Abstract
Respiratory syncytial virus (RSV) is the major viral cause of serious lower respiratory tract illness in infants and young children worldwide. RSV infection is limited to the superficial layers of the respiratory epithelium in immunocompetent individuals. Consistent with this in vivo observation, we and others have found that RSV buds preferentially from the apical surface of infected polarized epithelial cells. In contrast, directional budding is not observed in nonpolarized human epithelial cells. These findings suggest that RSV uses specific cellular trafficking pathways to accomplish viral replication. The host cell proteins that regulate directional budding of RSV are undefined. Apical sorting of cellular proteins in polarized epithelial cells involves the apical recycling endosome (ARE). To investigate whether ARE-mediated protein sorting plays a role during RSV replication, we expressed a fragment of the myosin Vb tail that functions as a dominant negative inhibitor of ARE-mediated protein sorting in polarized Madin-Darby canine kidney cells. When these cells were infected with RSV, a >9,000-fold reduction in viral yield was observed. A similar effect on virus replication was observed when a carboxyl-terminal fragment of another ARE-associated protein, the Rab11 family interacting protein 1, was expressed in Madin-Darby canine kidney cells. These data suggest that RSV requires proper ARE-mediated protein sorting for efficient egress from the apical surface of polarized epithelial cells.
Virtually all respiratory and enteric viruses infect polarized epithelial cells that line the respiratory or digestive tracts. Many of these viruses bud from infected polarized epithelial cells in a directional manner (1, 2). The majority of studies focused on polarized virus replication have examined apically or basolaterally targeted viral proteins that contribute to polarized virus budding (3–7). Few studies have examined the contribution of cellular proteins to this process. Identification of the host proteins and trafficking pathways that allow viruses to preferentially bud from a specific face of polarized epithelial cells will provide new insights regarding the life cycle of such viruses and may allow for the development of novel antiviral therapeutics.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract illness in infants and young children worldwide and has emerged as a significant cause of respiratory illness among elderly populations (8, 9). In vitro and ex vivo studies have shown that the virus exhibits a preference for apical budding from infected, polarized epithelial cells (10, 11). RSV replicates in these cells with relatively little evidence of cytopathology, contrary to the extensive morphologic changes observed in infected nonpolarized epithelial cell lines, such as HEp-2 cells. Furthermore, RSV infection is limited to the most superficial layer of ex vivo cultured stratified human respiratory epithelium (11). These findings are consistent with many clinical aspects of RSV infection. Infection can occur in both the upper and lower respiratory tract and may proceed for 5–7 days before the onset of clinical illness (12). Although viral antigens have been detected in circulating mononuclear cells, the virus does not cause a viremia (13). Autopsy histopathologic studies of infected individuals suggest that viral antigen does not penetrate further than the superficial layers of the respiratory epithelium in immunocompetent individuals (14). Additionally, the cellular syncytia that are frequently observed in nonpolarized cells in vitro are rarely noted in pathologic specimens. Collectively, these characteristics suggest that RSV cytopathic effect differs between polarized and nonpolarized cells and that infected polarized epithelial cell monolayer cultures may better reflect the nature of infected respiratory epithelium than nonpolarized cell monolayer cultures.
Epithelial cells at mucosal surfaces establish polarity and develop two distinct membrane domains, the particulars of which have been extensively reviewed (15). These membrane domains are exposed to very different physiological environments because the apical membrane faces the lumen, while the basolateral membrane abuts the underlying stratum of the epithelia. The two poles of the cell exhibit distinct profiles of proteins and lipids, which allow the cell to carry out surface-specific functions. Maintaining the composition of these membranes requires specialized protein-sorting endosomes, such as the basolateral early endosome, the common endosome, and the apical recycling endosome (ARE) (16). Collectively, these endosomes facilitate membrane-specific sorting of cellular proteins.
Apical host protein transport and recycling involve the ARE, a Rab11a-, Rab25-enriched, pericentrosomal tubulovesicular structure in polarized epithelial cells (16, 17). The role of the ARE in apical host protein sorting has been studied extensively, primarily by using the Madin-Darby canine kidney (MDCK) cell model of polymeric Ig receptor (pIgR)-mediated transcytosis of IgA (18). Although the degree to which the ARE controls apical transport of cellular proteins is unclear, pIgR-mediated transcytosis of IgA from the basolateral to the apical surface of polarized cells and apical recycling of IgA both involve ARE-mediated protein sorting. These experimental systems allowed for the identification of several cellular proteins that regulate ARE-mediated protein sorting. The actin motor protein myosin Vb, a member of the class V myosin family, is a central regulator of plasma membrane recycling (19). Myosin Vb interacts with the ARE-associated small GTPase Rab11a. A truncated form of myosin Vb (GFP-myosin Vb tail), composed of the carboxyl-terminal tail domain in the absence of the myosin motor domain, disrupts basolateral-to-apical transcytosis and apical recycling of IgA in polarized MDCK cells (20). Similarly, Rab11 family interacting protein (Rab11-FIP) members, such as Rab11-FIP1, pp75/Rip11, and Rab11-FIP2, all of which have similar carboxyl-terminal amphipathic α-helical Rab11 binding domains, also regulate ARE-mediated protein recycling (21, 22). These findings demonstrated an essential role for Rab11-associated proteins in ARE-mediated host protein transport.
We reasoned that viruses that are capable of budding preferentially from a particular membrane of polarized epithelial cells may use such elements of the host cell transport machinery to assemble at a specific cellular location. However, previous studies have not associated polarized budding of viruses with any such endosomal compartment. In the present study we test the hypothesis that RSV, which buds preferentially from the apical membrane of polarized epithelial cells, uses an ARE-mediated apical protein sorting pathway to facilitate polarized replication. To examine this question we studied the ability of RSV to replicate in polarized epithelial cells expressing dominant negative inhibitors of apical protein recycling. Our results demonstrate that RSV requires proper ARE function for efficient egress from the apical surface of polarized epithelial cells.
Materials and Methods
Mammalian Cell Lines and Virus. MDCK cells, type II (ATCC CCL-34), were transduced to express the murine pIgR (mpIgR) by using a replication defective retrovirus by subcloning the mpIgR cDNA (kindly provided by C. S. Kaetzel, University of Kentucky, Lexington) into the pBabe-puro retroviral vector. Production of transducing virus was carried out as described (23). Transduced cells were selected based on mpIgR expression and resistance to puromycin (10 μg/ml). Once isolated, the cells were no longer grown in the presence of puromycin. The MDCK T23 cell line (Clontech) was cotransfected with cDNA for myosin Vb tail or Rab11-FIP1 (576–651) cloned into the pTRE2 expression vector and pCB7. Transfected cell clones were selected by growth in the presence of hygromycin (150 μM). Induction and suppression of GFP fusion protein expression was tightly regulated by the absence or presence of doxycycline. These cell lines were maintained in DMEM and Ham's F-12 medium (Invitrogen) supplemented with 7% FBS, 320 μg/ml l-glutamine, 1% (vol/vol) nonessential amino acids, 2.7 μg/ml amphotericin B, and 45 μg/ml gentamicin. Additionally, media used for the GFP-myosin Vb tail and Rab11-FIP1 (576–651) cell lines were supplemented with 20 ng/ml of doxycycline as needed, to repress plasmid-based protein expression. HEp-2 cells (ATCC CCL-23) and BSC-40 cells were grown in Opti-MEM I (Invitrogen) supplemented with 2% FBS, 320 μg/ml l-glutamine, 2.7 μg/ml amphotericin B, and 45 μg/ml gentamicin. All cell lines were maintained at 37°C in 5% CO2.
The RSV WT strain A2, the vaccinia virus strain WR, and the WT influenza virus isolate A/Nashville/47/91 (an A/Beijing-like/H3N2 virus; gift of Peter Wright, Vanderbilt University, Nashville, TN) have been described (12, 24).
Viral Growth Assay. MDCK cells [WT, mpIgR-expressing, or transfected with GFP-myosin Vb tail or Rab11-FIP1 (576–651)] were seeded onto 48-well tissue culture plates (Costar) at ≈20% confluence and allowed to form fully confluent cell monolayers. GFP-myosin Vb tail-transfected MDCK cells or Rab11-FIP1 (576–651)-transfected MDCK cells were grown in the presence or absence of doxycycline to allow for the absence or presence of the transfected protein, respectively. Once confluent, the cell culture monolayers were infected with RSV, vaccinia virus, or influenza virus [multiplicity of infection = 0.25 plaque-forming unit (pfu) per cell]. Virus was allowed to adsorb for 1 h at 37°C. After adsorption, virus infection media were removed, tissue culture wells were washed three times with PBS, and 300 μl of growth medium (+/- doxycycline) was added. Infected cells were incubated at 37°C in 5% CO2.
Polarized Budding Assay. Growth assays that examined the apical and basolateral budding of RSV were performed by using 0.33-cm2 surface area polycarbonate tissue culture inserts with 3-μm pores (Costar). The transepithelial electrical resistance of each insert was measured to ensure the presence of polarized MDCK cell monolayers. We observed transepithelial electrical resistance measurements of 40–120 Ω·cm2, which were not noticeably altered because of expression of GFP-myosin Vb tail or Rab11-FIP1 (576–651). Maintenance and infection of insert-grown cell monolayer cultures was performed as described above.
Virus Recovery and Titration. Tissue culture supernatant containing virus was collected and brought to a volume of 300 μl, to correct for minor differences in evaporative loss. After the collection of the supernatant fraction, tissue culture wells were washed three times with 250 μl of PBS. Cell-associated virus was harvested in 300-μl samples by scraping MDCK cell culture monolayers into 150 μl of medium, followed by an additional washing of the well with 150 μl of medium. Virus was released from harvested cells by rapid freeze-thaw, which was repeated three times. Recovered RSV was quantified by plaque assay as described (25). Virus titers from vaccinia virus-infected cells were quantified by plaque assay on BSC-40 cell monolayers at 37°C as described; staining with crystal violet allowed for visualization of plaques (26). Virus titers from influenza virus-infected cells were quantified by determining the median tissue culture infectious dose (TCID50) on MDCK cell monolayers incubated for 5 days at 37°C as described (27).
Confocal Microscopy Analysis of RSV-Infected GFP-Myosin Vb Tail MDCK Cell Monolayers. MDCK cells (+/- doxycycline) were seeded onto polycarbonate tissue culture inserts (0.33 cm2) with 3-μm pores (Costar). Confluent MDCK cell monolayers were infected apically with RSV (multiplicity of infection = 1) as described above. At 24 h postinfection, cell monolayers were fixed and permeablized with 4% paraformaldehyde supplemented with 0.3% Triton X-100. After fixation, cell monolayers were blocked with 3% milk in PBS for 1 h at room temperature. Fixed cell monolayers were stained for the RSV fusion protein (F protein) with a mixture of three murine mAbs, followed by staining with Alexa 568-conjugated murine Ig-specific goat polyclonal antiserum (Molecular Probes). Z-section imaging was performed with a Zeiss LSM510 confocal microscope. Images were collected at a magnification of ×63, enhanced by a 2-fold zoom scan. Captured images were visualized by using Zeiss LSM image browser software, version 3.2.0.70.
Enumeration of RSV Fluorescent Foci. MDCK cell (+/- doxycycline) were seeded onto polycarbonate tissue culture inserts (0.33 cm2) with 3-μm pores (Costar). Confluent MDCK cell monolayers were infected apically with RSV (multiplicity of infection = 0.25) as described above. At 20 h postinfection, cell monolayers were fixed with 100% ice-cold methanol for 1 h at -20°C. After methanol fixation, cell monolayers were blocked with 3% milk in PBS for 1 h at room temperature. Fixed cell monolayers were stained for the RSV F protein with a mixture of three murine mAbs, followed by staining with Alexa 568-conjugated murine Ig-specific goat polyclonal antiserum (Molecular Probes). To enumerate fluorescent foci, infected cell monolayers were visualized at ×10 magnification, and four representative visual fields across the middle of the well were counted. The number of foci per well were estimated by multiplying by a factor of 4.125 to obtain an estimation of the number of foci per tissue culture insert, as one ×10 visual field approximates 1/16th of the growth surface area. Images were digitally captured with a Nikon D100 digital camera mounted on a Nikon Eclipse TE300 inverted microscope. Fluorescent images were acquired by using Apple IPHOTO 2.0 software.
Results
Polarized Budding of RSV. Previous studies demonstrated that polarized epithelial cells infected from the apical surface with RSV predominately shed RSV from their apical face at 24 h postinfection (10). We sought to extend these findings by determining whether the preference for apical budding is restricted to the first 24 h after infection and testing whether the apical route of infection is the determining factor that predisposes the virus to bud from the apical face of the cell. RSV infection (multiplicity of infection = 0.25 pfu per cell) of MDCK cells reaches peak titer 5 days postinfection (data not shown), therefore, we examined whether the preference for apical budding of RSV persisted over a 5-day period. Polarized MDCK cell monolayers were infected with RSV at the apical cell surface. During the 5-day period after infection, apical and basolateral tissue culture supernatants were collected separately. As shown in Fig. 1a, substantial virus was recovered from the apical supernatant at each time point, with a steady increase in yield from days 1 to 5. Conversely, virus was not detected in the basolateral medium at any time. To ensure that the tissue culture insert did not restrict the passage of virus to the basolateral chamber, we studied RSV infection of HEp-2 cell monolayers on tissue culture inserts. HEp-2 cells do not form tight junctions in culture or establish a high level of transepithelial electrical resistance. A restriction of virus to the apical chamber was not observed when HEp-2 cells grown on tissue culture inserts were infected. Thus, the tissue culture insert membrane did not influence the distribution of virus in the tissue culture supernatant (Fig. 1b). These findings indicate that preferential budding of RSV from the apical membrane of polarized epithelial cells is not restricted to early stages of infection, and that robust RSV production in polarized cells does not compromise cell integrity to the extent that cellular polarity is lost. Furthermore, the data indicate that the cell monolayer remains intact, because virus present in the apical medium was excluded from the basolateral compartment.
Fig. 1.
Directional release of RSV from polarized or nonpolarized cell culture monolayers. Polarized MDCK cell monolayers were infected with RSV from the apical (a) or basolateral surface (c). (b) Nonpolarized HEp-2 cell monolayers were infected with RSV from the apical surface. Apical (•) and basolateral (○) tissue culture supernatants were collected separately at the indicated times postinfection, and virus was quantified by plaque assay. The dashed line denotes the limit of detection for this assay. Titers at each time point are the average value of triplicate samples; the data are representative of at least two experiments.
We next tested whether the route of entry determines the polarity of budding by infecting polarized MDCK cell monolayers from the apical or basolateral surface. As Fig. 1c illustrates, substantially more virus was recovered from the apical supernatant than the basolateral supernatant on days 1–5 after basolateral inoculation. In addition, the amount of virus recovered from the basolateral supernatant was greatest on day 0, suggesting that virus recovered from the basolateral supernatant was part of the residual inoculum rather than progeny from infected cells. This result demonstrates that the preferential release of RSV from the apical face of polarized epithelial cells is not determined by the route of virus entry.
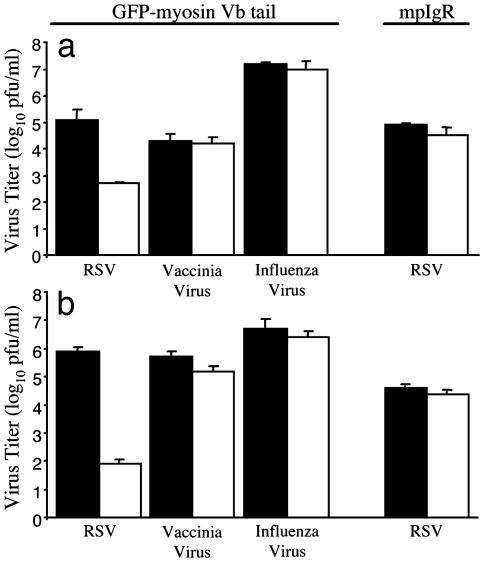
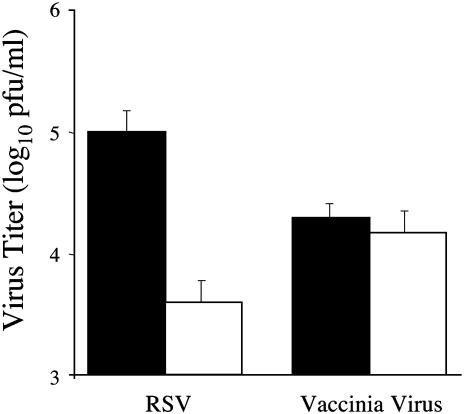
Expression of GFP-Myosin Vb Tail Disrupts RSV Replication. We next sought to identify the cellular components that influence polarized egress of RSV. We hypothesized that the ARE contributes to the apical release of RSV because of its role in apical protein sorting. To determine the effect of altered ARE-mediated protein sorting on RSV replication, we infected stably transfected MDCK cells grown under conditions allowing for the absence or presence (+/- doxycycline, respectively) of GFP-myosin Vb tail. Five days after infection, virus was collected separately from the tissue culture supernatant and cell monolayer and quantified by plaque assay. As Fig. 2a illustrates, we observed a 281-fold reduction in supernatant-associated virus. This reduction was accompanied by a 9,545-fold reduction in cell-associated infectious particles (Fig. 2b). In contrast, expression of GFP-myosin Vb tail did not inhibit the replication of vaccinia virus or influenza virus (Fig. 2). Furthermore, RSV replication was not influenced by high-level expression of another ARE-associated protein, mpIgR, grown in the presence or absence of doxycycline (Fig. 2). Together, these results indicate that proper ARE-mediated protein trafficking is required for efficient replication of RSV in polarized epithelial cells.
Fig. 2.
GFP-myosin Vb tail expression inhibits RSV replication. MDCK cell culture monolayers with (solid bars) or without (open bars) doxycycline were plated on 48-well tissue culture plates and infected with RSV, vaccinia virus, or influenza virus. MDCK cell culture monolayers expressing the mpIgR were plated on 48-well tissue culture plates and infected with RSV. Virus was recovered from infected cell culture supernatants (a) or cell monolayers (b) separately at 120, 48, and 72 h for RSV-, vaccinia virus-, or influenza virus-infected cells, respectively, and quantified as described in Materials and Methods. Results shown are the mean results of triplicate samples (±SD) and are representative of at least two experiments.
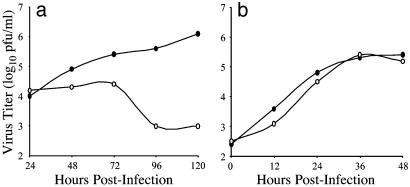
In the experiments described above, our measure of virus yield was determined by collecting samples at the time of peak virus production in WT MDCK cells. We next examined the kinetics of virus production of infected GFP-myosin Vb tail-expressing MDCK cells. Infected cell monolayers were collected at 12- or 24-h intervals for a period of 48 or 120 h, for vaccinia virus or RSV, respectively. Infectious viral particles associated with these monolayers were quantified by plaque assay (Fig. 3). RSV-infected MDCK cells expressing GFP-myosin Vb tail did not exhibit a logarithmic phase of virus production, in contrast with RSV-infected MDCK cells not expressing GFP-myosin Vb tail. In fact, the amount of virus associated with GFP-myosin Vb tail-expressing MDCK cells showed little change during the first 72 h after infection, which is consistent with the presence of residual virus inoculum. In contrast, the viral growth kinetics of vaccinia virus-infected MDCK cells expressing GFP-myosin Vb tail did not differ significantly from that of vaccinia virus-infected MDCK cell monolayers that were not expressing GFP-myosin Vb tail. These results indicate that expression of GFP-myosin Vb tail inhibits productive RSV infection but not vaccinia virus infection.
Fig. 3.
Kinetic aspects of virus infection of GFP-myosin Vb tail-expressing MDCK cells. MDCK cell culture monolayers with (•) or without (○) doxycycline were plated on 48-well tissue culture plates and infected with RSV (a) or vaccinia virus (b). Virus was recovered from infected cell culture monolayers at 24- or 12-h intervals for periods of 120 or 48 h, for RSV- or vaccinia virus-infected cells, respectively. Data shown are the average value of duplicate titration on samples from two experiments.
Expression of GFP-Myosin Vb Tail Reroutes Budding of RSV. To determine whether entry or egress of RSV was inhibited by GFP-myosin Vb tail, polarized MDCK cell monolayers (+/- expression of GFP-myosin Vb tail) grown on Transwell filters, were infected from the apical or basolateral side of the cell monolayer. Five days after the infection, the apical and basolateral supernatants were collected and analyzed separately for the presence of virus. Total virus yield (apical supernatant plus basolateral supernatant yields) and percent of total yield in the apical compartment are shown in Table 1. As expected, in the absence of GFP-myosin Vb tail expression, RSV was efficiently produced and preferentially released (>95%) from the apical membrane of infected cells regardless of the route of infection. Also, viral replication was inhibited in apically infected MDCK cell monolayers expressing GFP-myosin Vb tail, as evidenced by the 398-fold reduction in total RSV production for GFP-myosin Vb tail-expressing cells. Interestingly, total virus production in the culture was decreased only 3-fold for basolaterally infected MDCK cell monolayers expressing GFP-myosin Vb tail, as compared with cell monolayers not expressing GFP-myosin Vb tail.
Table 1. GFP-myosin Vb tail expression inhibits RSV replication and redirects budding to the basolateral cell surface.
| Total virus yield, log10 pfu/ml*
|
% Apical RSV‡
|
||||
|---|---|---|---|---|---|
| Route of infection | + Dox. | – Dox. | Fold reduction† | + Dox. | – Dox. |
| Apical | 4.2 | 1.6 | 398 | 97 | 24 |
| Basolateral | 5.2 | 4.7 | 3 | >99 | 10 |
Total virus yield was calculated by separately determining the titer of virus released into the apical or basolateral supernatants (pfu/ml), adding those values, and converting the total to a log10 pfu/ml value
Fold reduction was calculated by dividing the virus titer (pfu/ml) in the presence of doxycycline (Dox.) by that in the absence of doxycycline
Percentage of apical RSV was calculated by dividing the titer of virus recovered from the apical supernatant (pfu/ml) by the combined apical and basolateral supernatant titers (pfu/ml) and multiplying the quotient by 100
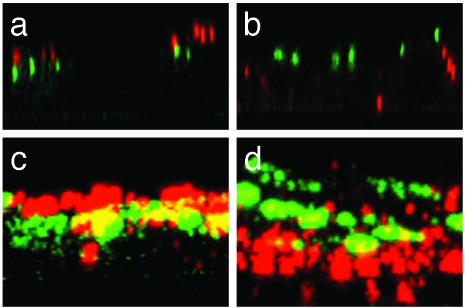
We observed that budding of RSV was rerouted to the basolateral membrane of basolaterally infected MDCK cell monolayers expressing GFP-myosin Vb tail. Under these conditions, RSV was preferentially released from the basolateral face of cells, as 90% of total virus produced was recovered from the basolateral cell culture medium. The site of virus budding for many enveloped viruses is determined by the cellular location of viral surface proteins and RSV requires the presence of a surface fusion protein to infect host cells. Therefore, we sought to determine whether the observed reduction in virus yield and rerouting of virus budding was accompanied by redistribution of the RSV F protein. Apically or basolaterally infected MDCK cell monolayers expressing GFP-myosin Vb tail were examined by indirect immunofluorescence for the distribution of the F protein 24 h postinfection (Fig. 4). We observed the F protein of apically infected cell monolayers to be associated with the apical face of infected cells, relative to subapically localized GFP-myosin Vb tail (Fig. 4 a and c). Conversely, the F protein was localized to the basolateral face of basolaterally infected cell monolayers (Fig. 4 b and d). However, neither condition resulted in appreciable reduction of F protein expression or colocalization with GFP-myosin Vb tail.
Fig. 4.
Confocal microscopy analysis of the cellular distribution of RSV F protein in the presence of GFP-myosin Vb tail. Polarized MDCK cell culture monolayers expressing GFP-myosin Vb tail were infected from the apical (a and c) or basolateral (b and d) surface with RSV and examined for the cellular distribution of RSV F protein. The cellular distribution of the F protein is depicted as a single X-Z section (a and b) or a X-Z projection of the cell monolayer (c and d). It should be noted that the apparent colocalization of GFP-myosin Vb tail and RSV F protein shown in the projection images are a consequence of the projection image rather than actual colocalization, which was rarely observed in individual X-Z sections. (Magnification: ×63.)
These findings are consistent with the hypothesis that proper ARE function is essential to apical egress of RSV and suggests that GFP-myosin Vb tail expression indirectly alters viral surface protein distribution within the cell, although it has no significant effect on viral transcription and translation. Furthermore, these data demonstrate that the previously described preferential release of RSV from the apical surface of infected WT MDCK cells (Fig. 1) is not required for efficient replication.
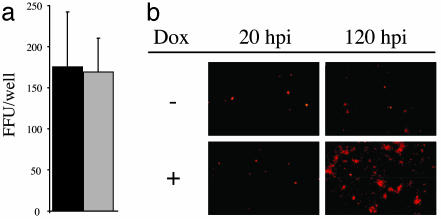
GFP-Myosin Vb Tail Reduces Local Spread of RSV. The above data suggest that total virus replication in the cell after the usual apical route of infection is inhibited by GFP-myosin Vb tail because virus infection from the apical cell surface does not allow for basolateral release of virus comparable to that after basolateral infection. To address more directly whether GFP-myosin Vb tail mediates an inhibition of viral attachment, entry, or spread, we examined RSV-infected MDCK cells expressing GFP-myosin Vb tail by fluorescence microscopy. If RSV infectivity were inhibited, a lower percentage of GFP-myosin Vb tail-expressing cells would become infected. MDCK cells, grown under conditions allowing for expression or repression of GFP-myosin Vb tail, were infected with RSV and immunostained for the RSV F protein 20 h after infection. Inspection of infected MDCK monolayers using fluorescence microscopy revealed that GFP-myosin Vb tail-expressing cells were infected at similar frequencies compared with nonexpressing MDCK cells (Fig. 5a). The data suggest that interruption of ARE-mediated protein sorting has little effect on RSV attachment or entry. However, local spread of virus was restricted at 120 h postinfection (Fig. 5b). Together, our data demonstrate that the mechanism of GFP-myosin Vb tail inhibition of RSV replication is complex, involving features that both reduce local virus spread in culture and redirect the polarity of virus assembly and budding.
Fig. 5.
GFP-myosin Vb tail reduces local spread of RSV. (a) Polarized MDCK cell culture monolayers with (solid bars) or without (open bars) doxycycline were infected from the apical surface with RSV and examined for the presence of RSV-infected cells by fluorescent focus assay. Data shown are the mean of four replicate samples (±SD) and are representative of two experiments. (b) Representative photomicrographs of RSV-infected MDCK cell monolayers +/- doxycycline (Dox) at 20 or 120 h postinfection. (Magnification: ×10.)
Rab11-FIP1 (576–651) Inhibits RSV Replication. We sought to determine whether the observed inhibition of RSV replication was restricted to an effect of GFP-myosin Vb tail or if alteration of another ARE-associated protein that participates in vesicular transport could produce a similar effect. We recently identified a group of Rab-11 interacting proteins, designated Rab11-FIP, which interact with each member of the Rab11 family of proteins (28). To examine the contribution of Rab11-FIPs to RSV replication we expressed a GFP-Rab11-FIP1 chimeric protein [Rab11-FIP1 (576–651)]. This chimeric protein contains the C-terminal 75 aa of Rab11-FIP1, which contains the conserved Rab11-binding domain common to all Rab11-FIPs. Therefore, this fusion protein should act as a general inhibitor of apical protein recycling because it retains the ability to interact with Rab11a, but does not contain putative Rab11-FIP effector domains. Similar truncations of Rab11-FIP2, Rab11-FIP3, and Rab11-FIP4, all of which retained the conserved C-terminal Rab11-binding domain, cause either redistribution of Rab11a or disruption of IgA transcytosis when expressed in MDCK cells (22, 29, 30). Therefore, we sought to determine whether Rab11-FIP1 (576–651) would have an inhibitory effect on RSV replication. MDCK cells stably transfected with Rab11-FIP1 (576–651) were grown under conditions allowing for the absence or presence (+/- doxycycline, respectively) of the transfected protein and then infected with RSV or vaccinia virus. Cell culture supernatants from RSV- or vaccinia virus-infected cells were collected 5 or 2 days after infection, respectively, and recovered virus was quantified by plaque assay. We observed a 25-fold reduction in RSV in the supernatant of infected MDCK cells expressing Rab11-FIP1 (576–651), compared with a <2-fold reduction in vaccinia virus yield (Fig. 6). This result supports the hypothesis that ARE-mediated protein sorting is essential to the normal apical polarity of RSV replication in polarized epithelial cells. Although the magnitude of reduction in virus yield was substantial, it was less dramatic than that caused by GFP-myosin Vb tail. This disparity suggests that the actin motor function of myosin Vb may be of greater importance to ARE function than that of the Rab11-FIP, which likely serves as a modulator of ARE-mediated trafficking.
Fig. 6.
Rab11-FIP1 (576–651) expression inhibits RSV replication. MDCK cell culture monolayers with (solid bars) or without (open bars) Rab11-FIP1 (576–651) expression were plated on 48-well tissue culture plates and infected with RSV or vaccinia virus. Virus was recovered from infected cell culture supernatants at 120 or 48 h for RSV- or vaccinia virus-infected cells, respectively. Virus was quantified as described in Materials and Methods. Results shown are the mean of triplicate samples (±SD) and are representative of two experiments.
Discussion
The mechanisms that facilitate virus budding in a directional manner from infected polarized epithelial cells are not well defined. Previous work examining the process of polarized virus infection has focused on the properties of viral proteins that contribute to this process. In this study, we examined the contribution of ARE-mediated protein sorting to the process of RSV replication in polarized epithelial cells. Our results show that RSV buds preferentially from the apical membrane of polarized MDCK cells for extended periods of infection, and that the preference for apical budding is independent of the route of virus entry. Efficient replication of RSV in polarized epithelial cells requires proper ARE function because expression of either GFP-myosin Vb tail or Rab11-FIP1 (576–651) in MDCK cells substantially reduced replication of RSV. Interestingly, when cells are infected from the basolateral surface expression of GFP-myosin Vb tail can redirect the viral F protein and virus budding to the basolateral surface of GFP-myosin Vb tail-expressing MDCK cells. Together, these data indicate proper ARE function is required for RSV egress from the apical face of polarized epithelial cells and that defective ARE-mediated protein sorting may affect more than one stage of the RSV life cycle.
There are several stages of the RSV life cycle that could be inhibited by interfering with ARE-mediated protein sorting, such as virus entry, viral genome and protein production, and viral egress. We tested whether GFP-myosin Vb tail inhibited virus infectivity by fluorescence focus assay. We found that a similar proportion of RSV infected GFP-myosin Vb tail-expressing or nonexpressing MDCK cells 20 h postinfection, suggesting that infectivity was not significantly inhibited (Fig. 5). Although these data suggest that myosin Vb tail does not restrict the ability of RSV to infect polarized cells from the apical surface, it does restrict local virus spread in culture. The greater reduction of total virus yield in the culture after apical infection (Table 1) suggests a role for the ARE in early events after infection. The data in Fig. 2 suggest that the observed reduction in virus yield is predominantly caused by an inhibition of virus assembly at the apical surface. In vitro, the majority of RSV remains associated with the cell monolayer, as is the case in our experiments in the absence of GFP-myosin Vb tail expression, where 80% of RSV was found to be associated with the cell monolayer. Conversely, only 20% of RSV was associated with the cell monolayer of infected MDCK expressing GFP-myosin Vb tail. This finding indicates that efficient virus budding occurs independently of myosin Vb, for the particles in which the requisite virion assembly steps are complete. These findings implicate virus assembly as the stage of the virus life cycle that is most likely inhibited by defective ARE-mediated protein sorting.
The observation that F protein targeting and virus budding is redirected to the basolateral face of GFP-myosin Vb tail-expressing MDCK cells after basolateral inoculation provides further insight into the basis for preferential apical budding of RSV from polarized epithelial cells. Because RSV is able to bud efficiently from the basolateral membrane of polarized cells when apical trafficking is disrupted, the cellular factors that participate in RSV assembly and budding must be present in the basolateral domain of the cell. However, substantial preferential budding from the basolateral membrane was observed only when cells were infected from the basolateral surface in the presence of GFP-myosin Vb tail. Therefore, RSV does not effectively access the basolateral cellular machinery when ARE-mediated sorting is not disrupted or when infection occurs from the apical face of the cell. This finding demonstrates that preferential apical budding of RSV is not a requirement for efficient replication of the virus in polarized epithelial cells but rather a favored route of egress. This finding also suggests that RSV uses particular cellular protein trafficking pathways efficiently, and that apical budding affords the virus some degree of enhanced fitness.
In summary, this study provides a deeper understanding of the process of directional virus budding in polarized epithelial cells. We have demonstrated that directional budding of RSV is a preferential route of egress rather than a requirement for virus replication. We have also identified the ARE as an essential component in the process of apical assembly of RSV. Additionally, this work demonstrates the utility of RSV replication as an indicator of proper ARE function.
Acknowledgments
We thank Drs. Christopher Aiken, Charlotte Kaetzel, and Peter Wright for providing reagents required for this work and Dr. Lynne Lapierre for helpful discussions. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Respiratory Pathogens Research Unit Grant NO1 AI-65298 (to J.E.C.), National Institutes of Health/National Institute of Allergy and Infectious Diseases Grants R21 DE-014039 (to J.E.C.) and DK48370 and DK43405 (to J.R.G.), and Viruses, Nucleic Acids, and Cancer Training Grant Program T32 CA09385 (to S.C.B.). Confocal microscopy studies were performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource, supported by National Institutes of Health Grants CA68485, DK20593, and DK58404.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RSV, respiratory syncytial virus; ARE, apical recycling endosome; MDCK, Madin-Darby canine kidney; pIgR, polymeric Ig receptor; mpIgR, murine pIgR; F protein, fusion protein; Rab11-FIP, Rab11 family interacting protein; pfu, plaque-forming unit.
References
- 1.Boulan, E. R. & Sabatini, D. D. (1978) Proc. Natl. Acad. Sci. USA 75, 5071-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Compans, R. W. (1995) Curr. Top. Microbiol. Immunol. 202, 209-219. [DOI] [PubMed] [Google Scholar]
- 3.Moll, M., Klenk, H. D., Herrler, G. & Maisner, A. (2001) J. Biol. Chem. 276, 17887-17894. [DOI] [PubMed] [Google Scholar]
- 4.Naim, H. Y., Ehler, E. & Billeter, M. A. (2000) EMBO J. 19, 3576-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth, M. G., Compans, R. W., Giusti, L., Davis, A. R., Nayak, D. P., Gething, M. J. & Sambrook, J. (1983) Cell 33, 435-443. [DOI] [PubMed] [Google Scholar]
- 6.Stephens, E. B., Compans, R. W., Earl, P. & Moss, B. (1986) EMBO J. 5, 237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQueen, N. L., Nayak, D. P., Stephens, E. B. & Compans, R. W. (1987) J. Biol. Chem. 262, 16233-16240. [PubMed] [Google Scholar]
- 8.Domachowske, J. B. & Rosenberg, H. F. (1999) Clin. Microbiol. Rev. 12, 298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey, A. R. & Walsh, E. E. (2000) Clin. Microbiol. Rev. 13, 371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts, S. R., Compans, R. W. & Wertz, G. W. (1995) J. Virol. 69, 2667-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, L., Peeples, M. E., Boucher, R. C., Collins, P. L. & Pickles, R. J. (2002) J. Virol. 76, 5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields, B. N., Knipe, D. M., Howley, P. M. & Griffin, D. E. (2001) Fields' Virology (Lippincott Williams & Wilkins, Philadelphia).
- 13.Domurat, F., Roberts, N. J., Jr., Walsh, E. E. & Dagan, R. (1985) J. Infect. Dis. 152, 895-902. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, P. S., McQuillin, J. & Court, S. D. (1970) Br. Med. J. 1, 327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeaman, C., Grindstaff, K. K. & Nelson, W. J. (1999) Physiol. Rev. 79, 73-98. [DOI] [PubMed] [Google Scholar]
- 16.Mostov, K. E., Verges, M. & Altschuler, Y. (2000) Curr. Opin. Cell Biol. 12, 483-490. [DOI] [PubMed] [Google Scholar]
- 17.Casanova, J. E., Wang, X., Kumar, R., Bhartur, S. G., Navarre, J., Woodrum, J. E., Altschuler, Y., Ray, G. S. & Goldenring, J. R. (1999) Mol. Biol. Cell 10, 47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostov, K. E. (1994) Annu. Rev. Immunol. 12, 63-84. [DOI] [PubMed] [Google Scholar]
- 19.Reck-Peterson, S. L., Provance, D. W., Jr., Mooseker, M. S. & Mercer, J. A. (2000) Biochim. Biophys. Acta 1496, 36-51. [DOI] [PubMed] [Google Scholar]
- 20.Lapierre, L. A., Kumar, R., Hales, C. M., Navarre, J., Bhartur, S. G., Burnette, J. O., Provance, D. W., Jr., Mercer, J. A., Bahler, M. & Goldenring, J. R. (2001) Mol. Biol. Cell 12, 1843-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prekeris, R., Klumperman, J. & Scheller, R. H. (2000) Mol. Cell 6, 1437-1448. [DOI] [PubMed] [Google Scholar]
- 22.Hales, C. M., Vaerman, J. P. & Goldenring, J. R. (2002) J. Biol. Chem. 277, 50415-50421. [DOI] [PubMed] [Google Scholar]
- 23.Morgenstern, J. P. & Land, H. (1990) Nucleic Acids Res. 18, 3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo, Y., Carroll, K. N., Ikizler, M. R. & Wright, P. F. (1996) J. Virol. 70, 2055-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy, B. R., Sotnikov, A. V., Lawrence, L. A., Banks, S. M. & Prince, G. A. (1990) Vaccine 8, 497-502. [DOI] [PubMed] [Google Scholar]
- 26.Ausubel, F. M. (1987) Current Protocols in Molecular Biology (Wiley, New York).
- 27.Schmidt, N. J. & Emmons, R. W. (1995) Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections (Am. Public Health Association, Washington, DC).
- 28.Hales, C. M., Griner, R., Hobdy-Henderson, K. C., Dorn, M. C., Hardy, D., Kumar, R., Navarre, J., Chan, E. K., Lapierre, L. A. & Goldenring, J. R. (2001) J. Biol. Chem. 276, 39067-39075. [DOI] [PubMed] [Google Scholar]
- 29.Meyers, J. M. & Prekeris, R. (2002) J. Biol. Chem. 277, 49003-49010. [DOI] [PubMed] [Google Scholar]
- 30.Wallace, D. M., Lindsay, A. J., Hendrick, A. G. & McCaffrey, M. W. (2002) Biochem. Biophys. Res. Commun. 299, 770-779. [DOI] [PubMed] [Google Scholar]