Abstract
Acute liver failure (ALF) is an uncommon disease in the United States, affecting more than 2 000 people each year. Of all the various causes, malignant infiltration is one of the least well known and carries with it a high mortality. We describe a case of ALF as the presenting manifestation of peripheral T-cell lymphoma in an elderly woman. By reporting this case, we hope to increase early recognition of this disease process in order to potentially improve treatment outcomes.
Keywords: Acute liver failure, Peripheral T-cell lymphoma
INTRODUCTION
Acute liver failure (ALF), defined as the presence of encephalopathy and coagulopathy in patients without preexisting liver disease, is an uncommon, although often fatal, condition in the United States[1]. There are over 2 000 cases of ALF annually and the overall mortality rate is 80%[2]. Drug-induced (chiefly acetaminophen toxicity) and viral hepatitis comprise the majority of causes of ALF[3]. Other etiologies include: autoimmune hepatitis; acute alcoholic hepatitis; toxins such as Amanita phalloides; hepatic circulatory compromise such as shock liver and Budd-Chiari; Wilson’s disease; and acute fatty liver of pregnancy.
Among the less common causes of ALF is neoplastic infiltration of the liver. Malignancy can involve the liver either as a primary or secondary site and may cause abnormal liver function tests. Acute liver failure as an initial manifestation of malignant infiltration, however, is quite rare, carries a high mortality, and the diagnosis is often made at autopsy[4-6]. We report a case of peripheral T-cell lymphoma resulting in ALF.
CASE REPORT
A 67-year-old African-American female presented to an outside hospital with a three-day history of progressive confusion, lethargy, and vague abdominal pain. Past medical history was significant only for hypertension, treated with diltiazem. Prior surgeries included cholecystectomy and hysterectomy. She denied any use of alcohol, acetaminophen, or over-the-counter herbs or supplements. She had no risk factors for viral hepatitis, had not travelled recently nor had any sick contacts. On physical examination she was somnolent but oriented. She was afebrile, but was markedly tachycardic and tachypneic. She had icteric sclerae, a distended, slightly tender abdomen with shifting dullness, and splenomegaly. There was no asterixis. The remainder of the examination was normal. Initial laboratory data were: total bilirubin 4.6 mg/dL, alkaline phosphatase 207 IU/L, aspartate aminotransferase (AST) 418 IU/L, alanine aminotransferase (ALT) 139 IU/L, creatinine 2.15 mg/dL, platelet count 113 × 109/L, white blood cell count 11 × 109/L, and international normalized ratio (INR) 2.15. She was admitted to the hospital and treated empirically with levofloxacin and intravenous fluids. Further laboratory studies including alcohol and acetaminophen levels, and serologies for hepatitis A, B, C, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus were negative. Iron and copper studies and autoimmune markers were all within normal limits. Abdominal ultrasound with dopplers and an abdominal computed tomography (CT) scan without intravenous contrast (not given because of impaired renal function) showed ascites and an enlarged heterogenous spleen with wedge-shaped infarcts; the hepatic parenchyma, vasculature, and biliary ducts were normal. Her clinical and laboratory status deteriorated, and she was transferred to our hospital for liver transplant evaluation one week after her initial presentation. On arrival, she had stage 2-3 encephalopathy and was intubated for airway protection and mechanical ventilation. Laboratory values on admission showed total bilirubin 9.5 mg/dL, alkaline phosphatase 344 IU/L, AST 128 IU/L, ALT 71 IU/L, platelet count 93 × 109/L, white blood cell count 35 × 109/L (77% neutrophils, 10% bands, 5% lymphocytes), glucose 19, pH 7.18, serum lactate 243 mmol/L and lactate dehydrogenase (LDH) 4660 IU/L. Diagnostic paracentesis revealed an ascitic leukocyte count of 1680 × 106/L (84% neutrophils) and serum-ascites albumin gradient (SAAG) 1.2 consistent with spontaneous bacterial peritonitis, and antibiotics were continued. A contrast-enhanced abdominal CT scan showed an enlarged and heterogeneously enhancing liver (Figure 1), with mediastinal, right hilar and chest wall lymphadenopathy, and, splenomegaly with splenic infarcts. A peripheral blood smear showed atypical lymphocytes. A transjugular liver biopsy (Figure 2) on hospital day 2 revealed diffuse infiltration of malignant-appearing lymphocytes with immunohistochemistry features supporting a T-cell phenotype. This was consistent with peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS). Due to her malignancy, she was therefore not a candidate for liver transplantation. Her clinical status continued to deteriorate, with worsening lactic acidosis, hypoglycemia, and disseminated intravascular coagulation, and she became comatose on hospital day 4. In agreement with her family’s wishes, aggressive chemotherapy was withheld and she was managed palliatively. She died 3 d later and 14 d after her initial presentation.
Figure 1.
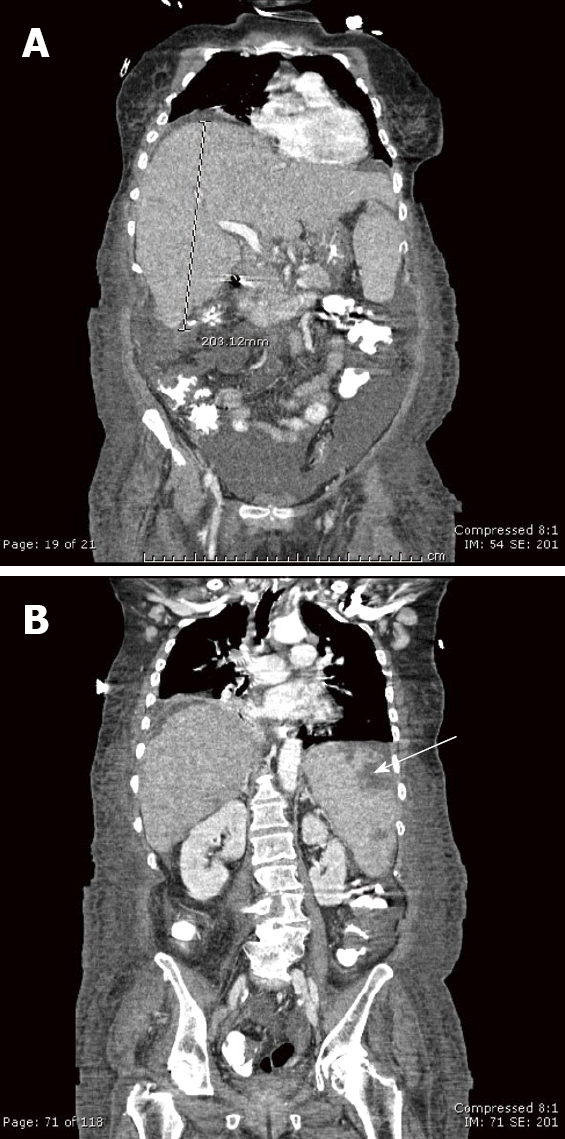
Contrast-enhanced computed tomography coronal reconstructions. A: An enlarged, heterogenous liver, 20 cm in the craniocaudad dimension; B: There are also multiple, subcapsular peripheral defects that lack enhancement in an enlarged spleen (arrow) representing splenic infarcts. Other computed tomography findings (not depicted) included mediastinal, right hilar, and chest wall lymphadenopathy as well as moderate ascites.
Figure 2.
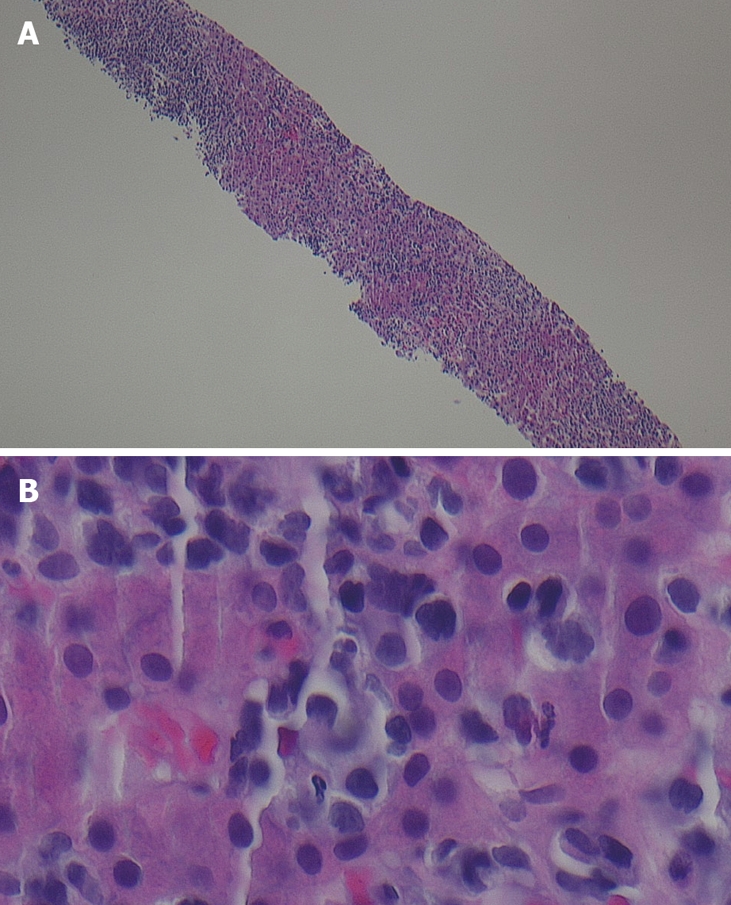
Views of liver biopsy demonstrating atypical mononucleate cells with very high nucleus/cytoplasmic ratios; hyperchromatic with irregularly distributed chromatin; and, irregular nuclear membranes. A: Low power view; B: High power view. Some nucleoli are infiltrating throughout the sinusoidal spaces. These are features of malignancy. The benign hepatocytes show eosinophilic granular cytoplasm and centrally placed round regular nucleus.
DISCUSSION
Acute liver failure is a rare initial presentation of lymphoma, with less than 40 cases reported in the literature[7]. This phenomenon is twice as likely to occur in men, and the mean age is 48.6 years. It carries an 83% mortality rate, with a mean survival of 10.7 d. Most diagnoses are established by liver biopsy and often occur postmortem. Chemotherapy has only been successful in inducing remission in 3 of 23 cases of non-Hodgkins lymphoma (NHL)-associated ALF[6]. Due to rapid recurrence rates with poor survivability, liver transplantation is contraindicated[8].
Peripheral T-cell lymphomas are malignancies of immunologically mature T-cells that arise in peripheral lymphoid tissues such as lymph nodes, spleen, gastrointestinal tract, and skin. They are a rare and heterogenous class comprising only 5%-10% of NHL in North America and Western Europe[9]. The PTCL-NOS classification represents the largest subgroup, and generally affects people over the age of 60 with a slight male predominance. Due to its aggressive nature, patients often present in advanced stages, with nodal and extranodal spread. In one series of 199 patients diagnosed with PTCL, overall liver involvement occurred in 10%, and, in 9% for the PTCL-NOS subtype[10]. The 5-year survival rate is 20%-30% in most series[9].
This case highlights the importance of considering all the possible causes of ALF once the more common ones have been excluded. A diagnosis of lymphoma should be entertained in any patient presenting with ALF, lactic acidosis, and, hepatomegaly with evidence of an infiltrative process[6]. Unfortunately, given the advanced stage of disease and the wishes of the patient’s family, it was decided not to subject our patient to chemotherapy and her condition rapidly deteriorated. Prompt recognition of lymphoma and initiation of chemotherapy, however, may result in better outcomes.
Footnotes
Peer reviewers: Smruti Ranjan Mohanty, MD, MS, Assistant Professor of Medicine, Department of Medicine, Section of Gastroenterology & Hepatology, Center for Liver Disease, 5841 S. Maryland Avenue, MC 7120, Chicago, IL 60637-1463, United States; Valentina Medici, MD, PhD, Department of Internal Medicine, University of California Davis, 4150 V Street, Suite 3500, Sacramento, CA 95817, United States; Jordi Muntane, PhD, Unidad de Investigacion, Hospital Universitario Reina Sofía, Av. Menéndez Pidal s/n, Cordoba 14004, Spain
S- Editor Zhang HN L- Editor Herholdt A E- Editor Liu N
References
- 1.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh P, Fox IJ, Rader AM, Sorrell MF. Fulminant hepatic failure as the initial manifestation of non-Hodgkins lymphoma. Am J Gastroenterol. 1995;90:2207–2209. [PubMed] [Google Scholar]
- 5.Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66–737. doi: 10.1007/s11894-008-0023-x. [DOI] [PubMed] [Google Scholar]
- 6.Lettieri CJ, Berg BW. Clinical features of non-Hodgkins lymphoma presenting with acute liver failure: a report of five cases and review of published experience. Am J Gastroenterol. 2003;98:1641–1646. doi: 10.1111/j.1572-0241.2003.07536.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhat YM, Krasinskas A, Craig FE, Shaw-Stiffel TA. Acute liver failure as an initial manifestation of an infiltrative hematolymphoid malignancy. Dig Dis Sci. 2006;51:63–67. doi: 10.1007/s10620-006-3085-3. [DOI] [PubMed] [Google Scholar]
- 8.Thompson DR, Faust TW, Stone MJ, Polter DE. Hepatic failure as the presenting manifestation of malignant lymphoma. Clin Lymphoma. 2001;2:123–128. doi: 10.3816/clm.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 9.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 10.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]


