Abstract
AIM: To investigate the proteolytic contribution of tumor-associated macrophages (TAM) in tumor invasion, we analyzed whether TAM at the invasive front of small HCC in Abcb4-/--mice show an enhanced expression of MMP-9.
METHODS: Liver cryosections of the hepatocellular carcinoma (HCC) invasive front from 12 mo old Abcb4-/--mice were stained for collagen type I and MMP-9 using Alexa488 and Alexa568 labeled secondary antibodies. Afterwards, the Alexa568 dye was bleached and the macrophage marker F4/80 was visualized using Alexa568 labeled secondary antibodies. Finally, photographs of the invasive tumor front were digitally overlaid and analyzed.
RESULTS: After complete bleaching of the primary dye, specific fluorescence staining of a third antigen, here F4/80, was successfully performed on the same histological section. With this method, we were able to identify conglomerates of matrix metalloproteinase (MMP-9) expressing macrophages within the tumor capsule of HCC.
CONCLUSION: MMP-9 expressing macrophages are involved in matrix remodelling at the invasive tumor front of HCC. The described staining protocol provides a simple yet powerful extension of conventional immuno-histochemistry, facilitating visualization of at least three different antigens plus nuclei in one single histological section.
Keywords: Fluorescence staining, Hepatocellular carcinoma, Matrix metalloproteinase, Tumor associated macrophages
INTRODUCTION
Abcb4 knockout mice (formerly called Mdr2-knockout mice) lack the liver-specific P-glycoprotein responsible for phosphatidylcholine transport across the canalicular membrane. The absence of phospholipids from biliary fluid in Abcb4-/--mice results in bile regurgitation and portal inflammation followed by development of dysplasia and hepatocellular carcinoma (HCC)[1,2]. Therefore, Abcb4-/--mice represent an ideal animal model mimicking cholangitis-associated carcinogenesis[1,2].
The initial step in carcinogenesis (i.e. invasive and metastatic cell behavior) is the proteolytic destruction of the extracellular matrix (ECM), including the basement membrane. Numerous studies have demonstrated the pivotal role of matrix metalloproteinases (MMPs) for tumor associated ECM degradation[3-5]. In particular, the gelatinases MMP-2 and MMP-9 have gained considerable attention: in various studies on tumor invasion and metastasis. MMP-2 and MMP-9 were shown to be able to cleave collagen type IV, the main component of the basement membrane[6-8].
Previous studies demonstrated abundant expression of MMP-9 in tumor cells of HCC[6,7]. Furthermore, MMP-9 expression was associated with growth and invasiveness of HCC[6,9,10]. Apart from the malignant cells themselves, growing attention is being paid to the tumor microenvironment as a mediator of invasive and metastatic behaviour[11,12]. Large quantities of tumor-associated macrophages (TAM) are associated with poor prognosis in various types of cancer, thereby suggesting relevance of these cells for tumor progression[13]. A number of studies were able to prove the expression of a broad range of tumor promoting factors including MMP-9 by which TAM may drive tumor angiogenesis[12,14,15].
Against this background, our aim was to analyze the expression patterns and cellular sources of MMP-9 within the tumor microenvironment of HCCs related to TAM in a mouse model of hepatocarcinogenesis. We developed a modification of the well-known and traditionally conducted immunohistochemistry. This allows the parallel visualization of at least three antigens within one histological section. The described approach may represent a powerful advancement in the analytical capabilities of immunohistochemistry.
MATERIALS AND METHODS
Animals and tissue preparation
FVB/N-Abcb4tm1bor gene-targeted mice were crossed back towards the fibrosis-susceptible BALB/cJ strain for ten generations as characterized recently[16,17]. Mice were killed at the age of twelve months and tissue samples of HCCs were fixed in 1% neutral buffered formalin for 16 h and afterwards embedded in Tissue-Tek (Sakura, Zoeterwoude, Netherlands) for cryopreservation at -80°C. The present study was performed with permission of the State of Hessen, regional council Giessen, according to section 8 of the German Law for the Protection of Animals and conforms to the Guide for the Care and Use of Laboratory Animals (Az: V54-19c20/15cGI20/10).
Immunofluorescence
3 µm frozen sections were blocked for 30 min with 5% bovine serum albumin, 2% goat serum (Biomeda, Foster City, USA) and 0.1% cold fish skin gelatine (Sigma-Aldrich) in PBS with 0.1% Triton (Roth, Karlsruhe, Germany) and 0.05% Tween 20 (Serva, Heidelberg, Germany). Sections were immunostained with goat anti-mouse MMP-9 antibodies, Alexa 568-conjugated donkey anti-goat IgG and afterwards with rabbit anti-mouse collagen type I antibodies and Alexa 488-conjugated goat anti-rabbit IgG. DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, Sigma) was used for nucleus staining. The stained probes were covered with Dako Cytomation mounting medium (Glostrup, Denmark) and glass cover slips for microscopic analysis. Fluorescence images of the tumor invasive front were obtained under a fluorescence microscope (Leica DMRB, Wetzlar, Germany; camera: Nikon Coolpix 5400, Düsseldorf, Germany). Afterwards, the Alexa568 dye was completely bleached by illumination with green light under the microscope for 2 h. Immunostaining of macrophage antigens was performed with rat anti-F4/80 primary and goat anti-rat Alexa568 secondary antibodies. The carefully adjusted selection of antibodies derived from different host-species was crucial to avoid unintended cross-stainings. Finally, photomicrographs of the invasive tumor front were taken and analyzed digitally using Photoshop software version 9.0.2. (Adobe, München, Germany).
Primary antibodies were diluted 1:50 and secondary antibodies 1:1000 prior to use. Specificity of immunohistological stainings was verified by the use of isotype control-IgGs instead of primary antibodies. MMP-9 IgGs were purchased from R&D-Systems (#AF909, Wiesbaden, Germany), collagen type I IgGs from Biodesign (#T40777R, Freiburg, Germany), and F4/80 IgGs from Dianova (#T-2006, Hamburg, Germany). Alexa 468- and Alexa 488-conjugated secondary antibodies were purchased from Molecular Probes (Eugene, USA).
RESULTS
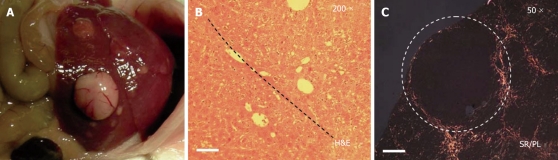
Within one year all BALB/c-Abcb4-/--mice (n = 17) had developed an HCC. On average 7 ± 4 tumors were detected per mouse, with a mean tumor size of 4.5 ± 2.5 mm. Figure 1 illustrates the macroscopic and histological aspect of HCC in Abcb4-/--mice.
Figure 1.
Macroscopic and histological aspect of HCC in BALB/c-Abcb4-/--mice. A: Representative macroscopic view of BALB/c-Abcb4-/--mouse liver at one year of age. Typically, one prominent tumor and a number of smaller tumors are visible; B: Hematoxylin and Eosin (H&E) stained liver section of the invasive tumor front (tumor on the upper right, fibrotic parenchyma on the lower left side, dashed line: HCC tumor capsule, bar represents 100 μm); C: Fifty-fold enlarged Sirius red (SR) stained liver section photographed under polarized light (PL, bar represents 400 μm). Fibrillar collagens appear in red. The tumor capsule is rich in collagen, whereas tumor stroma appears collagen-free.
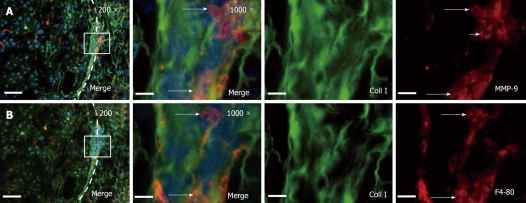
Following the modifications of conventional immunohistochemistry as described above, we successfully identified three antigens (MMP-9, collagen I and F4/80) plus nuclei within the same histological section (Figure 2).
Figure 2.
Conglomerates of tumor associated macrophages express MMP-9 within the HCC tumor capsule. A: Immunofluorescence co-staining of collagen type I (green) and MMP-9 (red) displayed clusters of MMP-9 expressing cells in the tumor capsule. B: After photobleaching of the red fluorescence dye, macrophage marker F4/80 was visualized. The long arrows indicate MMP-9 positive macrophages. The shot arrow indicates an MMP-9 expressing cell which is negative for F4/80. DAPI was used for nucleus staining. The left panels provide a section overview with 200-fold magnification and bars represent 100 μm (left side of the panel: Tumor; dashed line: Tumor capsule; box: This is enlarged to 1000 × magnification in the corresponding panel, bars represent 20 μm). Original green and red channel micrographs (1000 ×) were shown. Images were derived by conventional wide field fluorescence microscopy. Representative digital overlays are shown.
Immunofluorescence co-staining of collagen type I and MMP-9 visualized conglomerates of MMP-9 expressing cells within the tumor capsule of HCC. After photobleaching of the red fluorescent dye, staining with macrophage marker F4/80 identified macrophages to be the main source of MMP-9 expressing cells at the invasive tumor front. Representative results of the modified immunohistochemistry are shown in Figure 2.
DISCUSSION
Previous studies reported an enhanced expression of MMP-9 in HCC by the tumor cells themselves[6,7]. Furthermore, MMP-9 expression was associated with growth and invasiveness of HCC[6,9,10]. However, growing evidence exists that, apart from malignant cells, the tumor environment may play a pivotal role with regard to invasive and metastatic cell behaviour[11,12]. Accordingly, a number of in vitro and in vivo studies demonstrated that cancer cells were able to induce MMP expression in noncancerous cells thereby eventually promoting tumor progression[8,18-20]. Representing a part of the tumor microenvironment, TAM have been shown to be essential for both tumor growth and metastasis[21-23]. A recent study by Tsagozis and coworkers in prostate cancer revealed that the expression of MMP-9 by TAM is necessary to maintain their tumor promoting phenotype[24]. In murine studies on cervical cancer, Giraudo et al[14] showed that enhanced expression of MMP-9 contributed to cervical carcinogenesis by promoting tumor angiogenesis.
Herein, we analyzed the expression patterns and cellular sources of MMP-9 at the invasive front of HCC with respect to tumor associated macrophages by means of immunohistochemistry. Traditionally, double immunofluorescence staining with green and red fluorescent dyes allows minimal overlapping fluorescence emission spectra. The application of the blue fluorescence channel for antigen visualization in wide field fluorescence microscopy is not feasible yet, because of low fluorescence quantum yields of the correspondingly labeled secondary antibodies. Therefore, conventional immunohistochemistry was limited to the visualization of only two antigens plus nuclei within the same section.
To achieve within one histological section the combined illustration of the cellular sources of MMP-9 at the invasive front of HCC together with type I collagen representing the main component of fibrotic ECM the visualization of more than two antigens was essential .
We therefore developed a modification of conventional immunohistochemistry, facilitating the parallel visualization of three antigens using two fluorescent dyes. Essential for this approach is the complete wipeout of one dye (Alexa568) by green light. In contrast, green fluorescent Alexa488 dyes withstand the photobleaching without any loss of fluorescence intensity. Following the bleaching procedure, Alexa568 was re-used to tag the third antigen, the macrophage marker F4/80. Owing to the photo stability of Alexa488 towards green light, the defined region of interest was easily reproducible after the second staining.
Applying this approach, we have clearly visualized conglomerates of MMP-9 expressing macrophages within the capsule of HCCs. In accordance with data on TAM and MMP-9 expression for tumor invasion and angiogenesis in the literature, our results suggest an etiologic role for TAM and MMP-9 in the development of HCC[12,14,15,21-24].
However, whether MMP-9 expression in TAM within the HCC capsule leads to increased tumor invasiveness or eventually promotes angiogenesis remains unclear.
In summary, the staining procedure described herein has been shown to be a simple, feasible yet powerful advancement of the widely used technique of immunohistochemistry. This may be of the utmost interest for those lacking access to spectral imaging via confocal laser microscopy[25].
COMMENTS
Background
The initial step in carcinogenesis (i.e. invasive and metastatic cell behavior) is the proteolytic destruction of the extracellular matrix (ECM), including the basement membrane. Numerous reports were able to demonstrate the pivotal role of matrix-metalloproteinases (MMPs) for tumor associated ECM degradation. In particular, the gelatinases MMP-2 and MMP-9 have gained considerable attention: in various studies on tumor invasion and metastasis MMP-2 and MMP-9 were able to cleave collagen type IV, the main component of the basement membrane. Previous studies demonstrated abundant expression of MMP-9 in tumor cells of hepatocellular carcinoma (HCC). Furthermore, MMP-9 expression was associated with growth and invasiveness of HCC. Apart from the malignant cells themselves, growing attention is paid to the tumor microenvironment as a mediator of invasive and metastatic behaviour. Large quantities of tumor-associated macrophages (TAM) are related to poor prognosis in various entities of cancer, thereby suggesting relevance of these cells for tumor progression. A number of studies was able to prove the expression of a broad range of tumor promoting factors including MMP-9 by which TAM may drive tumor angiogenesis.
Research frontiers
It is unclear, whether MMP expression in TAM within the HCC may lead to increased tumor invasiveness or eventually promotes angiogenesis.
Innovations and breakthroughs
Representing a part of the tumor microenvironment, TAM have been shown to be essential for both, tumor growth and metastasis. A recent study by Tsagozis and coworkers in prostate cancer revealed that the expression of MMP-9 by TAM is necessary to maintain their tumor promoting phenotype. In murine studies on cervical cancer, Giraudo et al. showed that enhanced expression of MMP-9 contributed to cervical carcinogenesis by promoting tumor angiogenesis.
Applications
The staining procedure described herein proves to be a simple, feasible yet powerful advancement of the widely used technique of immunohistochemistry. This may be of utmost interest for those lacking access to spectral imaging via confocal laser microscopy. Applying this approach, we have clearly visualized conglomerates of MMP-9 expressing macrophages within the capsule of HCCs. In accordance with data on TAM and MMP-9 expression for tumor invasion and angiogenesis in the literature, our results suggest an etiologic role of TAM and MMP-9 for development of HCC.
Peer review
The rationale of this work is adequate. The methods employed clearly let the authors to reach the aims, and the results are appropriatle to sustain a discussion closer to the results showing well knowledge of the subject. This work is simple, direct, and interesting, and brings awareness to the subject.
Acknowledgments
The authors are grateful to Annette Tschuschner and Michaela Weiss for their technical support.
Footnotes
Supported by the Grants from the Deutsche Forschungsgemeinschaft (RO 957/8-1 and SFB/TRR 57) and by BMBF ZooMAP-TPC4; a Research Grant of the University Medical Center Giessen and Marburg (UKGM 10/2010 GI)
Peer reviewer: Juan Carlos Perazzo, MD, PhD, Professor, Department of Biological Sciences, Pathophysiology School of Pharmacy and Biochemistry, UBA, Junin 950, 5, Buenos Aires 1113, Argentina
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
References
- 1.Katzenellenbogen M, Pappo O, Barash H, Klopstock N, Mizrahi L, Olam D, Jacob-Hirsch J, Amariglio N, Rechavi G, Mitchell LA, et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 2.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 3.Classon M, Settleman J. Emerging concepts in tumor progression and therapy. Semin Cancer Biol. 2000;10:393–397. doi: 10.1006/scbi.2000.0338. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 5.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 6.Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, Mizumoto M, Fukumoto M, Imamura M. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316–322. doi: 10.1053/jhep.1996.v24.pm0008690399. [DOI] [PubMed] [Google Scholar]
- 7.Määttä M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726–2734. [PubMed] [Google Scholar]
- 8.Roeb E, Dietrich CG, Winograd R, Arndt M, Breuer B, Fass J, Schumpelick V, Matern S. Activity and cellular origin of gelatinases in patients with colon and rectal carcinoma differential activity of matrix metalloproteinase-9. Cancer. 2001;92:2680–2691. doi: 10.1002/1097-0142(20011115)92:10<2680::aid-cncr1622>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25:7009–7018. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Mafune K, Mori M, Shiraishi T, Imamura H, Mori M, Takayama T, Makuuchi M. Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. Int J Oncol. 2000;17:237–243. doi: 10.3892/ijo.17.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Marx J. Cancer biology. All in the stroma: cancer’s Cosa Nostra. Science. 2008;320:38–41. doi: 10.1126/science.320.5872.38. [DOI] [PubMed] [Google Scholar]
- 12.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 14.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 16.Henkel C, Roderfeld M, Weiskirchen R, Berres ML, Hillebrandt S, Lammert F, Meyer HE, Stühler K, Graf J, Roeb E. Changes of the hepatic proteome in murine models for toxically induced fibrogenesis and sclerosing cholangitis. Proteomics. 2006;6:6538–6548. doi: 10.1002/pmic.200600580. [DOI] [PubMed] [Google Scholar]
- 17.Hillebrandt S, Goos C, Matern S, Lammert F. Genome-wide analysis of hepatic fibrosis in inbred mice identifies the susceptibility locus Hfib1 on chromosome 15. Gastroenterology. 2002;123:2041–2051. doi: 10.1053/gast.2002.37069. [DOI] [PubMed] [Google Scholar]
- 18.Masson V, de la Ballina LR, Munaut C, Wielockx B, Jost M, Maillard C, Blacher S, Bajou K, Itoh T, Itohara S, et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–236. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mc Donnell S, Chaudhry V, Mansilla-Soto J, Zeng ZS, Shu WP, Guillem JG. Metastatic and non-metastatic colorectal cancer (CRC) cells induce host metalloproteinase production in vivo. Clin Exp Metastasis. 1999;17:341–349. doi: 10.1023/a:1006651019335. [DOI] [PubMed] [Google Scholar]
- 20.Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–829. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- 21.Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–482. doi: 10.1016/s1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsagozis P, Eriksson F, Pisa P. Zoledronic acid modulates antitumoral responses of prostate cancer-tumor associated macrophages. Cancer Immunol Immunother. 2008;57:1451–1459. doi: 10.1007/s00262-008-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roderfeld M, Matern S, Roeb E. [Confocal laser scanning microscopy: a deep look into the cell] Dtsch Med Wochenschr. 2003;128:2539–2542. doi: 10.1055/s-2003-44951. [DOI] [PubMed] [Google Scholar]