Abstract
Chronic infection with the protozoan parasite Trypanosoma cruzi is a major cause of morbidity and mortality in Latin America. Drug treatments for the associated illness, Chagas disease, are toxic and frequently unsuccessful. In a screening effort against the drug target protein farnesyltransferase, we identified a series of disubstituted imidazoles with highly potent anti-T. cruzi activity that apparently acted through a mechanism independent of protein farnesylation. Metabolic labeling studies of T. cruzi suggested that sterol biosynthesis was inhibited. Combined GC/MS analysis confirmed depletion of cellular sterols and suggested that the site of action was sterol 14-demethylase, a cytochrome P450 enzyme. Spectral studies with recombinant T. cruzi sterol 14-demethylase demonstrated that the compounds bind directly to this enzyme. Two of the compounds were well absorbed when given orally to mice, gave sustained plasma levels, and were well tolerated. The compounds were administered orally to mice with acute T. cruzi infection and caused dramatic decrease in parasitemia and led to 100% survival. These disubstituted imidazole compounds can be prepared by a relatively short synthetic route and represent a structural class with potent anti-T. cruzi activity.
As many as 200,000 new cases of Trypanosoma cruzi infection occur in Latin America each year (1). The protozoan, T. cruzi, is transmitted primarily by insect vectors and blood transfusion. The infected human becomes a lifelong host to the parasite, with ≈30% developing overt symptoms of Chagas disease. The disease typically manifests many years after the initial infection with cardiomyopathy or megasyndromes of the gastrointestinal tract, both of which are commonly fatal. Antiparasitic therapy with the principal clinical drugs, benznidazole or nifurtimox, is usually ineffective when given to the symptomatic patients in chronic stage of Chagas disease. The best results with benznidazole have been shown in acutely infected persons and in seropositive schoolchildren, with cure rates in the latter group up to 60% (1). Unfortunately, benznidazole is very poorly tolerated by adults, which greatly limits its usefulness. More effective and better tolerated anti-T. cruzi therapies are greatly needed.
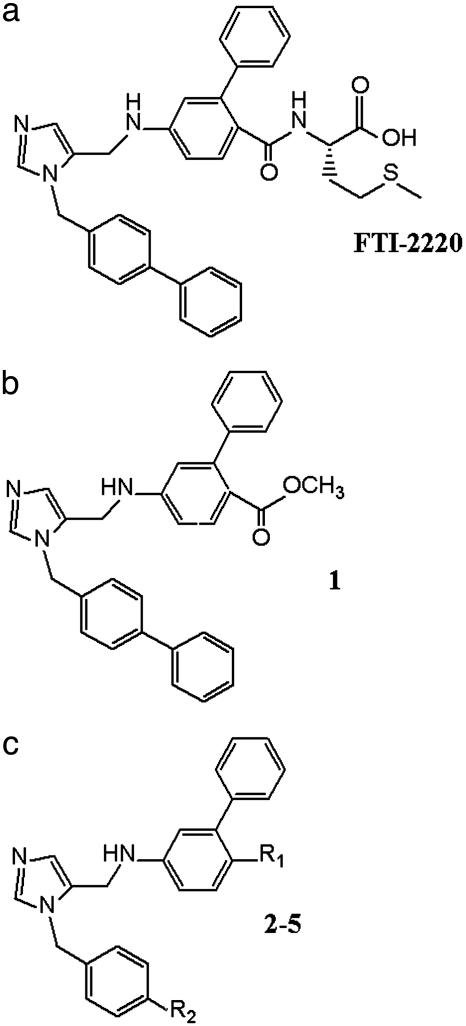
It follows that new biochemical targets for anti-T. cruzi therapy need to be identified. One such target is the enzymatic pathway involved in protein prenylation (2, 3). An advantage of this target is that a major effort is underway in the pharmaceutical industry to make prenylation inhibitors, particularly protein farnesylation inhibitors, as anticancer agents (4). Protein farnesylation is an attractive “piggy-back” target for antiprotozoan chemotherapy, because trypanosomatids and plasmodium are very sensitive to the effects of protein farnesyltransferase (PFT) inhibitors and this class of compounds appears to be fairly well tolerated in humans (5, 6). Through collaborations with academic and industrial partners we have an ongoing effort to screen PFT inhibitors against trypanosomatid parasites, including T. cruzi. Our screening efforts included FTI-2220 (Fig. 1a) which is a mimetic of the tetrapeptide substrate for PFT, CVIM (7). FTI-2220 inhibited Trypanosoma brucei PFT with an IC50 of 8 nM (23). The methyl ester of FTI-2220 was potent against T. brucei cells with an ED50 of 0.5 μM (23). This prodrug is better taken up by parasites than the free acid and presumably undergoes intracellular hydrolysis to the active PFT inhibitor (7).
Fig. 1.
Peptidomimetic compounds and derivatives. (a) Methionine containing FTI-2220 patterned after the tetrapeptide CVIM. (b) FTI-2220 with CO2CH3 group replacing the methionyl unit (compound 1). (c) Disubstituted imidazole compounds 2-5 (R1 and R2 substituents are defined in Table 1).
A screen of a series of compounds derived from FTI-2220 revealed a number of molecules with highly potent activity against T. cruzi cells. Notably, many of these compounds had almost no activity to inhibit the enzymatic function of T. cruzi PFT, suggesting that they worked through an independent mechanism of action. This article describes experiments that revealed the target of action of these compounds to be the cytochrome P450 enzyme, T. cruzi sterol 14-demethylase, of the sterol biosynthesis pathway. Subsequent experiments demonstrated potent antiparasitic activity of two compounds in this series when delivered orally in the mouse model of Chagas disease. The discovery introduces a set of chemical structures with potent anti-T. cruzi activity.
Materials and Methods
Compounds. The preparation of compounds included in this article will be described elsewhere (J.L., J.O., K.Y., F.B., R. Eastman, W.V., M.G., S.S., and A.H., unpublished work).
Cell Culture. Tulahuen strain T. cruzi were used in all experiments. Epimastigotes (the life cycle stage in the insect host) were cultured in liver infusion tryptone-based medium (8). The mammalian stages (amastigotes and trypomastigotes) of T. cruzi were grown in coculture with murine 3T3 fibroblasts (9).
Growth Inhibition Assays. Compounds were screened in microtiter plates against mammalian-stage T. cruzi that were engineered to express the Escherichia coli β-galactosidase gene (9). Growth inhibition of 3T3 fibroblasts was quantified by using Alamar Blue (Alamar Biosciences, Sacramento, CA) (9). An internal control of benznidazole was included in all T. cruzi assays, which consistently produced an ED50 of 0.6 ± 0.2 μM. The concentration causing 50% growth inhibition (ED50) was calculated from the dose–response curve by using PRISM (GraphPad, San Diego).
PFT Inhibition Assay. Partially purified T. cruzi PFT was prepared from T. cruzi epimastigotes as described (2). The standard reaction mixture contained 5 μM RAS1-CVIM (10), 0.75 μM (0.3 μCi) [3H]farnesyl pyrophosphate, and T. cruzi PFT in 20 μl of buffer (30 mM potassium phosphate/5 mM DTT/0.5 mM MgCl2/20 μM ZnCl2, pH 7.7). The mixture was incubated in the presence of 50 nM compound for 15 min at 30°C, and the amount of radioactive product was quantified by the glass-fiber filter method (11).
Radiolabeling of Prenylated Proteins. T. cruzi epimastigotes (1 × 107 cells in 1 ml of culture medium) were incubated for 24 h with 100 μCi (60 Ci/mmol) of racemic [5-3H]mevalonolactone (American Radiolabeled Chemicals, St. Louis) and compound or DMSO vehicle alone. The cell pellet was delipidated with 0.5 ml of chloroform/methanol (2:1, vol/vol). The resulting protein pellet was then washed once with ice-cold acetone, mixed with Laemmli sample buffer, and analyzed by SDS/PAGE and fluorography (12). Portions of the lipid fraction (in the chloroform/methanol extract) and the protein fraction (in Laemmli sample buffer) were subjected to scintillation counting to determine the relative incorporation of [3H]mevalonolactone into these cell components.
Analysis of Sterol Products. Epimastigotes (1 × 107 in 1 ml of culture medium) were incubated for 24 h with 100 μCi of [3H]mevalonolactone and compound or DMSO alone. The washed cell pellets were extracted with chloroform/methanol (2:1, vol/vol) (13). The resulting lipids were subjected to silica gel TLC plates by using toluene/dethyl ether (9:1, vol/vol) as solvent. Radioactive sterol standards produced from Candida albicans were kindly provided by T. White (Seattle Biomedical Research Institute, Seattle) (14). Radioactive lipid spots were visualized by fluorography using EN3HANCE (NEN).
GC-MS Analysis of Sterols. Epimastigotes (1 × 108) were cultured for 5 days in the presence of compound 2 in varying concentrations. Sterols were extracted from cell pellets (15) and dissolved in ethyl acetate, and 1 μl was injected splitless into a Hewlett–Packard GC-MS system with a 6890 series GC and 5973 mass selective detector (70 eV electron impact ionization). The HP-5MS column was used with helium carrier gas at constant flow of 1.1 ml/min; injector temperature was 250°. We compared the spectra with reference data from National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health 1998 Mass Spectral Library (Standard Reference Data Program: National Institute of Standards and Technology, Gaithersburg, MD) and published data (15).
Spectrophotometric Studies with T. cruzi Sterol 14-Demethylase. Recombinant T. cruzi sterol 14-demethylase protein was expressed by using the baculovirus/Sf9 cell system (16). Solubilized membranes containing 0.2 nmol of enzyme (ferric state) were placed in a cuvette in a volume of 0.6 ml and mixed with incremental amounts of compound 1 dissolved in DMSO. The sample was scanned from 450 to 390 nm with a Beckman DU-70 spectrophotometer. A difference spectrum was generated by measuring the absorbance in the sample cuvette and subtracting the absorbance of the enzyme mixed with DMSO alone.
Mouse Experiments. Eight-week-old female BALB/c mice (weight 21 ± 2 g) were given a s.c. injection of 2 × 103 tissue culture-derived T. cruzi trypomastigotes at the base of the tail. Parasitemia was determined by examining two drops of blood under a coverslip at ×400 magnification. Compounds were dissolved in DMSO at 200 mg/ml, then further diluted to 10 mg/ml in Trappsol hydroxypropyl β-cyclodextrin (Cyclodextrin Tecnologies Development, Gainesville, FL). The compounds were administered directly to the stomach by gavage in a volume of 100 μl.
Plasma Drug Level Determinations. Eight-week-old female BALB/c mice (weight 21 ± 2 g) were administered a single dose of compound at 30 mg/kg or 50 mg/kg by oral gavage. Blood was collected into heparinized capillary tubes 1, 3, and 7 h after dosing. The blood was centrifuged, and 12 μl of plasma was extracted with 50 μl of methanol containing 250 pmol of FTI-2148 (7) as an internal standard. The methanol extract (10 μl) was analyzed by liquid chromatography-MS using an Agilent (Palo Alto, CA) HP 1100 chromatograph and a Esquire-LC (Bruker, Billerica, MA) electrospray ion trap mass spectrometer. The sample was separated with a C18 reverse-phase column (TP218MS52, Grace-Vydac, Hesperia, CA) with water/5% acetonitrile, 1% acetic acid-acetonitrile, 1% acetic acid gradient. The compounds were quantified by the area under the corresponding peak in the extracted ion chromatogram by using the established response factors to the internal standard and Bruker QUANTANALYSIS software.
Results and Discussion
Growth Inhibition of T. cruzi Cultures by Disubstituted Imidazoles. The methyl ester of FTI-2220 (Fig. 1a) was found to be a potent inhibitor of T. cruzi amastigote growth in murine fibroblasts, displaying an ED50 of 0.1 μM. As a control, we studied the analog of FTI-2220, compound 1 (Fig. 1b), in which the methionyl unit is replaced by CO2CH3. Without the methionyl unit, compound 1 would not be expected to bind PFT, and, as predicted, this compound lacked significant in vitro inhibitory activity against T. cruzi PFT (Table 1). It was therefore a surprise that 1 had highly potent anti-T. cruzi activity in the cultured cell assay (ED50 = 0.5 nM) (J.L., J.O., K.Y., F.B., R. Eastman, W.V., M.G., S.S., and A.H., unpublished work). This compound was not toxic to murine fibroblast host cells when tested up to 10 μM. By evaluating a number of compounds related to 1, it became apparent that an anti-T. cruzi pharmacophore is represented by a class of disubstituted imidazoles (Fig. 1c). Compounds 2–5 were found to have potent anti-T. cruzi activity and low toxicity to mammalian cells (Fig. 1c and Table 1).
Table 1. Compound structures and activity against purified T. cruzi PFT, T. cruzi cells, and murine fibroblast cells.
| Compound | R1 | R2 | % inhibition of T. cruzi PFT at 50 nM of drug | ED50 against T. cruzi,* μM | ED50 against murine 3T3 fibroblasts, μM |
|---|---|---|---|---|---|
| 1 | CO2CH3 | Phenyl | 0.8 | 0.0005 | >10 |
| 2 | CO2CH3 | H | 4 | 0.08 | >10 |
| 3 | H | Phenyl | 10 | 0.01 | 10 |
| 4 | 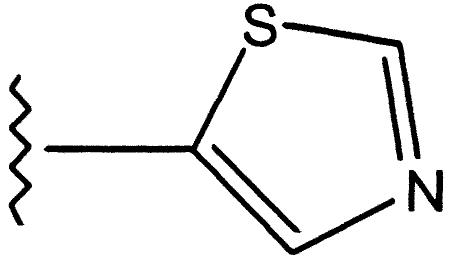 |
Phenyl | 23 | 0.05 | >10 |
| 5 | 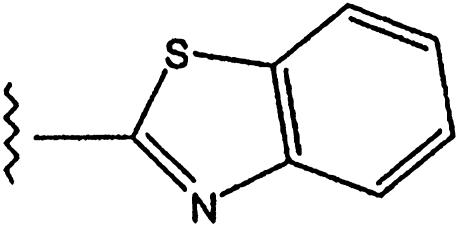 |
Phenyl | 16 | 0.02 | >10 |
R groups in reference to Fig. 1c.
Mammalian-stage parasites grown in 3T3 fibroblasts
Disubstituted Imidazole Compounds Block T. cruzi Synthesis of Sterols. To explore the possibility that these compounds were able to block the farnesylation of proteins in live parasites despite the fact that they lack in vitro PFT inhibition activity, we cultured T. cruzi cells with [3H]mevalonolactone (which is converted intracellularly to [3H]mevalonic acid, the precursor of farnesyl groups) in the presence of compound 2 and analyzed the uptake of radioactivity into prenylated proteins. In our previous studies of PFT inhibitors and T. brucei, we found a decrease in the amount of radioactivity from [3H]mevalonolactone incorporated into the protein fraction from cells treated with PFT inhibitors (10). However, for T. cruzi epimastigotes cultured with [3H]mevalonolactone, we found that addition of compound 2 caused a 4-fold increase of tritium incorporation into the protein fraction and a 5-fold decrease of tritium incorporation into lipids extracted with chloroform/methanol compared with the untreated control cells. These findings suggested the possibility that compound 2 blocked a step in the sterol biosynthetic pathway that lies downstream of farnesyl pyrophosphate formation. Such a block would elevate the levels of farnesyl pyrophosphate and thus tritrium incorporation into proteins and would reduce the amount of tritiated sterols.
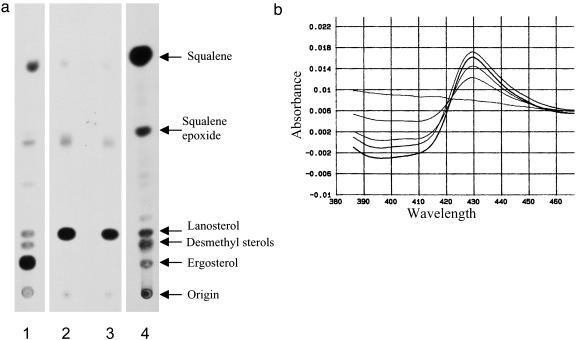
Like fungi, T. cruzi synthesize ergosterol and other C28 and C29 sterols from precursors derived from mevalonic acid (17). T. cruzi were labeled with 3H-mevalonolactone, and the lipids were analyzed by TLC. Cells treated with compound 2 had a complete block of sterol synthesis accompanied by accumulation of radioactive products comigrating with lanosterol, similar to the sterol profile observed with cells treated with the sterol 14-demethylase inhibitor, ketoconazole (Fig. 2a). Similar results were obtained with extracts from cells treated with compounds 1, 3, 4, and 5 (data not shown). GC-MS analysis of sterols extracted from cells showed that mature sterols disappeared with increasing doses of compound 2 or ketoconazole (data not shown). Missing or diminished sterols included ergosta 5,7,22-trien-3β-ol (ergosterol), 24-ethyl-cholesta-5,7,22-trien-3β-ol, ergosta-5,7-dien-3β-ol (dihydroergosterol), and 24-ethyl-cholesta-5,7-dien-3β-ol. Cholesterol was present in the cell membranes presumably from the serum in the culture medium (17). The analysis revealed accumulation of lanosterol and another major compound with a molecular weight of 440 (14 mass units larger than lanosterol). The latter compound is most likely 24-methylene-dihydrolanosterol (15). The data were consistent with a mechanism of sterol biosynthesis inhibition involving blockage of the enzyme sterol 14-demethylase, a heme-containing cytochrome P450 enzyme.
Fig. 2.
Disubstituted imidazole compounds block the synthesis of sterols in T. cruzi cells and bind to recombinant T. cruzi sterol 14α-demethylase. (a) TLC analysis of 3H-sterols from T. cruzi epimastigotes grown with [3H]mevalonactone and no drug (lane 1), 25 μM ketoconazole (lane 2), or 25 μM compound 2 (lane 3). Sterol standards are shown in lane 4. (b) Difference spectra of recombinant T. cruzi sterol 14-demethylase (ferric state) mixed with increasing amounts of compound 1. The flat line represents no added compound, and the subsequent plots that show serially increasing difference spectra represent additions of 0.05, 0.10, 0.15, and 0.20 nmol of compound 1.
Disubstituted Imidazole Compounds Bind Recombinant T. cruzi Lanosterol 14-Demethylase. The T. cruzi sterol 14-demethylase gene was recently cloned by our group (16). We tested the disubstituted imidazoles for their ability to bind to recombinant T. cruzi sterol 14-demethylase. Spectroscopy studies of the Soret peak from the heme-iron demonstrated a type II spectrum that is characteristic of inhibitor binding to the P450 enzyme (Fig. 2b). The higher concentrations of inhibitor 1 produced higher values of absorbance difference Δ432–410. Similar observations were made with compounds 2–5. The spectral shift suggests direct coordination of the unsubstituted imidazole nitrogen of the compounds to the heme iron. This is inferred from structural studies of the Mycobacterium tuberculosis CYP51 enzyme in complex with the triazole compound, fluconazole, that show binding of the nitroimidazole moiety of fluconazole to the heme iron of the enzyme (18).
Potent Activity of Compounds 4 and 5 in Murine Model of Chagas Disease. Compound 1 was highly active against cultured T. cruzi; however, the carboxyl-methyl ester group attached to the central aromatic ring was susceptible to hydrolysis by esterases in mouse serum (data not shown). We had established from other studies of PFT inhibitors that the carboxylic acid variants of the peptidomimetic compounds are too hydrophilic to efficiently pass through membranes (19). Therefore, we synthesized a series of compounds with varying groups at the R1 position, and we also explored variation of the R2 substituent to further improve potency (J.L., J.O., K.Y., F.B., R. Eastman, W.V., M.G., S.S., and A.H., unpublished work). These compounds were tested for in vitro activity against mammalian stage T. cruzi and compounds with ED50 values ≤50 nM were subsequently tested for pharmacokinetic properties in mice. Compound 3 (lacking a substituent at the R1 position) appeared to be poorly absorbed into the bloodstream after gastric lavage, with a comparatively low plasma concentration of 2.0 μM at 1 h after a dose of 50 mg/kg (≈100 μmol/kg). However, compounds 4 and 5 were determined to have comparatively high plasma levels of 8 and 16 μM, respectively, at 1 h after oral doses of 30 mg/kg (≈60 μmol/kg) and were found to have serum half-lives of ≈4 h.
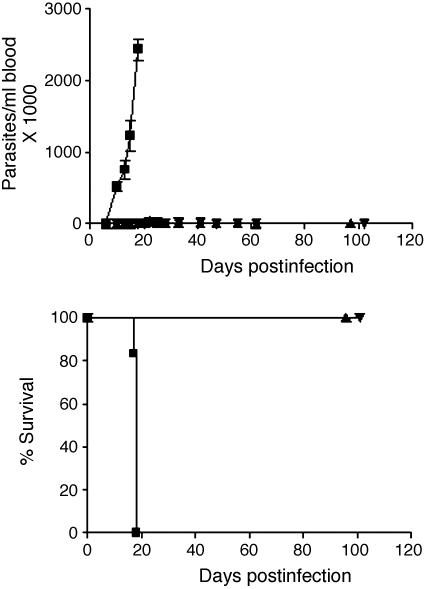
Experiments were performed with compounds 4 and 5 in the murine model of Chagas disease. Infected mice treated with these compounds for 14 days had maximum parasitemia levels of ≈1% of the maximum levels detected in the vehicle-treated mice (Fig. 3 Upper). It is possible that longer treatment courses would have resulted in parasitologic cure. All mice in the control group died from overwhelming infection by day 18 of the experiment, whereas all mice in the treatment groups survived to 101 days postinfection at which point they were killed (Fig. 3 Lower). The mice appeared completely healthy throughout the course of the experiment with no overt signs of toxicity from the compounds. The average weight of the treated mice was unchanged between days 0 and 15 of the experiment.
Fig. 3.
Reduced parasitemia and 100% survival in mice treated with compounds 4 or 5. BALB/c mice (six per group) were infected with T. cruzi on day 0 then dosed with vehicle or compounds from days 1 to 14. Mice received compounds at 50 mg/kg (≈100 μmol/kg) twice per day by oral gavage. (Upper) Parasitemia. (Lower) Survival. ▪, vehicle; ▴, compound 4; ▾, compound 5. (The experiment was also performed on a smaller scale with three mice per group, and similar results were observed.)
Concluding Remarks. The disubstituted imidazoles described here represent a structural class of compounds with potent anti-T. cruzi activity both in vitro and in an animal model of Chagas disease. The compounds are orally available and well tolerated in mice. It was fortuitous that our experiments testing for PFT inhibition in T. cruzi cells provided a clue to the mechanism of action of the compounds, as it is typically exceedingly difficult to delineate the mode of action of compounds that work by unknown mechanisms. Interestingly, other inhibitors of the sterol 14-demethylase enzyme, including the antifungal compounds, itraconazole, posaconazole, and ravuconazole, have also been shown to have anti-T. cruzi activity in murine models (20–22). Our data further establish the potential for exploiting this enzyme target and suggest that T. cruzi 14-demethylase may be susceptible to a variety of inhibitors in addition to the compounds optimized for antifungal activity. The inhibitors described here have significant potential advantages over many antifungal compounds in regard to significantly smaller size and structural complexity including the lack of chiral centers. These inhibitors are prepared by a relatively short synthetic route, thus minimizing the anticipated manufacturing costs. These compounds represent important leads toward an orally active, nontoxic, low-cost drug for the treatment of Chagas disease.
Acknowledgments
We thank Richard Eastman for technical assistance. This work was supported by National Institutes of Health Grants CA52874, AI48043, AI44199, and CA67771 and the University of Washington Keck Center for Microbial Pathogens.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: PFT, protein farnesyltransferase.
References
- 1.World Health Organization (2002) Control of Chagas Disease: Second Report of the Expert Committee (W.H.O., Geneva), W.H.O. Technical Report Series 905.
- 2.Buckner, F. S., Eastman, R., Speelmon, E., Nepomuceno-Silva, J. L., Myler P., Van Voorhis, W. C. & Yokoyama, K. (2002) Mol. Biochem. Parasitol. 122, 181-188. [DOI] [PubMed] [Google Scholar]
- 3.Buckner, F. S., Yokoyama, K., Nguyen, L. N., Grewal, A., Erdjument-Bromage, H., Tempst, P., Strickland, C. L., Xiao, L., Van Voorhis, W. C. & Gelb, M. H. (2000) J. Biol. Chem. 29, 21870-21876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haluska, P., Dy, G. K. & Adjei, A. A. (2002) Eur. J. Cancer 38, 1685-1700. [DOI] [PubMed] [Google Scholar]
- 5.Gelb, M. H., Buckner, F. S., Yokoyama, K., Ohkanda, J., Hamilton, A., Hguyen, L., Rossi-Bergmann, B., Sebti, S. M. & Van Voorhis, W. C. (2000) in Farnesyltransferase and Geranylgeranyltransferase-I: Targets for Cancer and Cardiovascular Therapy, ed. Sebti, S. M. (Humana, Totowa, NJ), pp. 221-232.
- 6.Karp, J. E., Kaufmann, S. H., Adjei, A. A., Lancet, J. E., Wright, J. J. & End, D. W. (2001) Curr. Opin. Oncol. 13, 470-476. [DOI] [PubMed] [Google Scholar]
- 7.Ohkanda, J., Lockman, J. W., Yokoyama, K., Gelb, M. H., Croft, S. L., Kendrick, H., Harrell, M. I., Feagin, J. E., Blaskovich, M. A., Sebti, S. M., et al. (2001) Bioorg. Med. Chem. Lett. 11, 761-764. [DOI] [PubMed] [Google Scholar]
- 8.Van Voorhis, W. C. & Eisen, H. (1989) J. Exp. Med. 169, 641-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner, F. S., Verlinde, C. L. M. J., La Flamme, A. C. & Van Voorhis, W. C. (1996) Antimicrob. Agents Chemother. 40, 2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama, K., Trobridge, P., Buckner, F. S., Van Voorhis, W. C., Stuart, K. D. & Gelb, M. H. (1998) J. Biol. Chem. 273, 26497-26505. [DOI] [PubMed] [Google Scholar]
- 11.Pompliano, D. L., Schaber, M. D., Mosser, S. D., Omer, C. A., Shafer, J. A. & Gibbs, J. B. (1993) Biochem. 32, 8341-8347. [DOI] [PubMed] [Google Scholar]
- 12.McGeady, P., Kuroda, S., Shimizu, K., Takai, Y. & Gelb, M. H. (1995) J. Biol. Chem. 270, 26347-26351. [DOI] [PubMed] [Google Scholar]
- 13.Haughan, P. A., Chance, M. L. & Goad, L. J. (1992) Biochem. Pharmacol. 44, 2199-2206. [DOI] [PubMed] [Google Scholar]
- 14.White, T. C. (1997) Antimicrob. Agents Chemother. 41, 1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbina, J. A., Vivas, J., Visbal, G. & Contreras, L. M. (1995) Mol. Biochem. Parasitol. 73, 199-210. [DOI] [PubMed] [Google Scholar]
- 16.Buckner, F. S., Joubert, B. M., Boyle, S. M., Eastman, R. T., Verlinde, C. L. & Matsuda, S. P. T. (2003) Mol. Biochem. Parasitol. 132, 75-81. [DOI] [PubMed] [Google Scholar]
- 17.Goad, L. J., Berens, R. L., Marr, J. J., Beach, D. H. & Holz, G. G. (1989) Mol. Biochem. Parasitol. 32, 179-190. [DOI] [PubMed] [Google Scholar]
- 18.Podust, L. M., Poulos, T. L. & Waterman, M. R. (2001) Proc. Natl. Acad. Sci. USA 98, 3068-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, J., Blaskovich, M. A., Knowles, D., Qian, Y., Ohkanda, J., Bailey, R. D., Hamilton, A. D. & Sebti, S. M. (1999) Cancer Res. 59, 4919-4926. [PubMed] [Google Scholar]
- 20.McCabe, R. E., Remmington, J. S. & Araujo, F. G. (1986) Am. J. Trop. Med. Hyg. 35, 280-284. [DOI] [PubMed] [Google Scholar]
- 21.Urbina, J. A., Payares, G., Contreras, L. M., Liendo, A., Sanoja, C., Molina, J., Piras, M., Piras, R., Perez, N., Wincker, P., et al. (1998) Antimicrob. Agents Chemother. 42, 1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbina, J. A., Payares, G., Sanoja, C., Lira, R. & Romanha, A. J. (2003) Int. J. Antimicrob. Agents 21, 27-38. [DOI] [PubMed] [Google Scholar]
- 23.Ohkanda, J., Buckner, F. S., Lockman, J. L., Yokoyama, K., Carrico, D., Eastman, R., Luca-Fradley, K., Davies, W., Croft, S., Van Voorhis, W. C., et al. (2004) J. Med. Chem., in press. [DOI] [PubMed]