Abstract
Conventional ultrasound (US) is the first-line imaging investigation for biliary diseases. However, it is lack of the ability to depict the microcirculation of some lesions which may lead to failure in diagnosis for some biliary diseases. The use of contrast-enhanced US (CEUS) has reached the field of bile duct disease in recent years and promising results have been achieved. In this review, the methodology, image interpretation, enhancement pattern, clinical usefulness, and indications for CEUS in the biliary system are summarized. CEUS may be indicated in the biliary system under the following circumstances: (1) Where there is a need to make a characterization of intrahepatic cholangiocarcinoma (ICC); (2) For differentiation diagnosis between ICC and other tumors (i.e. hepatocellular carcinoma or liver metastasis) or infectious diseases; (3) For differentiation diagnosis between biliary cystadenoma and biliary cystadenocarcinoma; (4) To detect malignant change in Caroli’s disease; (5) To depict the extent of Klatskin’s tumor with greater clarity; (6) To make a distinction between gallbladder cholesterol polyp, adenoma and polypoid cancer; (7) To make a distinction between chronic cholecystitis with thickened wall and gallbladder cancer; (8) For differentiation diagnosis between motionless sludge and gallbladder cancer; (9) For differentiation diagnosis between common bile duct cancer and sludge or stone without acoustic shadowing; and (10) In patients who are suspected of having a drop of their percutaneous transhepatic cholangiodrainage tube, US contrast agent can be administered to through the tube detect the site of the tube.
Keywords: Contrast-enhanced ultrasound, Bile duct, Gallbladder, Cholangiocarcinoma, Polypoid lesion, Ultrasound contrast agent
INTRODUCTION
Conventional ultrasound (US) is the first-line imaging investigation for diagnosis of biliary diseases, and most biliary diseases are firstly detected by conventional US. The continuous improvement in imaging quality and the advent of new techniques such as power Doppler imaging, tissue harmonic imaging, endoscopic US, and three-dimensional US have further enriched the application of US in the biliary system[1-3]. In recent years, a novel technique of contrast-enhanced US (CEUS) has been widely used in various applications and has been accepted in clinical practice. Regarding the abdominal organs, CEUS has been used in the liver, kidney, pancreas, and spleen[4-8]. However, in the ‘Guidelines and Good Clinical Practice Recommendations for CEUS - update 2008’ which was recently issued, the application of CEUS in the biliary system was not mentioned[9]. The use of CEUS has reached the field of bile duct disease in recent years and promising results have been achieved[10,11]. From 2004, we have carried out biliary CEUS examination in more than 400 patients. In this review, the methodology, image interpretation, enhancement pattern, clinical usefulness, and indications for CEUS in the biliary system are summarized.
CONTRAST AGENT
Ultrasound contrast agent (UCA) is a microbubble-based substance. The diameter of the microbubble is small enough to guarantee its passage through the pulmonary circulation and to reach various organs[12]. Currently, there are five commercially available UCAs that have been approved in clinic. They are Levovist® (air with galactose and palmitic acid as a surfactant; Schering, introduced in 1996), Optison® (octafluoropropane with an albumin shell; GE Healthcare, introduced in 1998), Luminity® (octafluoropropane perflutren with a lipid shell; Bristol-Myers Squibb, introduced in 2006), SonoVue® (sulfur hexafluoride with a phospholipid shell; Bracco, introduced in 2001), Sonazoid® (perfluorobutane with a phosphatidylserine shell; GE Healthcare, introduced in 2007). The diameters of these are approximately 2-8 μm, smaller than that of red cells in the blood. Levovist® works under high acoustic power and is only suitable for intermittent imaging, and is seldom used currently. Optison® and Luminity® are only licensed for cardiac use. Sonazoid® is only approved in Japan. The only licensed UCA in China is SonoVue®, which is also available in Europe[9].
UCA is administrated intravenously and is confined to the intravascular space, which is different from the contrast media for contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance imaging, where the contrast media is rapidly cleared from the blood pool into the extracellular space[9].
In addition to intravenous use, UCA intracavity applications can be performed in the biliary system. In patients who have undergone percutaneous transhepatic cholangiodrainage (PTCD), UCA can be administered through the drainage tube to visualize the bile duct tree and to locate the position of the tube[13].
IMAGING TECHNIQUE
When the intravenous UCAs are exposed to US, they strongly increase the US backscatter and therefore are useful in the enhancement of echogenicity for the assessment of blood flow, which enables the display of parenchymal microvasculature.
Real-time gray-scale CEUS examination is available with the combinations of UCAs and low acoustic power contrast-specific imaging (CSI) techniques. Under low acoustic power, which is expressed as mechanical index (MI) and is often lower than 0.2, most of the UCAs in the circulation will remain intact and will interact with the acoustic wave. In contrast, under high acoustic power, the microbubbles will be destroyed. Low MI techniques furthermore lead to effective tissue signal suppression, as the non-linear response from the tissue is minimal when low acoustic pressures are used. By using low MI CSI technique, CEUS enables effective investigations over several minutes with the visualization of the dynamic enhancement pattern in real time.
SCANNING METHOD
For all CEUS examinations, baseline US investigations are firstly performed. After identification of the target lesion, the transducer is kept in a stable position and the imaging mode is changed to low MI CSI. The tissue signals are eliminated and the depth, gain and focus are carefully adjusted. UCA is administered intravenously through the antecubital vein as a bolus (within 1-2 s), followed by a flush of 5 mL of normal saline. A stop clock should be started at time of UCA injection.
A simultaneous display of tissue and contrast signals has been implemented in many CSI modes. This modality is recommended in most cases, as it can ensure that the target lesion is kept within the scanning field during CEUS.
To continuously assess the vascular enhancement change, continuous scanning for 60-90 s is recommended in each case. In addition, in the late phase, the scanning may be used intermittently until the disappearance of the UCA from the region of interest (ROI). In patients suspected of malignancy, thorough liver scanning in the late phase is necessary to exclude liver metastasis.
INTRAHEPATIC BILIARY SYSTEM
The intrahepatic bile duct is hard to visualize under normal circumstances, and most lesions of the intrahepatic bile duct are difficult to demarcate from the adjacent parenchyma. Therefore, the entire CEUS process for the intrahepatic biliary system is performed with reference to that for liver, i.e. including arterial (8-30 s from the beginning of contrast agent administration), portal (31-120 s), and late (121-360 s) phases[9]. Also, the enhancement extent is compared with the adjacent liver parenchyma.
Intrahepatic cholangiocarcinoma (ICC)
ICC is a malignant epithelial tumor that originates at the second branch (segmental branch) or the proximal branch of the intrahepatic bile ducts and is the second most common primary malignant tumor in the liver. ICC often appears as a large mass because the tumor does not cause clinical symptoms at its early stage. The US features of peripheral cholangiocarcinomas are nonspecific. Most of them show a mass-forming lesion as a hypoechoic mass, and satellite nodules can be seen. Other echo patterns such as isoechoic or hyper-echoic and inhomogeneous masses can also be visualized. Sonographically, it is difficult to differentiate peripheral cholangiocarcinoma from more common diseases such as metastatic liver cancer and hepatocellular carcinoma (HCC) other than the finding that peripheral cholangiocarcinoma is more likely to be associated with peripheral bile duct dilatation.
Xu et al[14] summarized the CEUS findings of ICC and Chen et al [15] compared the enhancement patterns of ICC between CEUS and contrast-enhanced CT. These authors found that during the arterial phase four enhancement patterns were present on CEUS; these were (1) peripheral irregular rim-like hyper-enhancement (47.5%), (2) diffuse heterogeneous hyper-enhancement (22.5%), (3) diffuse heterogeneous hypo-enhancement (17.5%), and (4) diffuse homogeneous hyper-enhancement (12.5%) (Figure 1). In comparison with CT, the enhancement patterns of ICC on CEUS were consistent with those on CECT in the arterial phase, whereas in the portal phase ICC faded out more obviously on CEUS than on CECT. Chen et al[15] reported that CEUS made a correct diagnosis in 80.0% of ICCs before pathological examination and CECT made a correct diagnosis in 67.5%. CEUS therefore had the same accuracy as CECT in diagnosing ICCs, and so can be used as a new modality for the characterization of ICC. Although baseline US has low ability in differentiating ICC and HCC, Chen et al[16] found that CEUS greatly improved the diagnostic performance in this use.
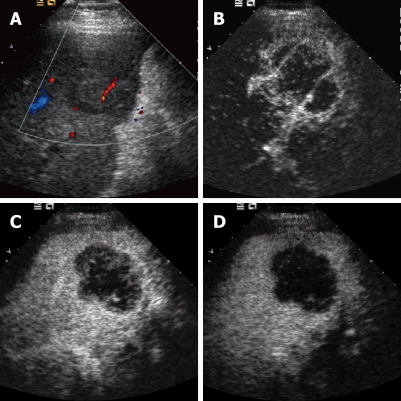
Figure 1.
Intrahepatic cholangiocarcinoma. A: Baseline ultrasound shows an isoechoic mass in segment 5 of the liver; B: The lesion shows peripheral rim-like hyper-enhancement 26 s after contrast agent injection on CEUS; C: The lesion becomes hypo-enhanced 52 s after contrast agent injection; D: The lesion continues to be hypo-enhanced 121 s after contrast agent injection.
The enhancement patterns of ICC correlate with tumor size. That is, most small ICCs (≤ 3 cm) are homogeneously enhancing, while those > 3 cm enhance heterogeneously or show a peripherally enhancing rim. This reflects the intratumoral hemodynamic changes in the course of tumor growth. When an ICC is small, it may be abundant in tumor cells with little fibrous tissue, which leads to homogeneous hypervascularity similar to that of HCC. When grown large, more fibrous tissue and central necrosis appear, and the ICC would show peripheral hypervascularity or overall hypovascularity. Consequently, it is difficult to distinguish small ICC from HCC on CEUS, especially in those with homogeneous hyper-enhancement. Under such circumstances, it is important to refer to the clinical background such as liver cirrhosis, blood test for viral hepatitis, and the tumor markers.
In portal and late phase on CEUS, most ICCs show up as hypo-enhancing, which is a prominent feature of malignancy on CEUS. However, on enhanced CT or MRI, most ICCs show delayed hyper-enhancement in the late phase. This phenomenon might be explained by the fact that the UCA is a real blood pool agent and thus it does not diffuse through the vascular endothelium into the interstitium. Conversely, the CT contrast agent can diffuse into the interstitial spaces of the tumor slowly from the intratumoral vessels, and clear up slowly owing to the abundant fibrous tissue and slow blood flow in ICCs; therefore, even delayed tumor enhancement is visualized.
Intrahepatic biliary cystadenoma and cystadenocarcinoma
Intrahepatic biliary cystadenoma is an uncommon multilocular cystic liver mass with malignant potential that usually arises from the epithelium of the intrahepatic bile duct, and which represents less than 5% of intrahepatic cystic masses of biliary origin. Conventional US always reveals a well-defined anechoic cystic mass with echogenic septations or papillary infoldings[6,17].
On CEUS, hyper-enhancement of the cystic wall, internal septations, or a papillary infolding during the arterial phase are seen. The enhancement washes out progressively and is depicted as iso- or hypo-enhancement during the portal and late phases[6,17].
Intrahepatic biliary cystadenocarcinoma is the malignant counterpart of cystadenoma. On CEUS, mural nodule-like hyper-enhancement, thick septa hyper-enhancement, enlarged solid portion hyper-enhancement and non-enhanced central area in the arterial phase are always seen. During portal and late phases, the hyper-enhanced areas wash out and show hypo-enhancement[6].
Biliary epithelial dysplasia of the intrahepatic bile duct
Biliary epithelial dysplasia of the intrahepatic bile duct is a premalignant lesion that arises from the epithelium of the intrahepatic bile duct. The imaging features of this entity have seldom been described. In our series, we found one lesion that was slightly hyperechoic and was within a dilated bile duct on baseline US. A peripheral dilated bile duct was visible. On CEUS, the lesion showed homogeneous hyper-enhancement during the arterial phase before liver parenchyma enhancement, which washed out continuously so that the lesion appeared as hypo-enhanced during the portal and late phases and was more conspicuous during those phases[17]. Sonographically, it was hard to differentiate it from its malignant counterpart, ICC.
Periductal inflammation
In patients with intrahepatic cholelithiasis, the continuous irritation of the stone on the wall of the bile duct will cause inflammation, and even lead to periductal inflammation. The patients always complain of fever and right upper quadrant pain. On baseline US, the periductal inflammatory lesion always shows hypoechogenicity around the intrahepatic bile duct. The lesion may be regular or irregular. On CEUS, hyper-enhancement during arterial phase and hypo-enhancement during portal or late phase are the most common findings for this entity. It is difficult to make a distinction between this and liver malignancy solely depending on the findings on US.
HILAR BILE DUCT
The entire CEUS process for the hilar bile duct is also performed with reference to that for liver, i.e. including arterial, portal, and late phases. In addition, the enhancement extent is compared with the adjacent liver parenchyma. Since the lesion in the hilar region is always small, the ROI can be zoomed to display the lesion and the relationship between it and the surrounding vessels more carefully. Simultaneous display of the contrast and tissue mode is recommended to avoid loss of the target lesion.
Klatskin tumor
The most common disease entity in the hilar bile duct is hilar cholangiocarcinoma (i.e. Klatskin tumor). Transabdominal US examination is the primary investigation for suspected Klatskin tumor, which is highly sensitive for confirming biliary duct dilatation, localizing the site of obstruction and excluding gallstones. However, it has a limited role in determining the nature of the obstruction and defining the extent of tumor involvement since the Klatskin tumor is always isoechoic to surrounding liver and the infiltrative nature of the lesion. The use of CEUS in evaluating Klatskin tumor has been investigated. In comparison with the adjacent liver parenchyma, the enhancement of the lesion was earlier (37.5%) or simultaneous (50.0%), and only 12.5% enhanced later. During the arterial phase, hyper-enhancement, iso-enhancement, and hypo-enhancement were visualized in 43.8%, 43.8%, and 12.4% of the lesions, respectively, on CEUS (Figure 2). As to enhancement pattern, peripheral rim-like hyper-enhancement, homogeneous enhancement, and inhomogeneous enhancement were illustrated in 9.4%, 34.4%, and 56.2% of the lesions, respectively. During the portal and late phases of CEUS, 93.8% lesions appeared as hypo-enhancement and the tumor margins became more conspicuous[18].
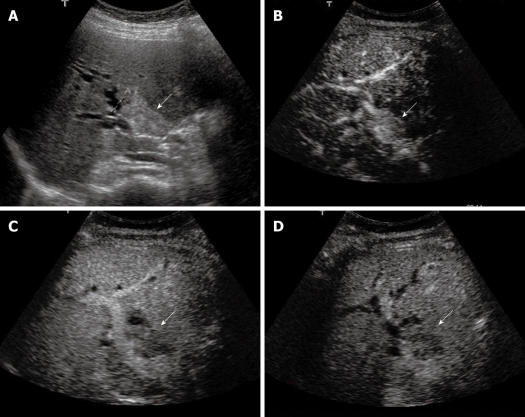
Figure 2.
Klatskin tumor. A: Baseline ultrasound shows a hyperechoic mass (arrow) in the hilar bile duct; B: The lesion (arrow) shows homogeneous hyper-enhancement 14 s after contrast agent injection on CEUS; C: The lesion (arrow) becomes hypo-enhanced 45 s after contrast agent injection; D: The lesion (arrow) continues to be hypo-enhanced 126 s after contrast agent injection.
Xu et al[18] compared the enhancement pattern of Klatskin tumor between CEUS and CECT. They found that the enhancement pattern of hilar cholangiocarcinoma on CEUS was similar to that on CECT in the arterial phase, whereas in portal phase hilar cholangiocarcinoma was more likely to show hypo-enhancement on CEUS. CEUS and CECT led to similar results when evaluating portal vein infiltration. CEUS achieved a correct diagnosis in 93.8% of cases whereas CECT was correct in 78.1% of cases. No significant difference was found between these two modalities in diagnosis of this entity. Therefore, CEUS has potential to be a tool for characterization of Klatskin tumor.
GALLBLADDER
The enhancement process of gallbladder lesions on CEUS is classified as early phase (10-30 s after contrast injection) and late phase (31-180 s after contrast injection) since the blood supply of the gallbladder is entirely arterial. The late phase persists for a short time in comparison with that for the liver[19].
Conventional grey-scale US is the first-line imaging investigation for diagnosis of gallbladder diseases, whereas it is insufficient to determine the nature of some complicated gallbladder diseases. Most of the gallbladder cancers present as either a solid mass that occupies the whole gallbladder or as a focal polypoid lesion on conventional US. However, some biliary sludge can generate similar US images and no movement is found[20]. Focal or diffuse thickening of the gallbladder wall is also a common, though nonspecific, US finding. Abnormal thickness can be attributed to diseases such as acute or chronic cholecystitis, benign or malignant tumor. The differentiation between these will become difficult, which is particularly true when the lesions fill the gallbladder or more than two types of lesions are present in gallbladder diseases. The insufficiency of conventional US is largely due to the fact that it has low ability in depicting vascularity in gallbladder diseases. CEUS can thus be applied to overcome the limitations of conventional US, since it can depict the hemodynamics in micro- and macro-circulation[21].
Gallbladder carcinoma
On conventional US, a solid mass that occupies the whole gallbladder, a sessile polypoid mass, focal or diffuse wall thickening, intralesional hypervascularity or infiltration to adjacent liver may be found. On CEUS, branch-like flow pattern or hyper-enhancement during the early phase is always recognized (Figure 3). The arterial branches supplying a gallbladder carcinoma tend to show irregularly tortuous extension. A lesion with tortuous-type tumor vessels on CEUS may be a clue for gallbladder carcinoma[10]. On the other hand, washout of the contrast agent within 35-60 s after contrast agent administration occurs in most gallbladder carcinomas, which seems to be a useful clue for differentiation between malignant and benign gallbladder diseases. Gallbladder carcinoma is usually at an advanced stage when it is firstly detected because it is asymptomatic at early stage. The infiltration to the gallbladder wall and the adjacent liver tissue is common. Accordingly, destruction of the gallbladder wall integrity is also a major sign of gallbladder carcinomas on CEUS[19].
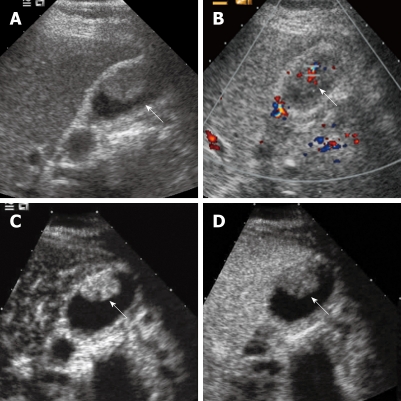
Figure 3.
Gallbladder carcinoma. A: Baseline ultrasound shows an isoechoic mass (arrow) in the gallbladder cavity; B: The lesion (arrow) shows hypervascularity on color Doppler flow imaging; C: The lesion (arrow) shows homogeneous hyper-enhancement 19 s after contrast agent injection on CEUS; D: The lesion (arrow) becomes hypo-enhanced 34 s after contrast agent injection.
Gallbladder adenomas
Gallbladder adenomas are relatively uncommon, with an incidence ranging from 0.3% to 0.5% in gallbladders removed by cholecystectomy. The lesions are polypoid, protrude into the gallbladder lumen, and have lobular or cauliflower-like surface. Ninety percent of gallbladder adenomas are single, and 60% are associated with cholecystolithiasis. Gallbladder adenoma appears on US as a sessile polypoid mass, a lesion of an echogenicity slightly greater or similar to the liver, with a smooth or lobulated surface and a relatively homogeneous internal texture, without infiltration to adjacent tissue. On CEUS, homogeneous hyper-enhancement (78%) and iso-enhancement (22%) during the early phase is found. In the late phase, iso- (56%) and hypo-enhancement (44%) are visualized[19].
Cholesterol polyp
Cholesterol polyp shows a pedicle polypoid lesion, a tiny echogenic spot or an aggregation of echogenic spots within the lesion, with no or scarce vascularity on conventional US. On CEUS, hyper-enhancement is found in the majority (93%) of the lesions and the remaining 7% show iso-enhancement during the early phase. In cholesterol polyp, almost normal-caliber arteries taper normally and subdivide normally into small vessels, and the lesion may show dotted- or branched-type tumor vessels on CEUS. In the late phase, hypo-enhancement is found in 64% and iso-enhancement in 36% of the lesions[19].
Inflammatory polyp
Inflammatory polyp is also a hypervascular lesion which shows hyper-enhancement in the early phase on CEUS. So far no data are available about its pattern in the late phase on CEUS.
Adenomyomatosis
Adenomyomatosis shows focal or diffuse thickened wall, multiple microcysts or comet tail artifact on conventional US. On CEUS, the lesions shows hyper- (20%) or iso-enhancement (80%) in the early phase and all the lesions show hypo-enhancement in the late phase[19].
Biliary sludge
Sometimes, motionless biliary sludge in the gallbladder mimics gallbladder polypoid lesion or cancer on transabdominal US. CEUS makes it very easy to differentiate biliary sludge from other polypoid gallbladder diseases on the basis of the finding of the absence of tumor enhancement. This means that evaluating the vascularity of the abnormality may be more useful in defining sludge than evaluating the lesion alterations induced by posture change.
Cholecystitis
Chronic cholecystitis shows thickened wall and no infiltration to adjacent tissue on conventional US and CEUS. When the gallbladder cavity is filled with gallstone or biliary sludge, it is always difficult to differentiate chronic cholecystitis with thickened gallbladder wall from gallbladder carcinoma with conventional US. The intactness of wall is the criterion to differentiate these two diseases. On CEUS, hyper- (86%), iso- (7%), and hypo-enhancement (7%) of the gallbladder wall is found in the early phase and all show hypo-enhancement in the late phase[19].
Baseline US can provide a definite diagnosis in most acute cholecystitis cases, while CEUS may enhance the diagnosis of the complications such as gallbladder perforation. It is easy to show the perforation site on the wall by CEUS, which is visualized as a non-enhancing area. Hypervascularization of the wall may be seen. The hepatic surface of the gallbladder is drained by vessels that communicate with adjacent hepatic veins. Acute inflammation of the gallbladder can extend to the adjacent liver parenchyma through this connection, resulting in contrast enhancement of liver parenchyma in acute cholecystitis.
CEUS may be able to help in differentiation between acute and chronic cholecystitis. Adamietz et al[22] found that in 16/20 cases with histologically proven acute cholecystitis, the gallbladder wall showed a strong enhancement. Low enhancement was found in 4/20 patients with acute, and in 6/8 patients with chronic cholecystitis. The gallbladder wall of 2/8 patients with chronic inflammation and all patients (30/30) of the control group showed no enhancement.
Differentiation between benign and malignant gallbladder diseases
The value of CEUS in the differential diagnosis of benign and malignant gallbladder diseases was investigated by Xie et al[19]. The extent of enhancement in the early phase did not achieve significant difference between benign and malignant gallbladder diseases. In the early phase at CEUS, hyper-, iso-, hypo-, and non-enhancement were found in 84.8%, 9.1%, 6.1% and 0% of gallbladder carcinomas, and 70.3%, 17.0%, 2.1%, and 10.6% of benign diseases, respectively. Pathologically, gallbladder carcinomas, adenomas, cholesterol polyps, and inflammatory polyps are supplied by arterial flow from branches of the cystic artery. It is therefore not surprising that both lesions show hyper-enhancement. On the other hand, the blood vessel distribution in the lesion may be helpful in differentiation, for benign lesions often show dotted vessel enhancement whereas malignant lesions often show tortuous vessel enhancement.
Hyper- or iso-enhancement in the early phase and then fading out to hypo-enhancement within 35-60 s after contrast agent administration was found in the majority of carcinomas and minority of benign lesions. Destruction of the gallbladder wall intactness was absent in benign diseases, whereas it was present in most carcinomas. It was suggested that the CEUS features of washout within 35-60 s after contrast agent administration and destruction of gallbladder wall intactness are highly suggestive of gallbladder malignancy. CEUS is particularly useful in differentiating gallbladder carcinomas and motionless biliary sludge or chronic cholecystitis with thickened gallbladder wall.
EXTRAHEPATIC BILIARY SYSTEM
Defining the enhancement process of the extrahepatic system is complicated. The blood supply of the extrahepatic bile duct is entirely arterial. It is recommended that the process is classified as early phase (10-30 s after contrast injection) and late phase (31-180 s after contrast injection). However, the enhancement extent of the upper extrahepatic bile duct can be compared with the liver and the lower portion can be compared with the pancreas so as to facilitate the image interpretation.
Villous adenoma
It is not easy to differentiate villous adenoma in the extrahepatic bile duct from biliary sludge since they have similar echogenicity on conventional US. We have reported a case of villous adenoma in the extrahepatic bile duct that was successfully diagnosed with CEUS before surgical resection[23]. On baseline US, the lesion appeared as a homogeneously isoechoic mass filling the bile duct from the confluence of the right and left hepatic ducts to the distal common bile duct. No intralesional flow signal was found on color Doppler imaging and power Doppler imaging. On CEUS, the mass showed homogeneous enhancement during the arterial phase, thus confirming the neoplastic nature of the lesion. The enhancement decreased gradually so that the mass became hypo-enhanced during the late phase.
Extrahepatic cholangiocarcinoma
The extrahepatic cholangiocarcinomas may show hyper-, iso-, or hypo-enhancement in the early phase of CEUS and most of them show hypo-enhancement in the late phase (Figure 4). The major role of CEUS in this regard may be in distinguishing between tumor and debris or stone without obvious acoustic shadowing.
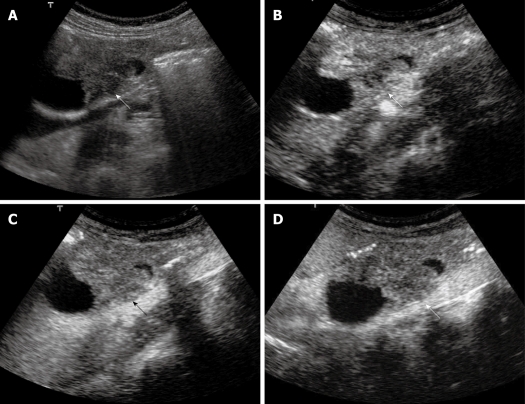
Figure 4.
Extrahepatic cholangiocarcinoma. A: Baseline ultrasound shows an isoechoic mass (arrow) in the lower portion of the common bile duct; B: The lesion (arrow) shows heterogeneous iso-enhancement 13 s after contrast agent injection on CEUS; C: The lesion (arrow) continues to be iso-enhanced 56 s after contrast agent injection; D: The lesion (arrow) becomes hypo-enhanced 107 s after contrast agent injection.
CONCLUSION
In addition to transabdominal CEUS, CEUS can also be performed intraoperatively. Intraoperative CEUS may provide more details about the biliary diseases; however, experience is limited[24-26]. In summary, the application of CEUS in the biliary system is under investigation and needs further validation. CEUS may be indicated under the following circumstances: (1) Where there is a need to make a characterization of ICC; (2) For differentiation diagnosis between ICC and other tumors (i.e. HCC or liver metastasis) or infectious diseases; (3) For differentiation diagnosis between biliary cystadenoma and biliary cystadenocarcinoma; (4) To detect any malignant change in Caroli’s disease; (5) To depict the extent of Klatskin’s tumor with great clarity; (6) To make a distinction between gallbladder cholesterol polyp, adenoma and polypoid cancer; (7) To make a distinction between chronic cholecystitis with thickened wall and gallbladder cancer; (8) For differentiation diagnosis between motionless sludge and gallbladder cancer; (9) For differentiation diagnosis between common bile duct cancer and sludge or stone without acoustic shadowing; and (10) In patients who are suspected of having a drop of their PTCD tube, UCA can be administered to through the tube detect the site of the tube.
Footnotes
Supported by (in part) Grant No. NCET-06-0723 from the Chinese Ministry of Education and grant 2008-2-10 of Public Welfare Research Special Project from the Chinese Ministry of Health
Peer reviewer: Herwig R Cerwenka, Professor, MD, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Wang JL L- Editor Logan S E- Editor Zheng XM
References
- 1.Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295–314; questionnaire, 549-555. doi: 10.1148/radiographics.21.2.g01mr16295. [DOI] [PubMed] [Google Scholar]
- 2.Hirooka Y, Naitoh Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hashimoto S, Hayakawa T. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest Endosc. 1998;48:406–410. doi: 10.1016/s0016-5107(98)70012-4. [DOI] [PubMed] [Google Scholar]
- 3.Xu HX, Yin XY, Lu MD, Liu L, Yue DC, Liu GJ. Comparison of three- and two-dimensional sonography in diagnosis of gallbladder diseases: preliminary experience. J Ultrasound Med. 2003;22:181–191. doi: 10.7863/jum.2003.22.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Leen E. The role of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Eur Radiol. 2001;11 Suppl 3:E27–E34. doi: 10.1007/pl00014128. [DOI] [PubMed] [Google Scholar]
- 5.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349–361. doi: 10.7863/jum.2006.25.3.349. [DOI] [PubMed] [Google Scholar]
- 6.Lin MX, Xu HX, Lu MD, Xie XY, Chen LD, Xu ZF, Liu GJ, Xie XH, Liang JY, Wang Z. Diagnostic performance of contrast-enhanced ultrasound for complex cystic focal liver lesions: blinded reader study. Eur Radiol. 2009;19:358–369. doi: 10.1007/s00330-008-1166-8. [DOI] [PubMed] [Google Scholar]
- 7.Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Liang JY, Lu MD. Renal cell carcinoma: real-time contrast-enhanced ultrasound findings. Abdom Imaging. 2009:Epub ahead of print. doi: 10.1007/s00261-009-9583-y. [DOI] [PubMed] [Google Scholar]
- 8.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261–272. doi: 10.1002/jcu.20234. [DOI] [PubMed] [Google Scholar]
- 9.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Kitano M, Kudo M, Sakamoto H, Kawasaki T, Yasuda C, Maekawa K. Diagnosis of gallbladder diseases by contrast-enhanced phase-inversion harmonic ultrasonography. Ultrasound Med Biol. 2007;33:353–361. doi: 10.1016/j.ultrasmedbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Numata K, Oka H, Morimoto M, Sugimori K, Kunisaki R, Nihonmatsu H, Matsuo K, Nagano Y, Nozawa A, Tanaka K. Differential diagnosis of gallbladder diseases with contrast-enhanced harmonic gray scale ultrasonography. J Ultrasound Med. 2007;26:763–774. doi: 10.7863/jum.2007.26.6.763. [DOI] [PubMed] [Google Scholar]
- 12.Correas JM, Bridal L, Lesavre A, Méjean A, Claudon M, Hélénon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11:1316–1328. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 13.Ignee A, Baum U, Schuessler G, Dietrich CF. Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD) Endoscopy. 2009;41:725–726. doi: 10.1055/s-0029-1214956. [DOI] [PubMed] [Google Scholar]
- 14.Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23–33. doi: 10.7863/jum.2006.25.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Chen LD, Xu HX, Xie XY, Lu MD, Xu ZF, Liu GJ, Liang JY, Lin MX. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881–889. doi: 10.1259/bjr/22318475. [DOI] [PubMed] [Google Scholar]
- 16.Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2009:Epub ahead of print. doi: 10.1007/s00330-009-1599-8. [DOI] [PubMed] [Google Scholar]
- 17.Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Liang JY, Chen LD. Unusual benign focal liver lesions: findings on real-time contrast-enhanced sonography. J Ultrasound Med. 2008;27:243–254. doi: 10.7863/jum.2008.27.2.243. [DOI] [PubMed] [Google Scholar]
- 18.Xu HX, Chen LD, Xie XY, Xie XH, Xu ZF, Liu GJ, Lin MX, Wang Z, Lu MD. Enhancement pattern of hilar cholangiocarcinoma: Contrast-enhanced ultrasound versus contrast-enhanced computed tomography. Eur J Radiol. 2009:Epub ahead of print. doi: 10.1016/j.ejrad.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Xie XH, Xu HX, Xie XY, Lu MD, Kuang M, Xu ZF, Liu GJ, Wang Z, Liang JY, Chen LD, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2009:Epub ahead of print. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 20.Anastasi B, Sutherland GR. Biliary sludge-ultrasonic appearance simulating neoplasm. Br J Radiol. 1981;54:679–681. doi: 10.1259/0007-1285-54-644-679. [DOI] [PubMed] [Google Scholar]
- 21.Hattori M, Inui K, Yoshino J, Miyoshi H, Okushima K, Nakamura Y, Naito T, Imaeda Y, Horibe Y, Hattori T, et al. [Usefulness of contrast-enhanced ultrasonography in the differential diagnosis of polypoid gallbladder lesions] Nippon Shokakibyo Gakkai Zasshi. 2007;104:790–798. [PubMed] [Google Scholar]
- 22.Adamietz B, Wenkel E, Uder M, Meyer T, Schneider I, Dimmler A, Bautz W, Janka R. Contrast enhanced sonography of the gallbladder: a tool in the diagnosis of cholecystitis? Eur J Radiol. 2007;61:262–266. doi: 10.1016/j.ejrad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Xu HX, Chen LD. Villous adenoma of extrahepatic bile duct: contrast-enhanced sonography findings. J Clin Ultrasound. 2008;36:39–41. doi: 10.1002/jcu.20361. [DOI] [PubMed] [Google Scholar]
- 24.Torzilli G, Del Fabbro D, Palmisano A, Donadon M, Montorsi M. Contrast-enhanced intraoperative ultrasonography: a valuable and not any more monocentric diagnostic technique performed in different ways. Ann Surg. 2007;245:152–153; author reply 152-153. doi: 10.1097/01.sla.0000250940.21627.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torzilli G, Del Fabbro D, Olivari N, Calliada F, Montorsi M, Makuuchi M. Contrast-enhanced ultrasonography during liver surgery. Br J Surg. 2004;91:1165–1167. doi: 10.1002/bjs.4628. [DOI] [PubMed] [Google Scholar]
- 26.Cerwenka H. Intraoperative ultrasonography during planned liver resections remains an important surgical tool. Surg Endosc. 2008;22:1137–1138. doi: 10.1007/s00464-008-9797-z. [DOI] [PubMed] [Google Scholar]