacent organs. The site chosen for the creation of the gastrotomy was the gastric antrum in all patients to facilitate access to the right hypochondrium and the gallbladder. Additional transparietal assistance was mandatory in all patients to retract the gallbladder and to achieve a safe exposure of Calot’s triangle. Dissection of the gallbladder was completely achieved with flexible endoscopic instruments in 2 patients while a combination with laparoscopic hook dissector was used in the other patients. A 5-mm laparoscopic clip applier was systematically used to secure the cystic pedicle. Single port gastric closure was successfully achieved in 10/11 patients. One patient required an additional 5 mm trocar because of technical failure of the instrumentation.
No trauma, vascular or biliary injury to the adjacent organs occurred during the procedure.
The mean operative time was 132 min (range 90-180 min) and this was mainly due to the longer time needed to accomplish the dissection of the gallbladder and closure of the gastrotomy without any new dedicated technology.
All patients recovered promptly. Postoperative pain evaluated using the VAS, Visual Analog Scale, a tool that allows to objectify pain intensity on a 0-10 scale (0 being no pain, 10 being extreme pain), was 2/10 at 24 h and 0/10 at 48 h under usual immediate postoperative analgesia with paracetamol. One patient required additional analgesia with morphine on day 1. They were allowed fluids the very evening of the procedure and resumed a normal diet on the first postoperative day. No gastric or biliary leaks occurred. Mean hospital stay was 2 d (range 2-3 d). The bacteriological analysis of the peritoneal aspirates showed no significant contamination of the peritoneal cavity for both aerobic and non-aerobic species. One patient was readmitted 8 d after the operation for epigastric pain. Workup, including gastroscopy, did not reveal any complications.
PERSPECTIVE AND FUTURE PROSPECTS
One of the most frequent criticisms of laparoscopy in the early 1990s was the absence of scientific background based on experimental work and the fact that the majority of surgeons entered this new surgical approach without any previous training. One of the most popular and frequent operations, cholecystectomy, rapidly became the procedure to be done laparoscopically. There was no scientific evidence of the superiority of the laparoscopic approach over the open operation but every single surgeon thought that if he was not performing laparoscopic surgery he would be brought off a market driven and largely supported by the industry. Some years later, it became evident that the rate of iatrogenic injuries of the biliary tract had more than doubled compared to open surgery. This finding stimulated the surgical scientific community and teaching programs were started all over the world. Scientific societies were created to drive education and training and stimulate scientific support and criticism. Guidelines were established.
Twenty years later, after the first report on transgastric peritoneoscopy using a flexible endoscope, the lesson was learned. There was no question about starting this new technique in a clinical setting without a strong and systematic evaluation of the feasibility and safety of the technique and consistent training in the laboratory. Scientific societies reacted immediately and organized joint associations between gastroenterologists and laparoscopic surgeons which proposed prerequisites and guidelines for experimental development and clinical implementation[23].
An extensive research program was developed in IRCAD Strasbourg to evaluate the feasibility and potential of NOTES. The numerous challenges generated an impressive number of experiments that provided endoscopic and surgical training to gastroenterologists and surgeons involved in the program. All natural orifice access and potential surgical procedures were reviewed and studied[24-31]. Transgastric access was the most widely used and several surgical applications were tested. Cholecystectomy was then defined as the application that would be transferred to human application.
Various transgastric surgical procedures via natural orifices have proven to be feasible in animal models. Survival studies on cholecystectomy were a prerequisite before clinical implementation[17]. These procedures are technically challenging given the current instrumentation that is available. The choice of the adequate gastric exit site and the creation and closure of the gastrotomy are all parameters that still need to be standardized. An additional challenge is obtaining adequate spatial orientation and retraction with the endoscope in a retroflexed position when the image is upside down and an off-axis manipulation is required[32]. Although some of this spatial incongruity can be overcome with experience and exposure, interpretation of the anatomy and identification of the structural landmarks are still quite challenging. In cholecystectomy, one of the major limitations is to obtain satisfactory exposure of the gallbladder and Calot’s triangle. Research programs have identified possible solutions but none is currently available for clinical application[18]. So transparietal assistance is still mandatory. These “hybrid” techniques are a cross between NOTES and laparoscopy.
The passage to the clinical setting relied on the experimental work and the surgical steps reproduced closely the techniques that were performed many times in the laboratory: creation of the gastrotomy, introduction of the endoscope in the peritoneal cavity, exposure of the gallbladder and technique of dissection with the flexible double channel endoscope[4]. For obvious safety reasons, all these steps were completed under the surveillance and assistance of a 5-mm umbilical trocar. Indeed, clipping of the cystic elements and closure of the gastrotomy were achieved with laparoscopic instruments. In two of our patients, a laparoscopic view was necessary to verify the biliary anatomy that was not clearly understood. One of the lessons of this experience is that there can be some distortion of the anatomical landmarks related to a different angle of view.
Bacterial contamination of the abdomen was also of great concern. The peritoneal fluid samples taken before and after the gastrotomy’s closure did not reveal any significant contamination of the peritoneal cavity and no clinical infection occurred. These findings confirm the results reported by Narula et al[21,22] who investigated the bacterial load and contamination in patients during laparoscopic Roux-en-Y gastric bypass and transgastric diagnostic peritoneoscopy.
Finally, an unsuspected limitation of the transgastric cholecystectomy technique may be related to the size of the gallstone which should not be over 20 mm in diameter. Retrieval of larger stones, although feasible by enlarging the gastrotomy, could result in impaction and/or injury of the oesophagus and oro-pharynx. In one patient we had to change the 5 mm umbilical trocar to a 10-mm one to extract the gallbladder containing a 25-mm stone. In all but this patient, the gallbladder was extracted orally through the gastrotomy. No complications occurred during our initial clinical series and this is more than probably related to the intensive training program in the laboratory.
CONCLUSION
Although NOTES hybrid techniques seem to diverge from the philosophy pursued at the beginning in the lab, they have the great merit of allowing the application of this revolutionary approach in clinical settings to explore the potential benefits for patients waiting for technological development that will facilitate the performance of pure NOTES “no scar surgery”.
Footnotes
Peer reviewer: Miroslav N Milicevic, Professor, Department of HPB Surgery and Liver Transplant, The First Surgical Clinic University of Belgrade Clinical Center, Belgrade 11000, Yugoslavia
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
References
- 1.Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 2.Sohn DK, Turner BG, Gee DW, Willingham FF, Sylla P, Cizginer S, Konuk Y, Brugge WR, Rattner DW. Reducing the unexpectedly high rate of injuries caused by NOTES gastrotomy creation. Surg Endosc. 2010;24:277–282. doi: 10.1007/s00464-009-0570-8. [DOI] [PubMed] [Google Scholar]
- 3.Sumiyama K, Gostout CJ. Techniques for transgastric access to the peritoneal cavity. Gastrointest Endosc Clin N Am. 2008;18:235–244; vii. doi: 10.1016/j.giec.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Dallemagne B, Perretta S, Allemann P, Asakuma M, Marescaux J. Transgastric hybrid cholecystectomy. Br J Surg. 2009;96:1162–1166. doi: 10.1002/bjs.6704. [DOI] [PubMed] [Google Scholar]
- 5.Dray X, Giday SA, Buscaglia JM, Gabrielson KL, Kantsevoy SV, Magno P, Assumpcao L, Shin EJ, Reddings SK, Woods KE, et al. Omentoplasty for gastrotomy closure after natural orifice transluminal endoscopic surgery procedures (with video) Gastrointest Endosc. 2009;70:131–140. doi: 10.1016/j.gie.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Katsarelias D, Polydorou A, Tsaroucha A, Pavlakis E, Dedemadi G, Pistiolis L, Karakostas N, Kondi-Paphiti A, Mallas E, et al. Endoloop application as an alternative method for gastrot¬omy closure in experimental transgastric surgery. Surg Endosc. 2007;21:1862–1865. doi: 10.1007/s00464-007-9281-1. [DOI] [PubMed] [Google Scholar]
- 7.Magno P, Giday SA, Dray X, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Kalloo AN, Pasricha PJ, White JJ, et al. A new stapler-based full-thickness transgastric access closure: results from an animal pilot trial. Endoscopy. 2007;39:876–880. doi: 10.1055/s-2007-966896. [DOI] [PubMed] [Google Scholar]
- 8.McGee MF, Marks JM, Jin J, Williams C, Chak A, Schomisch SJ, Andrews J, Okada S, Ponsky JL. Complete endoscopic closure of gastric defects using a full-thickness tissue plicating device. J Gastrointest Surg. 2008;12:38–45. doi: 10.1007/s11605-007-0371-0. [DOI] [PubMed] [Google Scholar]
- 9.Meireles OR, Kantsevoy SV, Assumpcao LR, Magno P, Dray X, Giday SA, Kalloo AN, Hanly EJ, Marohn MR. Reliable gastric closure after natural orifice translumenal endoscopic surgery (NOTES) using a novel automated flexible stapling device. Surg Endosc. 2008;22:1609–1613. doi: 10.1007/s00464-008-9750-1. [DOI] [PubMed] [Google Scholar]
- 10.Sporn E, Bachman SL, Miedema BW, Loy TS, Calaluce R, Thaler K. Endoscopic colotomy closure for natural orifice transluminal endoscopic surgery using a T-fastener prototype in comparison to conventional laparoscopic suture closure. Gastrointest Endosc. 2008;68:724–730. doi: 10.1016/j.gie.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Trunzo JA, Mcgee MF, Cavazzola L. A Comparison of Three Endoscopic Suturing Devices for Natural Orifice Translumenal Endoscopic Surgery Gastrotomy Closure. Gastrointest Endosc. 2009;69:AB304. [Google Scholar]
- 12.Voermans RP, Worm AM, van Berge Henegouwen MI, Breedveld P, Bemelman WA, Fockens P. In vitro comparison and evaluation of seven gastric closure modalities for natural orifice transluminal endoscopic surgery (NOTES) Endoscopy. 2008;40:595–601. doi: 10.1055/s-2008-1077409. [DOI] [PubMed] [Google Scholar]
- 13.von Renteln D, Schmidt A, Vassiliou MC, Gieselmann M, Caca K. Natural orifice transluminal endoscopic surgery gastrotomy closure with an over-the-endoscope clip: a randomized, controlled porcine study (with videos) Gastrointest Endosc. 2009;70:732–739. doi: 10.1016/j.gie.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Chiu PW, Lau JY, Ng EK, Lam CC, Hui M, To KF, Sung JJ, Chung SS. Closure of a gastrotomy after transgastric tubal ligation by using the Eagle Claw VII: a survival experiment in a porcine model (with video) Gastrointest Endosc. 2008;68:554–559. doi: 10.1016/j.gie.2008.03.1110. [DOI] [PubMed] [Google Scholar]
- 15.Asakuma M, Perretta S, Cahill RA, Solano C, Pasupathy S, Dallemagne B, Tanigawa N, Marescaux J. Peroral dual scope for natural orifice transluminal endoscopic surgery (NOTES) gastrotomy closure. Surg Innov. 2009;16:97–103. doi: 10.1177/1553350609337309. [DOI] [PubMed] [Google Scholar]
- 16.Perretta S, Sereno S, Forgione A, Dallemagne B, Coumaros D, Boosfeld C, Moll C, Marescaux J. A new method to close the gastrotomy by using a cardiac septal occluder: long-term survival study in a porcine model. Gastrointest Endosc. 2007;66:809–813. doi: 10.1016/j.gie.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Perretta S, Dallemagne B, Coumaros D, Marescaux J. Natural orifice transluminal endoscopic surgery: transgastric cholecystectomy in a survival porcine model. Surg Endosc. 2008;22:1126–1130. doi: 10.1007/s00464-007-9582-4. [DOI] [PubMed] [Google Scholar]
- 18.Scott DJ, Tang SJ, Fernandez R, Bergs R, Goova MT, Zeltser I, Kehdy FJ, Cadeddu JA. Completely transvaginal NOTES cholecystectomy using magnetically anchored instruments. Surg Endosc. 2007;21:2308–2316. doi: 10.1007/s00464-007-9498-z. [DOI] [PubMed] [Google Scholar]
- 19.Forgione A, Maggioni D, Sansonna F, Ferrari C, Di Lernia S, Citterio D, Magistro C, Frigerio L, Pugliese R. Transvaginal endoscopic cholecystectomy in human beings: preliminary results. J Laparoendosc Adv Surg Tech A. 2008;18:345–351. doi: 10.1089/lap.2007.0203. [DOI] [PubMed] [Google Scholar]
- 20.Marescaux J, Dallemagne B, Perretta S, Wattiez A, Mutter D, Coumaros D. Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg. 2007;142:823–826; discussion 826-827. doi: 10.1001/archsurg.142.9.823. [DOI] [PubMed] [Google Scholar]
- 21.Narula VK, Happel LC, Volt K, Bergman S, Roland JC, Dettorre R, Renton DB, Reavis KM, Needleman BJ, Mikami DJ, et al. Transgastric endoscopic peritoneoscopy does not require decontamination of the stomach in humans. Surg Endosc. 2009;23:1331–1336. doi: 10.1007/s00464-008-0161-0. [DOI] [PubMed] [Google Scholar]
- 22.Narula VK, Hazey JW, Renton DB, Reavis KM, Paul CM, Hinshaw KE, Needleman BJ, Mikami DJ, Ellison EC, Melvin WS. Transgastric instrumentation and bacterial contamination of the peritoneal cavity. Surg Endosc. 2008;22:605–611. doi: 10.1007/s00464-007-9661-6. [DOI] [PubMed] [Google Scholar]
- 23.Rattner D, Kalloo A. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. October 2005. Surg Endosc. 2006;20:329–333. doi: 10.1007/s00464-005-3006-0. [DOI] [PubMed] [Google Scholar]
- 24.Allemann P, Perretta S, Asakuma M, Dallemagne B, Mutter D, Marescaux J. Multimedia manuscript. NOTES retroperitoneal transvaginal distal pancreatectomy. Surg Endosc. 2009;23:882–883. doi: 10.1007/s00464-008-0297-y. [DOI] [PubMed] [Google Scholar]
- 25.Allemann P, Perretta S, Marescaux J. Surgical access to the adrenal gland: the quest for a "no visible scar" approach. Surg Oncol. 2009;18:131–137. doi: 10.1016/j.suronc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Cahill RA, Asakuma M, Perretta S, Dallemagne B, Marescaux J. Gastric lymphatic mapping for sentinel node biopsy by natural orifice transluminal endoscopic surgery (NOTES) Surg Endosc. 2009;23:1110–1116. doi: 10.1007/s00464-008-0124-5. [DOI] [PubMed] [Google Scholar]
- 27.Cahill RA, Perretta S, Leroy J, Dallemagne B, Marescaux J. Lymphatic mapping and sentinel node biopsy in the colonic mesentery by Natural Orifice Transluminal Endoscopic Surgery (NOTES) Ann Surg Oncol. 2008;15:2677–2683. doi: 10.1245/s10434-008-9952-8. [DOI] [PubMed] [Google Scholar]
- 28.Leroy J, Cahill RA, Perretta S, Forgione A, Dallemagne B, Marescaux J. Natural orifice translumenal endoscopic surgery (NOTES) applied totally to sigmoidectomy: an original technique with survival in a porcine model. Surg Endosc. 2009;23:24–30. doi: 10.1007/s00464-008-0102-y. [DOI] [PubMed] [Google Scholar]
- 29.Nassif J, Zacharopoulou C, Wattiez A. Staging of gynaecological malignancies by natural orifice transluminal endoscopic surgery (N.O.T.E.S.) Surg Oncol. 2009;18:147–152. doi: 10.1016/j.suronc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Perretta S, Allemann P, Asakuma M, Dallemagne B, Marescaux J. Adrenalectomy using natural orifice translumenal endoscopic surgery (NOTES): a transvaginal retroperitoneal approach. Surg Endosc. 2009;23:1390. doi: 10.1007/s00464-009-0367-9. [DOI] [PubMed] [Google Scholar]
- 31.Perretta S, Allemann P, Dallemagne B, Marescaux J. Natural orifice transluminal endoscopic surgery (N.O.T.E.S.) for neoplasia of the chest and mediastinum. Surg Oncol. 2009;18:177–180. doi: 10.1016/j.suronc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Swanstrom L, Zheng B. Spatial orientation and off-axis challenges for NOTES. Gastrointest Endosc Clin N Am. 2008;18:315–324; ix. doi: 10.1016/j.giec.2008.01.015. [DOI] [PubMed] [Google Scholar]