Abstract
AIM: To evaluate the direct binding of two main chlamydial biovars (C. trachomatis and C. pneumoniae) to plasma lipoproteins and its effect on chlamydial infection rate in human hepatoma cell line (HepG2 cells).
METHODS: Murine plasma lipoproteins were fractionated and isolated using fast-performance liquid chromatography (FPLC), spotted on nitrocellulose membrane and incubated with chlamydial suspensions. Direct binding of chlamydial particles to lipoprotein fractions has been studied using lipopolysaccharide-specific antibodies in immuno-dot blot binding assay and immunoprecipitation analysis. Immunostaining protocol as well as flow cytometry analysis have been employed to study the infectivity rate of chlamydial species in HepG2 cells.
RESULTS: Elementary bodies of both C. trachomatis and C. pneumoniae bind ApoB-containing fractions of plasma lipoproteins. That binding becomes stronger when heat-denatured FPLC fractions are used, suggesting a primary role of apolipoproteins in interaction between chlamydial particle and lipoprotein. Both chlamydial biovars efficiently propagate in human hepatoma cell line - HepG2 cells even in serum free conditions forming late-stage inclusion bodies and releasing extracellular elementary bodies. Preincubation of C. trachomatis and C. pneumoniae with native ApoB-containing lipoproteins enhances the rate of chlamydial infection in HepG2 cells.
CONCLUSION: A productive infection caused by C. trachomatis and C. pneumoniae may take place in human-derived hepatocytes revealing hepatic cells as possible target in chlamydial infection. Obtained results may suggest the participation of lipoprotein receptors in the mechanism of attachment and/or entry of chlamydial particles into target cells.
Keywords: ApoB-containing lipoproteins, Chlamydial trachomatis, Chlamydial pneumoniae, Human hepatoma cell line, Liver infection
INTRODUCTION
Although plasma constituents are known to have an enormous impact on the initiation, development and outcomes of many infections, the role of plasma lipoproteins in the pathogenesis of infectious diseases remains poorly understood. However, at least in case of bacterial sepsis, the severity and clinical manifestation of the disease largely depends on the plasma lipoprotein profile of patients. Survival rate in bacterial sepsis is higher in hypercholesterolemic individuals presumably due to ability of plasma lipoproteins to scavenge lipopolysaccharide from the infected cells[1]. High density lipoproteins are considered to be a mayor acceptor of lipopolysaccharide (LPS) in vivo and in vitro systems preventing tissue damage in sepsis[2]. LPS avidly binds two major high density lipoproteins (HDL)-specific apolipoproteins - A1 and Apo C I[3,4]. Subsequent binding of HDL-LPS complexes to the scavenger receptor SR-BI in the liver promotes hepatic clearance of LPS from the blood stream[5].
Much less information is available about the possible role of plasma lipoproteins in dissemination mechanisms of infectious agents. Most of our knowledge in that field relies on the well characterized association between plasma lipoproteins and hepatitis C virus. The majority of hepatitis C viral particles are bound to ApoB-containing very low density lipoproteins (VLDL) and low density lipoproteins (LDL) and can be immunoprecipitated with ApoB-specific antibody[6]. Complexes LDL-Hepatitis C virus, elsewhere termed viral lipoparticles, interact with the LDL-receptor as well as with surface receptor CD81, providing a dual receptor mechanism for viral attachment and entry in the target cells[7].
However, interactions between chlamydial species and plasma lipoproteins remain completely unknown. A published paper on this issue[8] demonstrates that LDL promotes foam cell formation in the macrophage cell line preincubated with chlamydial trachomatis (C. pneumoniae). The objective of this study was to initiate an investigation of direct interaction between chlamydial particles and plasma lipoproteins and its role in infecting host cells. Here we show, for the first time, that the elementary body of C. trachomatis and C. pneumoniae directly binds apoB-containing lipoproteins, promoting the infection rate in human hepatoma cell line (HepG2 cells).
MATERIALS AND METHODS
Reagents
All reagents were from Sigma-Aldrich unless otherwise stated. Fast-performance liquid chromatography (FPLC) was performed using Superose 6HR 10/30 column (Pharmacia, Sweden) as described[9,10]. Cholesterol content in the FPLC fractions was measured using Cholesterol/Cholesteryl Quantification Kit (Calbiochem, UK).
Gradient gel electrophoresis of FPLC fractions was performed as published by Ordovas JM[11]. Protein level was measured using BCA kit from Pierce (Cramlington, UK). HepG2 cells were obtained from “European Collection of Cell Cultures” (Salisbury, UK).
Genus-specific monoclonal antibodies against chlamydial LPS and chlamydial major outer membrane protein (MOMP) were described previously[12]. Polyclonal antibody against apolipoprotein B (ab20737) was purchased from Abcam (Cambridge, UK). Anti-mouse IgG horseradish-peroxidase linked secondary antibody was obtained from Amersham (Buckinghamshire, UK).
Cell culture and organisms
The following chlamydial organisms were used: C. trachomatis strain L2/Bu434 and C. pneumoniae strain Kajjani-6. Both of them were kindly provided by Dr. P. Saikku (University of Oulu, Finland).
Chlamydial strains were propagated in Hep2 cells and purified by Renografin gradient centrifugation as described[13]. Purified elementary bodies were suspended in sucrose-phosphate-glutamic acid buffer[13]. Chlamydial titers were determined by infecting Hep2 cells with 10-fold dilutions of thawed stock suspension.
HepG2 cells were maintained in Dulbecco's Modified Eagle Media (DMEM) with 10 % fetal calf serum until subconfluence was reached. Monolayers of HepG2 cells in 24-well plates containing cover slips were washed and infected with C. trachomatis or C. pneumoniae at multiplicity 1:1. Infected plates were centrifuged 1 h at 1500 g and kept in serum-free DMEM supplemented with 2 μg/mL of cycloheximide for 48 h (C. trachomatis) or 72 h (C. pneumoniae). Cover slips were fixed with acetone and stained by direct immunofluorescence using fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody against chlamydial LPS. To evaluate the contribution of ApoB-containing lipoproteins into infectivity rate of C. trachomatis and C. pneumoniae, titrated stock suspensions containing elementary bodies of each chlamydial type were incubated (1 h, 37°C) with 0.1 mg/mL of native ApoB-containing lipoproteins dissolved in Public Broadcasting Service (PBS). After removal of unbound lipoproteins (2 washes in PBS, 15 000 g, 5 min each), the volume of the stock suspensions was readjusted to the initial value to commence immediately with the cell infection protocol.
Animals
C57BL/6 male mice 6-8 wk old from Pushino Animal Breeding Facility (Moscow, Russia) were kept in the animal facility in compliance with “Declaration of Helsinki and Guiding Principles in the Care and Use of Animals” under approved institutional protocol. Mice were kept on the synthetic atherogenic high-fat diet (ICN Biochemicals Inc, Aurora, Ohio) for 4 wk with free access to the food and water before collection of blood via retro orbital sinus puncture under anesthesia. Plasma obtained from inbred mice was considered as the preferred source of lipoproteins to avoid any variables related to the genetic background and/or dietary status of human individuals.
Isolation of native ApoB-containing lipoproteins
A low-density fraction of plasma lipoproteins was isolated by centrifugation of mouse plasma at the density of 1.055 g/mL for 4 h, 4°C and 543 000 g TL100, Beckman Instruments, USA[14]. The upper layer was dialyzed overnight against PBS supplemented with 0.01% sodium EDTA (pH 7.4), filtered through 0.22 μm pore-sized membranes and stored at 4°C for no longer than 3 wk.
FPLC and gel electrophoresis analysis
Pooled plasma (2.5 mL) obtained from 5 mice was subjected to ultracentrifugation at density of 1.215 g/mL. Purified lipoproteins were loaded on FPLC column equilibrated with PBS containing 0.01% EDTA and 0.01% sodium azide. Plasma lipoproteins were eluted from the column at room temperature and flow rate 0.2 mL/min with the same buffer. Elution fractions (0.3 mL each, 46 fractions total) were monitored at 280 nm and analyzed for cholesterol content. Plasma lipoprotein fractions were stored at 4°C and used within 3 wk after preparation. For gel electrophoresis, each three consecutive FPLC fractions were pooled and delipidated with chloroform/methanol mixture (1:1). After centrifugation (5 000 g, 10 min) the pellet was dissolved, vortexed and boiled in 50 mmol/L Tris-HCL (pH 7.8) containing 8 mol/L urea, 10% SDS, 10 % Glycerol and 0.05% bromophenol blue. Aliquots of reconstituted FPLC fractions were loaded on 4%-15% gradient SDS-polyacrilamide gel, which was stained after an overnight run with Coomassie Blue.
Immuno-dot blot binding assay
20 μL aliquotes of FPLC fractions (native or heat-denatured) containing 100 ng of total protein were loaded under vacuum on to nitrocellulose membrane (Amersham, UK). Crosslinked membranes were blocked in 3% gelatin in 20mmol/L Tris-buffer (pH 7.5) with 0.05% Tween 20 (TBST) for 1 h at room temperature and incubated overnight at 4°C with suspensions of UV-inactivated C. trachomatis and C. pneumoniae in TBST (100 μg of total protein/mL). After three TBST washings (5 min each), the membranes were incubated with monoclonal antibodies against chlamydial LPS (2 h, 5 μg/mL) and blotted for 15 min with horse-radish peroxidase conjugated secondary antibody (Amersham, UK). After 3 final washes in TBST, membranes were developed in the Enhanced Chemiluminescent Substrate (Pierce, UK) and exposed to X-ray films for 5-7 s.
Immunoprecipitation analysis
Immunoprecipitation studies were done using Seize® X Bacterial Immunoprecipitation kit (Pierce, Cramlington, UK). Briefly, elementary bodies of C. trachomatis or C. pneumoniae (0.1 mg/mL in PBS) were thoroughly mixed with native LDL (0.1 mg/mL in PBS) and incubated at 37°C for 1 h under continuous gentle shacking. Bacterial cells were pelleted by centrifugation (15 000 g, 10 min) washed 3 times with ice-cold PBS to remove unbound lipoproteins and kept frozen at -80°C. After thawing, soluble proteins from the bacterial pellet were extracted with B-Per® reagent and applied to the affinity mini column with immobilized polyclonal ApoB-specific antibody. Uncoated beads and beads with irrelevant polyclonal antibody were used in control experiments. After 2 h of incubation at room temperature, the columns were centrifuged and the resulting flow-through was designated as supernatant. Following washing procedure, immunoprecipitated protein was eluted with 150 μL of the elution buffer and referred as a pellet fraction. Supernatant and pellet fractions were analyzed in 8% SDS-PAGE and further immunoblot analysis was conducted with monoclonal MOMP-specific antibody.
Flow Cytometry analysis
Flow Cytometry analysis was performed according to the method described by Dessus-Babus S[15]. Briefly, HepG2 monolayers were trypsinized and removed from the dishes, centrifuged at 400 g for 5 min. The cell pellet was resuspended and washed twice in PBS. After fixation in 70% ethanol, washed cells were incubated with chlamydial LPS-specific antibody labeled with FITC. The number of cells with fluorescent inclusions was determined with BD FACSCalibur Flow Cytometer (BD Biosciences, USA) with the support of CellQuest Pro Software. All experiments shown below were repeated at least twice. Representative sets of results are submitted here.
RESULTS
It is known[10] that normal mouse plasma contains almost undetectable amounts of ApoB-containing lipoproteins - VLDL, LDL and IDL (results not shown). As expected, dietary treatment of mice with high-fat diet promoted appearance in ApoB isoforms in FPLC fractions (Figure 1). As can be seen from the gradient gel image, ApoB-100 and ApoB-48 were almost equally represented in VLDL, LDL and IDL fractions, while ApoA1 was a major apolipoprotein present in HDL fractions. Cholesterol profile pinpoints the specific fractions and validates efficiency of FPLC protocol used in our study. The highest cholesterol content was detected in HDL fraction whereas the lowest cholesterol value was measured in VLDL fraction. Those tendencies are consistent with characteristics of the mouse plasma lipoprotein profile induced by high-fat diet published elsewhere[10].
Figure 1.
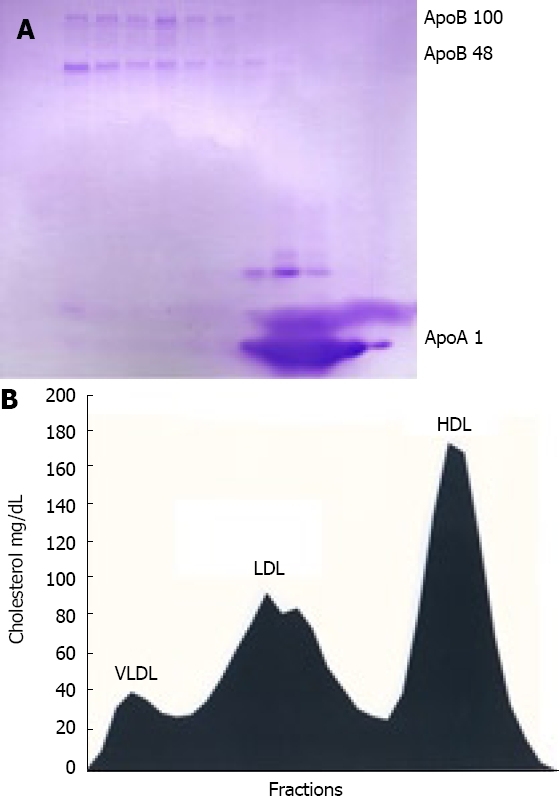
FPLC fractionation of mouse plasma. Mice kept on high-fat diet were bled and pooled plasma was adjusted with KBr to d = 1.215 g/mL and ultracentrifuged. Resulting lipoprotein fraction with density d < 1.215 g/mL was subjected to FPLC gel filtration and cholesterol content was measured in each fraction. Three of each consecutive fraction were pooled, delipidated, heat-denatured and subjected to SDS PAGE gel electrophoresis in gradient gel as described in “Material and Methods”. A: Coomassie blue-stained gel with ApoB-100, ApoB-48 and ApoAI indicated by arrow; B: Cholesterol levels in FPLC fractions aligned to apolipoprotein profile of mouse plasma.
Lipoprotein purification protocol implemented in this work allowed us to use pure murine lipoprotein fractions for dot-blot binding assay. Dot-blot immunodetection analysis showed that elementary bodies of C. trachomatis and C. pneumoniae directly interact with purified undenatured and denatured lipoproteins (Figure 2). As can be seen from our results, C. trachomatis predominantly binds native lipoproteins belonging to VLDL and LDL fractions. That interaction becomes much stronger and broader when lipoprotein fractions were denatured. This fact reveals that apolipoprotein component of plasma lipoproteins is crucial for lipoprotein attachment to the chlamydial particles. Similar pattern of binding has been found for C. pneumoniae. That interaction appears to be very similar at exactly the same conditions of binding assay used (duration of incubation and exposure time, concentration of basic reagents). Therefore, the major feature of chlamydial-lipoprotein interaction is clear. Both chlamydial species bind ApoB-containing lipoproteins, with no detectable affinity to HDL fraction. To confirm our observation we employed immunoprecipitation analysis. Protein extracts obtained from C. trachomatis and C. pneumoniae after preincubation with native LDL were immunoprecipitated with ApoB-specific antibody. As can be seen from Figure 3, ApoB-specific antibody pulls down MOMP from protein extracts of both chlamydial species, when bacterial particles were pre-exposed to LDL.
Figure 2.
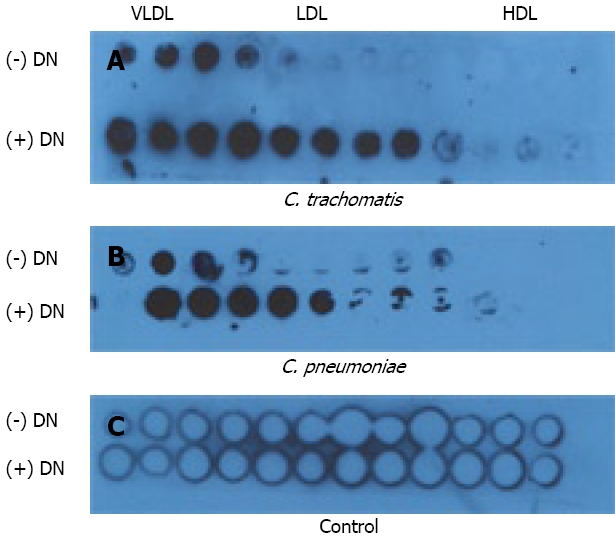
Immuno-dot blot binding assay. Heat-denatured (+ DN) or non-heat-denatured (- DN) FPLCS fractions of mouse plasma lipoproteins were applied to nitrocellulose membrane and incubated with elementary bodies of C. trachomatis (Panel A) or C. pneumoniae (Panel B) and blotted with chlamydial LPS-specific antibodies as described in “Material and Methods”. Control membrane (Panel C) treated similarly except addition of chlamydial suspension.
Figure 3.
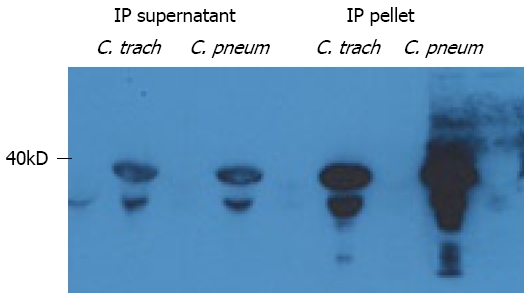
Immunoprecipitation analysis. Soluble membrane extracts of C. trachomatis and C. pneumoniae preincubated with native Apo-B containing lipoproteins were immunoprecipitated with Apo-B specific polyclonal antibody and subjected to SDS PAGE. Immunoprecipitated (IP) supernatant and pellet were obtained as described in Materials and Methods. Membranes were immunoblotted with MOMP-specific monoclonal antibodies.
The validity of immunoprecipitation analysis was confirmed in the control experiments by immunoblotting of supernatant fractions (Figure 3) as well as by using irrelevant antibody and uncoated beads (results not shown).
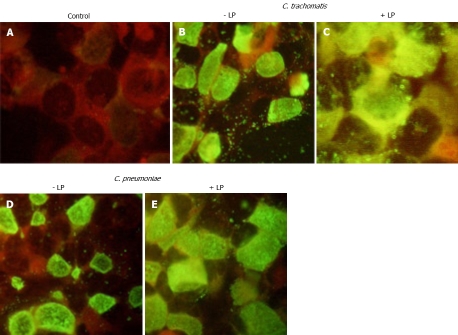
The results presented above showed that there is distinct binding of chlamydial species to the ApoB-containing plasma lipoproteins. Therefore, we decided to test if complexes containing chlamydial particles and plasma lipoproteins may infect LDL-receptor expressing cell line. HepG2 cells were used for that purpose. As can be seen from Figure 4, both C. trachomatis and C. pneumoniae may efficiently propagate in immortalized human hepatocytes infected in serum-free conditions. After infecting the cells with chlamydial species, late stage inclusions and extracellular elementary bodies were seen in approximately 60% of the cells. Inoculums containing chlamydial particles preincubated with ApoB-containing lipoproteins promoted intensity of staining and number of infected HepG2 cells.
Figure 4.
HepG2 cells. Direct immunofluorescence with FITC-conjugated antibody against chlamydial LPS. HepG2 cells were infected with inoculants containing: A: No chlamydial particles, B: C. trachomatis alone; C: C. trachomatis preincubated with native Apo-B containing lipoproteins; D: C. pneumoniae alone; E: C. pneumoniae preincubated with native Apo-B containing lipoproteins. Immunofluorescence protocol is detailed in Materials and Methods. Original magnification × 40.
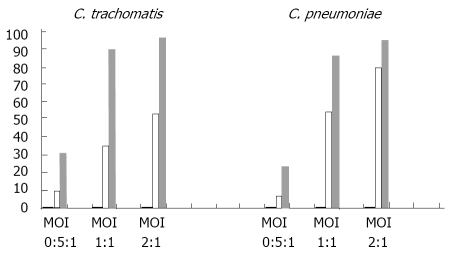
To quantify the effect of native Apo-B containing lipoproteins on infectivity rate of C. trachomatis and C .pneumoniae, flow cytometric analysis has been used (Figure 5). As follows from our results, ApoB-containing lipoproteins consistently enhanced the infectivity rate of C. trachomatis at different levels of infection multiplicity (MOI 0.5:1; 1:1; 2:1). The same tendency has been seen in the case of C. pneumoniae. HDL fraction did not affect infection rate in HepG2 cells incubated with chlamydial biovars (results are not shown).
Figure 5.
Flow Cytometry Analysis of HepG2 cells. Infectivity of C. pneumoniae and C. trachomatis preincubated with native ApoB-containing lipoproteins. HepG2 cells were infected with C. pneumoniae (left side of diagram) or C. trachomatis (right side of diagram) with or without preincubation of bacteria with native ApoB-containing lipoproteins as described in Material Methods. Three different levels of infection multiplicity were studied for each pathogen (MOI 0.5:1; 1:1; 2:1). Percentage of the infected cells shown on value Y axis was determined by flow cytometry (see Materials and Methods) for control (uninfected) HepG2 suspensions (hardly seen black columns), HepG2 suspensions infected with chlamydia bacteria alone (white columns) and HepG2 suspensions infected with chlamydial bacteria preincubated with native ApoB-containing lipoproteins (gray columns).
DISCUSSION
In the present paper we show for the first time that both C. trachomatis and C. pneumoniae interact directly with plasma lipoproteins and may infect a novel cell target type for chlamydial infection - hepatic cells. That interaction enhances the ability of chlamydial particles to enter and/or propagate inside of the hepatocytes, increasing the infection rate in the hepatic cell line HepG2. The ability of Chlamydia trachomatis to propagate in HepG2 cells has been reported recently by G Wang et al[16].
The precise molecular mechanisms responsible for chlamydial invasion of the host cells have yet to be identified. Several receptors, including mannose 6-phosphate/insulin-like growth factors (IGF-2)[17] and eukaryotic lipid membrane domains[18], are proposed to play a significant role in the entrance of chlamydial particles in the host cells. On the other hand, a variety of other polyvalent interactions between chlamydial particles and host cell membrane deserves detailed considerations[19]. An initially surprising finding about direct interaction between chlamydial particles and plasma lipoproteins may represent a case of metabolic mimicry, when a bacterial particle acquires mammalian ligand for specific membrane receptor, allowing subsequent endocytosis and initiation of infectious cycle inside of the host cell. Association of chlamydial particles with plasma lipoproteins does not seem to be an absolute requirement for initiation of the chlamydial infection in the hepatocytes since chlamydial infection in HepG2 cells can be initiated and sustained in serum-free medium. However, if binding chlamydial particles to plasma lipoproteins takes place in vivo situation, it may have significant pathophysiological consequences especially in hyperlipidemic and atherosclerotic patients. ApoB-containing lipoproteins are known to be efficiently internalized not only by hepatocytes, but also by macrophages and endothelial cells residing in atherosclerotic lesions[20]. Therefore, persistent increase in plasma ApoB - containing lipoproteins may promote targeted delivery of chlamydial particles and aggravate inflammatory response in the atherosclerotic plaque. This hypothesis and a molecular mechanism of interaction between chlamydial biovars and lipoproteins are currently under investigation.
COMMENTS
Background
Two major chlamydial biovars - C. trachomatis and C. pneumoniae cause a wide range of urogenital and respiratory infections. Possible role of C. pneumoniae in atherosclerosis is currently discussed. Participation of chlamydial species in hepatic diseases is suspected but not proven yet.
Research frontiers
It has been shown recently that C. trachomatis may propagate in hepatocytes. On the other hand, a productive infection by C. pneumoniae is reported in Kupffer cells isolated from liver. The relevance of these observations for hepatobiliary pathology remains to be elucidated.
Innovations and breakthroughs
This study is first to show that two major chlamydial species (C. trachomatis and C. pneumoniae) can bind plasma lipoproteins and efficiently propagate in immortalized human hepatocytes. Plasma ApoB-containing lipoproteins promote the infection rate in human hepatoma cell line (HepG2 cells), suggesting possible role of lipoproteins in the mechanism of attachment and/or entry of bacteria into the host cell.
Applications
Understanding of the precise molecular mechanism predetermining interaction of chlamydial biovars with the host cells is essential for the development of new strategies in treatment of chlamydial infections.
Peer review
The authors showed that C. trachomatis and C. pneumoniae can bind ApoB-containing plasma lipoproteins. Both chlamydial biovars are shown to propagate in human hepatoma cell line - HepG2 cells. Preincubation of chlamydial particles with ApoB-containing lipoproteins promotes the infectivity rate of chlamydial biovars in HepG2 cells, suggesting possible participation of lipoprotein receptor in interaction of chlamydial species with the host cells. The results are interesting and may bring new insight into pathogenesis of chlamydial infections.
Acknowledgments
The authors are thankful to Drs E Fedina, E Tokarskaya and N Kolkova for challenging discussions. Ms Agni Roce is appreciated for invaluable help during experimental work and manuscript preparation.
Footnotes
Supported by Cambridge Theranostics Ltd
Peer reviewer: Lucia Malaguarnera, Associate Professor, MD, PhD, Department of Biomedical Sciences, University, Via E. De Amicis, 24 Trecastagni, Catania 95039, Italy
S- Editor Wang YR L- Editor Roemmele A E- Editor Liu N
References
- 1.von Haehling S, Schefold JC, Springer J, Anker SD. The cholesterol paradox revisited: heart failure, systemic inflammation, and beyond. Heart Fail Clin. 2008;4:141–151. doi: 10.1016/j.hfc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Murch O, Collin M, Hinds CJ, Thiemermann C. Lipoproteins in inflammation and sepsis. I. Basic science. Intensive Care Med. 2007;33:13–24. doi: 10.1007/s00134-006-0432-y. [DOI] [PubMed] [Google Scholar]
- 3.Berbee JF, van der Hoogt CC, Kleemann R, Schippers EF, Kitchens RL, van Dissel JT, Bakker-Woudenberg IA, Havekes LM, Rensen PC. Apolipoprotein CI stimulates the response to lipopolysaccharide and reduces mortality in gram-negative sepsis. FASEB J. 2006;20:2162–2164. doi: 10.1096/fj.05-5639fje. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Liao XL, Lou B, Wu MP. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim Biophys Sin (Shanghai) 2004;36:419–424. doi: 10.1093/abbs/36.6.419. [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- 7.Wunschmann S, Muller HM, Stipp CS, Hemler ME, Stapleton JT. In vitro interaction between hepatitis C virus (HCV) envelope glycoprotein E2 and serum lipoproteins (LPs) results in enhanced cellular binding of both HCV E2 and LPs. J Infect Dis. 2006;194:1058–1067. doi: 10.1086/507647. [DOI] [PubMed] [Google Scholar]
- 8.Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae--induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–759. doi: 10.1128/IAI.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res. 2000;41:1020–1026. [PubMed] [Google Scholar]
- 10.Gallou-Kabani C, Vigé A, Gross MS, Boileau C, Rabes JP, Fruchart-Najib J, Jais JP, Junien C. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab. 2007;292:E1095–E1100. doi: 10.1152/ajpendo.00390.2006. [DOI] [PubMed] [Google Scholar]
- 11.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutlin AV, Drobyshevskaia EI, Shatkin AA. [The immunochemical and biological properties of monoclonal antibodies to Chlamydia trachomatis] Zh Mikrobiol Epidemiol Immunobiol. 1996:3–6. [PubMed] [Google Scholar]
- 13.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz O, Delers F, Maynard M, Demignot S, Zoulim F, Chambaz J, Trépo C, Lotteau V, André P. Preferential association of Hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87:2983–2991. doi: 10.1099/vir.0.82033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dessus-Babus S, Belloc F, Bébéar CM, Poutiers F, Lacombe F, Bébéar C, de Barbeyrac B. Antibiotic susceptibility testing for Chlamydia trachomatis using flow cytometry. Cytometry. 1998;31:37–44. [PubMed] [Google Scholar]
- 16.Wang G, Burczynski F, Anderson J, Zhong G. Effect of host fatty acid-binding protein and fatty acid uptake on growth of Chlamydia trachomatis L2. Microbiology. 2007;153:1935–1939. doi: 10.1099/mic.0.2006/003491-0. [DOI] [PubMed] [Google Scholar]
- 17.Puolakkainen M, Kuo CC, Campbell LA. Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect Immun. 2005;73:4620–4625. doi: 10.1128/IAI.73.8.4620-4625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jutras I, Abrami L, Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect Immun. 2003;71:260–266. doi: 10.1128/IAI.71.1.260-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dautry-Varsat A, Balañá ME, Wyplosz B. Chlamydia--host cell interactions: recent advances on bacterial entry and intracellular development. Traffic. 2004;5:561–570. doi: 10.1111/j.1398-9219.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 20.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]