Abstract
Attrition and eventual loss of articular cartilage are important elements in the pathophysiology of osteoarthritis (OA). Preventing the breakdown of cartilage is believed to be critical to preserve the functional integrity of a joint. Chondral injuries are also common in the knee joint, and many patients benefit from cartilage repair. Magnetic resonance imaging (MRI) and advanced digital post-processing techniques have opened possibilities for in vivo analysis of cartilage morphology, structure, and function in healthy and diseased knee joints. Techniques of semi-quantitative scoring of human knee cartilage pathology and quantitative assessment of human cartilage have been developed. Cartilage thickness and volume have been quantified in humans as well as in small animals. MRI detected cartilage loss has been shown to be more sensitive than radiographs detecting joint space narrowing. It is possible to longitudinally study knee cartilage morphology with enough accuracy to follow the disease-caused changes and also evaluate the therapeutic effects of chondro-protective drugs. There are also several MRI methods that may allow evaluation of the glycosaminoglycan matrix or collagen network of articular cartilage, and may be more sensitive for the detection of early changes. The clinical relevance of these methods is being validated. With the development of new therapies for OA and cartilage injury, MR images will play an important role in the diagnosis, staging, and evaluation of the effectiveness of these therapies.
Keywords: Animal model, Cartilage, Osteoarthritis, Joint space narrowing, Knee, Magnetic resonance imaging, Radiography
INTRODUCTION
Osteoarthritis (OA) is viewed as the clinical and pathological outcome of a range of disorders that result in structural degradation and functional failure of synovial joints. OA occurs when the dynamic equilibrium between the breakdown and repair of joint tissues becomes unbalanced, often in a situation in which the mechanical loads applied exceed those that can be tolerated by the joint tissues. This progressive joint failure may cause pain and disability and is being ranked as the leading cause of disability in the elderly. Although articular cartilage lacks nerves, recent studies have shown that cartilage pathology is associated with clinical symptoms[1,2]. Currently, no well accepted medical treatment for OA with structure or disease modification efficacy exists. Attrition and eventual loss of articular cartilage are crucial elements in the pathophysiology of OA. Because of the avascular nature and small chondrocyte population in adults, the capacity of injured or degenerated cartilage to synthesize and secrete its extracellular matrix is poor. The healing response to cartilage injury and degeneration also decreases with age. Preventing the breakdown of cartilage is believed to be critical to preserve the functional integrity of a joint. Chondral injuries are common in the knee joint, and many patients benefit from cartilage repair. A number of promising therapeutic agents and surgical procedures are currently under development in this regard. In addition to investigating the pathophysiology of cartilage generation, there is a significant need for a non-invasive method of monitoring OA to judge the success of potential chondroregenerative and surgical treatments. Magnetic resonance imaging (MRI) offers a unique opportunity to characterize various pathologies of articular cartilage in vivo.
MRI detected cartilage loss has been shown to be more sensitive than radiographs detecting joint space narrowing (JSN). Amin et al[3] reported that cartilage loss was significantly associated with semi-quantitatively graded JSN of weight-bearing radiographs in the femoral-tibia joint, however, there was a substantial proportion of knees in which cartilage loss was detected with MRI but no radiographic JSN was observed. Raynauld et al[4] described no significant change in the medial femoral-tibia compartment of weight-bearing semiflexed radiographs positioned with fluoroscopy in 32 patients with OA over 2 years, but reported a highly significant change in cartilage volume from MRI both in the medial and lateral femoral-tibia compartment. The knee is the largest weight-bearing joint in the body and therefore most commonly affected by OA. Chondral injury is a frequent cause of pain and knee-function limitation. Cartilage repair surgery is a highly dynamic research field, and there is a pressing need for reliable and objective monitoring in order to evaluate and compare various surgical treatment options. This review discusses in vivo MRI methodology of the morphological assessment of knee cartilage, both in clinical studies and experimental settings. Novel techniques with potential of assessing macromolecular matrix of cartilage are also briefly discussed.
MR IMAGE ACQUISITION TECHNIQUES FOR HUMAN KNEE
MR images capable of resolving various structures of the knee joint require a good signal-to-noise ratio, good spatial resolution, and good tissue contrast. Given that knee cartilage is only 1.3-2.5 mm thick in healthy human subjects, and because of its complex morphology, knee cartilage presents a challenge in MRI[5-8]. The challenges are even greater in OA patients, as a decrease in signal and thinning make delineation of the articular cartilage more difficult.
MRI assessment of cartilage repair requires cartilage-sensitive sequences such as fat-suppressed (FS) 3D T1-weighted gradient echo (GE) sequences and proton-density and T2- or intermediate weighted fast spin echo (FSE) techniques. The pattern of joint structures as seen on MR images can be modified in various ways by the choice of MR pulse sequences. The MR sequences that have been most commonly used for cartilage assessment are FS T1-weighted spoiled GE sequences. FS is important for increasing the dynamic range between cartilage and adjacent structures and to eliminate chemical shift artifacts at the cartilage-bone interface. FS enhances the contrast for the cartilage and it has been reported that it can lead to better reproducibility for volumetric measurements[9]. GE sequences allow very short time of echo (TE), and this improves signal sensitivity when small structures are imaged. FS GE sequences with short TEs and relatively large flip angles provide T1-weighted images where the intra-articular fluid is less intense than the cartilage and fat is suppressed therefore maximizing the contrast between cartilage, fluid and marrow, with cartilage showing a bright signal. Because of the relatively short T2 relaxation times in articular cartilage, especially in deep cartilage adjacent to the bone interface, MR sequences for quantitative volume measurements should be used with TE as short as possible, preferably below 10 ms. A longer TE leads to cartilage signal decay. Eckstein et al[10] reported a significant underestimation of tibial cartilage thickness compared to CT arthrography, when using a GE sequence with a TE of 11 ms. It is particularly important to avoid this confounding effect of T2 relaxation on volume measurement in longitudinal studies of OA progression.
In general, 3D-GE sequences with FS allow the exact depiction of the thickness and surface of cartilage, whereas dual FSE sequences outline the normal and abnormal internal structure of hyaline cartilage. Cartilage demonstrates intermediate signals in intermediate - and T2-weighted sequences, whereas synovial fluid is bright and the internal structure of the cartilage displays a more heterogeneous signal and ‘internal’ pathological changes may be more readily displayed (Figure 1).
Figure 1.
Comparison of gradient echo (GE) sequence and fat-suppressed (FS) sequence in depicting intra-cartilage lesion. A: Sagittal fat saturated spoiled GE image of the knee in a patient with early OA and a cartilage fissure (arrow); B: Sagittal FS intermediate weighted image of the same patient with the fissure (arrow). Note the difference in contrast with bright cartilage signal in (A) and intermediate cartilage signal in (B). Joint effusion in (B) with bright signal improves visualization of the cartilage fissure (Reproduced by permission of John Wiley & Sons, Ltd from Reference 8).
Sagittal scan plane is commonly used for cartilage evaluation. Although there is no current consensus on the optimal resolution for imaging knees in OA, 1.5 mm section thickness and 0.3 mm in-plane resolution has been commonly used, as these allow total coverage of the knee with imaging times of 10-12 min. Rubenstein et al[11] demonstrated that a voxel size under 300 μm is required to reveal fraying of the articular surface of cartilage. In a systematic comparison of images with different in-plane resolutions, Hardya et al[12] reported significantly larger precision errors for cartilage volume measurements derived from the lower-resolution (0.55 mm × 0.55 mm) images than for those from the higher-resolution (0.28 mm × 0.28 mm) images in the femur and tibia. Wluka et al[13] and Cicuttini et al[14] measured the rate of progression in tibial cartilage using sagittal images and also using reformatted coronal images. They reported a higher rate of progression of tibial cartilage loss in the reformatted coronal vs original sagittal images. These findings indicate that changes may be more readily detected in coronal views of the knee. Which protocol (sagittal or a combination of axial and coronal scans) is preferable remains to be confirmed by future studies.
For human studies, MR images should be acquired within reasonable examination times (< 20 min per pulse sequence), in order to avoid movement artifacts, maintain patient comfort, and contain costs. Recently developed high-resolution 3D isotropic cartilage-sensitive sequences at 3 Tesla will further improve the assessment of quantitative morphologic aspects of volumetric cartilage[15]. New coil technologies with multi-element design allow the use of parallel imaging, which can additionally decrease the scan time. Higher magnetic field could potentially increase the quality of cartilage images, while scan times can be kept well below 10 min. At 3.0T, it was found that the signal to noise ratio and contrast to noise ratio efficiency for cartilage increased by a factor of 1.8 vs 1.5T for spoiled GE sequences. Cartilage volume and thickness measurements at 3.0T showed only small (non-significant) differences as compared with measurements at 1.5T[16,17]. Using a porcine model of artificial cartilage lesions, it was reported that the highest lesion detection rate was found with an intermediate-weighted FSE sequence at 3.0T (90% vs 62% at 1.5T), whereas the lesion grade was most accurately evaluated with spoiled GE sequences at 3.0T (83% vs 70% at 1.5T). Receiver operator characteristics analyses in the same model confirmed improved diagnostic performance in detecting cartilage lesions at 3.0T if high-resolution imaging protocols (slice thickness ≤ 2 mm and in-plane resolution ≤ 0.39 mm) were used[18,19]. Low-field systems should not be used for cartilage imaging as previous studies have shown that low-field MR scanners operating at field strengths of 0.18-0.20T have substantial limitations compared to high-field systems (1.5T) in visualizing cartilage pathology[20]. Further studies to improve both spatial and contrast resolution using novelly designed sequences are still ongoing[20,21].
Studies have shown that knee bends and squatting can cause a reduction of approx. Five percent in patellar cartilage volume and thickness and this effect can last for approx. 90 min[6]. To avoid differences in subject conditions due to differences in levels of physical activity prior to imaging, for cartilage volume and thickness measurements, study subjects need to rest for 1 h prior to image acquisition.
MR IMAGE ACQUISITION TECHNIQUES FOR ANIMAL KNEE
A number of animal models have been devised to investigate the pathogenesis of OA and cartilage trauma. It is a great advantage for research that the time course of OA in animal models is much more rapid than the development of OA pathology in humans. The knee is the mostly used joint for OA induction. MRI has already been applied to investigate a variety of OA animal models, including mouse, rat, guinea pig, rabbit, monkey, goat and dog[5]. The high resolution requirement for small animal knee MRI demands high performance of MR instruments. For imaging of large animals like dog and goat, clinical human scanners are commonly used, mostly together with a RF coil designed for human knees or wrists. For signal optimization, suitable RF coils for animal knee can be custom-made and interfaced to clinical human scanners.
High field research MR scanners, usually with a magnetic field of 4.7T or 7T, tend to be equipped with small bores which can hold up to the size of rabbits or rats. The RF coils are usually home-made, or made by some small specialist companies. The most commonly used is single-turn solenoid RF coils[8]. They are designed to open at the top. Animals can be placed on a Perspex platform with one hind leg extending through the RF coil, with the knee centered in it. While designed to completely cover the knee joint of the animal species imaged, the length and diameter of the coil are optimized to minimize the image field of view so as to obtain a good filling factor. To prevent motion, the animal’s paw on the leg being scanned can be secured to a secondary lower platform. With clinical scanners, RF coils suitable for imaging human fingers can be used for imaging rat knee, although quantification of cartilage thickness or volume using this set-up remains challenging.
Similar MR sequences for human studies are used for animal studies. With high field MR scanners it is feasible to obtain 3D data sets of less than 100 micro meter resolution with scanning duration less than 1 h[22,23]. In the study by Tessier et al[24], MR image acquisition protocol for guinea pig knee cartilage evaluation was detailed. A 4.7T magnet and an FS 3D-GE sequence were used. The length and diameter of the solenoid RF coil used were optimized to minimize the image field of view (30 mm × 30 mm × 30 mm). A transverse image of the knee was used to select the orientation of the sagittal view of the 3D images such that they were parallel to the medial condyle. The image matrix was zero-filled to 512 × 256 × 128 after 3D Fourier transform and an apparent image resolution of 59 mm × 117 mm × 234 mm was achieved. The highest resolution (59 mm) was chosen across the cartilage thickness (approx 330 mm). For assessment of rat knee joint, Wang et al[22] used a 4.7T magnet; the RF coil was an in-house built double-balanced matched 3 cm diameter copper sheet solenoid, and was 1 cm in length. 3D data set at the sagittal plane was acquired using a spoiled multi-echo FS 3D-GE (TR = 75 ms, flip angle = 30°, 5 echoes TE1 = 2.8 ms, TE2 = 6.0 ms, TE3 = 9.2 ms, TE4 = 12.5 ms, TE5 = 15.7 ms). Echo summation provided a means of enhancing SNR and enabled acquisition of a high resolution 3D image of the rat knee in approximately 50 min. The images covered the entire knee joint with a resolution of 59 mm × 117 mm × 234 mm.
TECHNIQUES FOR ASSESSING CARTILAGE COMPOSITION
During the early stages of OA, articular cartilage constituents may degenerate before any substantial morphological changes occur. In recent years, a significant amount of research has been directed toward the development of techniques for assessing the loss of the macromolecular matrix of articular cartilage in the absence of macroscopic lesions. One of the main motivations behind this work has been the ongoing development of drugs designed to slow or reverse the development of OA at this early stage. During disease progression, changes in the tissue MR relaxation values [T1, T2, and T1 rho (or, T1 in the rotating frame)], diffusion-coefficient, magnetic transfer, sodium MRI and ultra-short TE imaging, and delayed gadolinium enhanced MRI of cartilage (dGEMRIC) may reflect early alterations in the tissue architecture and biochemical composition[5,25-27]. Among them, the most promising three techniques include (1) dGEMRIC; (2) T1 rho (spin-lock) imaging, and (3) T2 maps[20,21,25,28].
dGEMRIC works by allowing negatively charged gadolinium-diethylenetriamine pentaacetic acid (DTPA)-2 to distribute in cartilage in inverse proportion to the negatively charged glycosaminoglycans. One of the most common MR contrast agents, gadopentetate dimeglumine (Gd-DTPA-2; Magnevist®, Schering, Berlin, Germany), has a negative charge and will therefore show a lower concentration in cartilage areas of high glycosaminoglycan concentrations following penetration via diffusion. Double-dose Gd-DTPA-2 is injected intravenously, and a delay of 90 min is used to allow the contrast material to diffuse into the cartilage. A map of the T1s in the cartilage can be computed, which reflects the underlying glycosaminoglycan content. Areas of glycosaminoglycan depletion can be seen in diseased cartilage. T1 rho (spin-lock) imaging employs a long, low-power, RF pulse at the resonant frequency that follows a 90° pulse and that is applied along the axis of the magnetization vector in the transverse plane. This pulse serves to “lock” the spins along the pulse, reducing the T2 decay that would normally cause the magnetization to dephase in the plane. Despite the reduction of T2 decay, there is still a loss of magnitude of the magnetization that is characterized by the time constant T1 rho (i.e. “T1 in the rotating frame”). This sequence is sensitive to the loss of proteoglycans, which is detected as an increase in T1 rho. The actual calculation of the spatial distribution of T1 rho values (maps) requires that the sequence be repeated several times while systematically varying the time length that the spin-lock pulse is applied. The T2 maps of articular cartilage are a function of the water content of the tissue. Measurement of the spatial distribution of the T2 may reveal areas of increased or decreased water content, which correlate with cartilage damage. Focal increases in T2 within cartilage have been associated with matrix damage, particularly loss of the collagen matrix.
Intuitively, compositional measures may have a significant role to play in examining changes that occur in early disease before gross defects are apparent, whereas morphologic measures - both semiquantitative and quantitative - may have a greater role in the later stages of disease. Overall, these cartilage composition imaging techniques require dedicated staff and careful attention to the MRI parameters and timings. The major drawback of the dGEMRIC technique is the long period of time, between the time of contrast agent injection and the commencement of imaging, needed to allow diffusion of the contrast into the cartilage. A lesser problem is the relatively long acquisition time needed to acquire sufficient data to create the T1 maps using a series of inversion-recovery sequences, during which the subject must remain motionless. The one limitation of T1 rho technique is that it is not commonly available on commercial MR systems. Custom software is also necessary to reconstruct and view the T1 rho maps. With T2 maps, correlation between T2 times and the changes in the macromolecular matrix is less certain. Further research is needed to show how clinically feasible these new biomarkers are as measures of early cartilage degeneration. Which of these techniques alone or perhaps in combination will prove most useful remains to be determined. Particularly for supposedly ‘early’ changes of OA, however, the natural course of these changes and the relationship with clinical outcome remain to be established. Additional research and validation is needed to guide interpretation of the results of these techniques.
MORPHOLOGICAL EVALUATION OF HUMAN KNEE CARTILAGE
Conventional radiography is the least expensive method for imaging joint structure. The progression of knee OA has been assessed by measuring changes in the width of the space between the medial femoral condyle and medial tibial plateau on plain radiographs, as the medial femorotibial compartment is the most common site of involvement in knee OA. A reduction in cartilage thickness is inferred from a reduction in this space. Observational studies show a weak relationship between radiographic structural change, pain, and function in OA[29]. Measurement errors related to the variability in knee positioning required considerable effort for the standardization of radiographic protocols, including the use of fluoroscopy. Progression in JSN also reflects OA changes in joint tissues other than articular cartilage, particularly extrusion and degenerative changes of the menisci. Data also suggest that knee pain itself can modify the appearance of joint space width in weight-bearing extended view radiographs[30].
Knee cartilage morphological evaluation with MRI includes qualitative assessment of articular cartilage pathology, semi-quantitative scoring of articular cartilage pathology, and quantitative assessment of articular cartilage volume and thickness. It has been reported that MRI based volume measurement of knee cartilage can demonstrate change undetectable with radiographs[4]. Using MRI assessment as the gold standard for cartilage loss, the specificity of radiography in detecting cartilage loss in the medial compartment was 91%; however, the sensitivity was only 23%[31]. MRI also has important applications in the study of cartilage repair. Specifically, MRI may (1) help to estimate the size, nature, and location of lesions preoperatively, in order to optimize surgical planning; (2) help to evaluate the quality and success of tissue repair processes after surgical treatment; and (3) allow one to monitor changes in the joint after cartilage repair. An excellent review on MRI monitoring of surgical repair of cartilage has been reported elsewhere[32].
Early degenerative disease may be seen on MRI as early alterations in cartilage contour morphology (fibrillation, surface irregularity); changes in cartilage thickness, including cartilage thinning or thickening, which may be an early feature predating cartilage volume loss; or intrachondral alterations in signal intensity potentially related to premorphologic intrasubstance collagen degeneration and increased free-water content. Advanced degenerative chondral lesions typically manifest on MRI as multiple areas of cartilage thinning of varying depth and size, usually seen on opposing surfaces of an articulation. Cartilage defects typically illustrate obtuse margins and may be associated with corresponding subchondral regions of increased T2-weighted signal reflective of subchondral edema or cysts or a low signal intensity reflective of subchondral fibrosis or trabecular sclerosis. Other associated MRI findings of degenerative cartilage disease include central and marginal articular osteophytes, joint effusion, and synovitis. Traumatic cartilage fragments may remain in situ, become partially detached, or become loose and displaced into the joint space.
Quantitative measurements of cartilage lesion depth, diameter, area, and volume have been validated in a porcine experimental model of OA[33]. Satisfactory specificity and sensitivity for detecting chondral lesions have been demonstrated in knee specimens and in vivo with arthroscopic verification. Bredella et al[34] reported a sensitivity of 93% and a specificity of 99% in detecting chondral lesions with MRI vs athroscopy when axial and coronal images were combined, and values of 94% and 99% when images in all three planes were used. In that study, accuracy was highest for severe cartilage lesions and lowest for smaller lesions, particularly for signal intensity alterations. In human knee cartilage, the mean difference between measured and actual artificial cartilage defect diameters was reported to be < 0.1 mm, whereas the lesion depth was underestimated in MRI by > 0.4 mm[35]. Graichen et al[36] reported an overestimation of the true size of artificial cartilage defects in the human knee, which decreased from 42% in 3 mm defects to 4% in 8 mm defects. It is expected as MRI field and gradient strength increase and RF coil techniques and sequences improve, the performance of lesion detection with MRI will further increase.
Semi-quantitative scoring of human knee cartilage pathology
A number of semi-quantitative scoring methods have been developed for evaluation of articular cartilage on MR images[37,38]. Most of these methods grade the severity of cartilage thinning from 0 to 3 or 4 based on subjective evaluations by one or more experienced readers. These systems commonly differentiate between cartilage lesions of < 50% depth, > 50% depth and full thickness cartilage lesions.
In an observer-dependent semi-quantitative manner, novel scoring approaches are used to assess a variety of features that are currently believed to be relevant to the functional integrity of the knee or potentially involved in the pathophysiology of OA. Peterfy et al[39] described a scoring system in which cartilage lesions are graded according to both depth and extent along the joint surface. This score is part of a more comprehensive scoring system, in which multiple features are graded within the knee, such as articular cartilage integrity, subarticular bone marrow abnormality, subarticular cysts, subarticular bone attrition and marginal osteophytes. The surface areas of the knee joint are subdivided into 15 different regional anatomical landmarks in the extended knee. Cartilage signal and morphology are scored in each of the articular-surface regions using FS T2-weighted FSE images and the FS 3D spoiled GE images. An eight-point scale for semi-quantitative scoring of articular cartilage signal and morphology was used[27]: 0 = normal thickness and signal; 1 = normal thickness but increased signal on T2-weighted MR images; 2.0 = partial-thickness focal defect < 1 cm in greatest width; 2.5 = full-thickness focal defect < 1 cm in greatest width; 3 = multiple areas of partial-thickness (grade 2.0) defects intermixed with areas of normal thickness, or a grade 2.0 defect wider than 1 cm but < 75% of the region; 4 = diffuse (≥ 75% of the region) partial-thickness loss; 5 = multiple areas of full-thickness loss (grade 2.5) or a grade 2.5 lesion wider than 1 cm but < 75% of the region; 6 = diffuse (≥ 75% of the region) full-thickness loss. Peterfy et al[39] reported that despite the complexity of the system, the inter-observer agreement among two trained readers was high. However, Conaghan et al[40] reported some limitations when applying the WORMS grading system to knees of 336 subjects assessed by three readers. The authors commented that adding up individual scoring subscales, as recommended by WORMS, is problematic, and that several subscales (in particular those for cartilage signal and morphology and for osteophytes) may need to be redeveloped[40]. Other compartment-based knee OA scoring systems have also been published. Kornaat et al[41] reported a knee OA scoring system termed KOSS, with intraobserver reproducibility of 0.76-0.96 (intraclass correlation coefficient: ICC) and interobserver reproducibility amongst two independent observers of 0.63-0.91. In a recent report the reliability of a further novel MRI scoring system for evaluating OA of the knee was explored[42]. Nine intra-articular anatomical divisions and eight items were tested, including features of cartilage, bone-marrow lesions (BML), osteophytes, synovitis, effusions, and ligaments, and a scale of 0-3 was applied for each of these to yield the Boston-Leeds Osteoarthritis Knee Score (BLOKS). A series of iterative reliability exercises was performed to reduce the initial items. The interreader reliability for the final BLOKS items ranged from 0.51 for meniscal extrusion to 0.79 for meniscal tear, with that for cartilage morphology being 0.72. In another sample, both BLOKS and WORMS were used to score BML. Maximum BML size in BLOKS had a positive linear relationship with pain, whereas in WORMS it did not. Baseline BML was associated with cartilage loss on both the BLOKS and WORMS scale, but the association was stronger for BLOKS than for WORMS. These MRI semi-quantitative scoring approaches on OA have shown the ability to detect lesion progression over 1-2 years[43]. Although the sensitivity to change observed can sometimes be small[44], the ability to measure individual characteristics of OA is appealing in delineating structural risk factors for both pain and OA progression. The identification of early pathology in the course of disease may be enhanced by using these measures. For example, recent data suggest that full thickness defects may occur as part of early disease and that quantitative morphometry appears most useful (sensitive to change) in persons with late stage disease[45,46].
Quantitative assessment of human cartilage
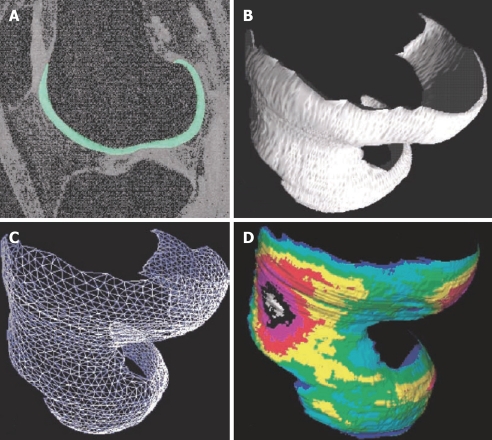
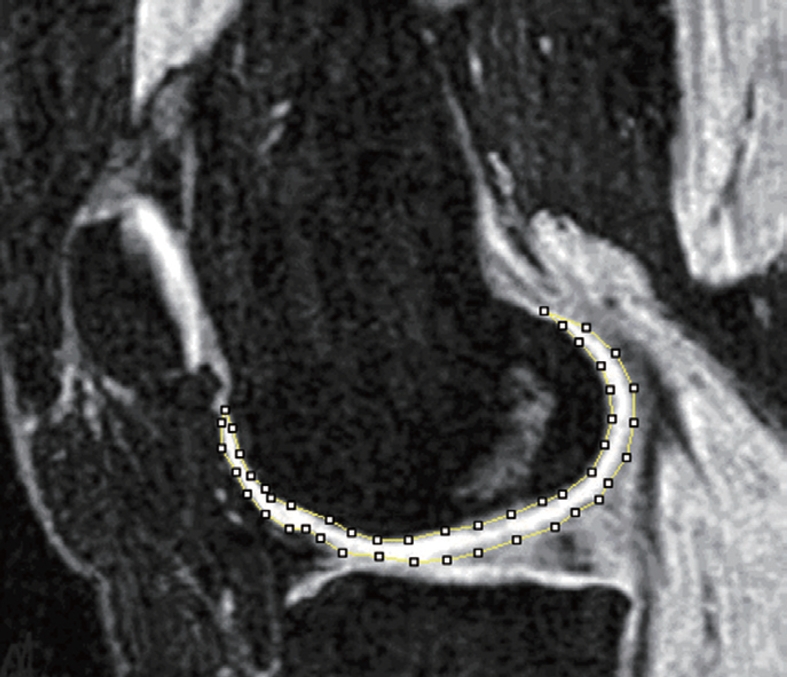
Due to the relatively low contrast in some areas of the joint surface, fully automated segmentation of knee cartilage volume from MR images has not yet been achieved. Computer-generated measurements based on signal intensity or predefined shape is not always reliable. Editing of automatically generated segmentations, or complete manual segmentation by experienced readers, is frequently necessary, therefore cartilage segmentation remains time-consuming (Figure 2). After segmentation, computation of the cartilage volume is achieved by simply summing the voxels attributed to the segmented cartilage (Figure 3). Osteophytes are excluded from segmentation.
Figure 2.
3D GE fat suppressed magnetic resonance imaging (MRI) at the sagittal plane. Bone cortex, bone marrow, and fat tissue appear to have a dark signal. Muscle appears to have a gray signal and cartilage a bright signal. The femur cartilage has been manually segmented at this slice.
Figure 3.
Quantitative 3D analysis of cartilage morphology from MRI. A: Sagittal MR image of human knee obtained with a fat-suppressed GE sequence, femoral cartilage is segmented; B: 3D volume reconstruction of the femoral cartilage; C: Analysis of joint surface area by a triangulation technique; D: Computation of 3D thickness distribution, independent of section orientation. (Reproduced from reference 6, with kind permission of Springer Science and Business Media).
In the absence of OA, Hudelmaier et al[7,47] reported a 0.3%-0.5% reduction of cartilage thickness per annum due to cartilage thinning during normal aging. Other authors reported a faster rate of cartilage loss. Hanna et al[48] found a significant (-2.8% annual) reduction in total tibial cartilage volume over a 2 years period. With data summarized from studies on OA patients in the published literature, Eckstein et al[8] reported that the annual loss of cartilage volume was -136 μL (-4.1%) in the patella, -90 μL (-5.6%) in the medial tibia, and -107 μL (-6.0%) in the lateral tibia. The annual changes in cartilage volume/thickness exceeded the precision errors and appeared to be associated with clinical symptoms as well as with time to knee arthroplasty. However, results varied between published studies, the annual rate of change ranged from -0.3% to -7.4% in the medial tibia[8]. It has been reported that in early OA, cartilage may not be thin but rather thicker and swollen with water[49,50].
Because attrition in cartilage volume occurs at a very slow rate, measurement precision is of critical importance. Precision errors can be expressed as the standard deviation (SD) or coefficient of variation (CV%, SD divided by the mean value) of repeated measurements. Use of the CV% is most appropriate when SD is proportional to volume, for instance when comparing precision in different joint surfaces of the knee (i.e. medial tibia vs total femur). If the SD is independent of volume, it is more appropriate to compare SD values directly. It should be noted that when examining patients with severe OA, the CV% will be larger than that in healthy volunteers, even if the absolute error (SD) is similar. This is because patients with severe OA have less cartilage volume. CV% of cartilage volume in the medial tibia in healthy volunteers has been reported to be in the range of 2%-3.5%. Precision errors for cartilage volume across studies at 1.5T for each cartilage component have been recently summarized[8]. Several studies examined the inter-observer and intra-observer precision of repeated analyses. It has been confirmed that segmenting all images in a subject’s series by the same skilled reader is more accurate than segmenting by different readers. To alleviate reader bias, the results of several readers can be averaged. Re-segmentation of the same data sets over a period of 1 year by the same user involved larger errors than those involved by segmentation of different data sets immediately after each other[46]. Therefore, to ensure the smallest coefficient of variation, in longitudinal studies comparative analyses should be performed in one post-processing session. Blinding the user to the order of the exams is necessary in order to avoid bias.
Cartilage volume provides a first step in the analysis of cartilage morphology. More comprehensive information can be derived from separating the cartilage volume into its two factors, namely the cartilage thickness and the size of the joint surface area. Specific algorithms are required to differentiate between these factors. Other variables such as percent cartilaginous (or denuded) joint surface area, cartilage surface curvature, lesion size and depth can also be investigated. As overall volume and mean thickness for an entire cartilage plate may be relatively insensitive to regional/focal changes, several investigators developed techniques for displaying regional thickness patterns. Measuring cartilage volume in regions of the knee (e.g. medial tibia) and regional mean thickness as distinct from focal measures of change (region of interest analysis centered around focal defects) and measures of denuded cartilage may provide very different measurements about the important pathologic changes occurring in early disease. Distinguishing what measures are most discriminatory at each stage of the disease process is essential for utilizing these measures appropriately. Kshirsagar et al[51] suggested that analyzing subvolumes within the joint surface by such techniques can reduce precision errors relative to those from analyses of the entire cartilage plate and this was recently confirmed in a study by Koo et al[52] for the central weight-bearing regions of the femoral condyles. These types of ‘local’ approaches to measuring changes in cartilage morphology are technically challenging, but they can be important, because the overall cartilage volume can remain constant even if there are focal changes in cartilage thickness and denuded bone. In order to track local/regional thickness changes over time, the bone interfaces from two data sets are “matched”, so that the thickness distribution can be compared on a point-by-point basis. Koo et al[52] suggested a ‘trimmed’ region defined to avoid errors arising at the edges of articulating surfaces, which are difficult to segment yet may be involved to only a minor degree in the disease process.
A substantial percentage of the variability in cartilage volume is determined by joint surface area[53]. Wang et al[54] demonstrated that the medial and lateral tibial plateau area increased over a 2-year period (2.2% and 1.5% per annum, respectively), with increases at the medial (but not lateral) tibia being stronger in male patients, in participants with high body mass index, and in patients with higher baseline grade of medial JSN. Metaphyseal enlargement with age and OA might create problems for cartilage volume measurements that did not adjust for bone size[55], and either adjustment for subchondral bone area or the direct measurement of cartilage thickness has thus been recommended.
Quantitative MRI of cartilage is currently used in ongoing studies to monitor cartilage volume and will be used to assess structural effects of pharmacological therapies on cartilage in order to prove their effectiveness. However, until now the measurement of cartilage volume has not gained widespread usage in routine clinical practice partly due to its time-consuming nature. Although the volume measurements are typically semi-automated, they still require some degree of manual segmentation. Segmentation of cartilage will become easier with sequences that create improved contrast between cartilage and the surrounding bone and fluid, but will still likely require a high degree of user interaction for at least the near future.
MORPHOLOGICAL EVALUATION OF ANIMAL KNEE CARTILAGE
Cartilage thickness and volume have been quantified in animals as small as guinea pigs and rabbits. Cartilage of OA involves an initial swelling phase, and later attrition and loss, defect phases. These phases have been demonstrated in experimental animal models by sequential MRI[24,56]. Tessier et al[24] studied 19 male Dunkin-Hartley guinea pigs with MRI at 3, 6, 9, 10.5 and 12 mo of age. They found that at 6 mo, swollen cartilage was observed in all animals; at 9 mo, marked fragmentation of the medial tibial cartilage was seen in the areas not covered by the meniscus; at 12 mo, focal thinning of the cartilage was apparent with occasional full cartilage loss.
Tessier et al[24] detailed their segmentation methodology for guinea pig tibia cartilage. Segmentation of the cartilage of the medial plateau was performed on sagittal slices covering the medial side only. These slices were selected on the basis that they covered the ‘flattest’ region of the medial tibial surface. The slices covering the inner side where the cruciate ligament is attached were excluded because the images are subject to significant partial volume averaging. Slices at one time-point were matched as close as possible to those obtained at subsequent time-points based on anatomical references. Thus for any animal, the number of slices analyzed were equal for all time-points. However, the number of segmented slices was different between animals to account for the difference in the overall size of the tibial plateau. Their data showed that maximal cartilage loss (36%) occurs from the medial side of the tibial plateau from 9 to 12 mo of age.
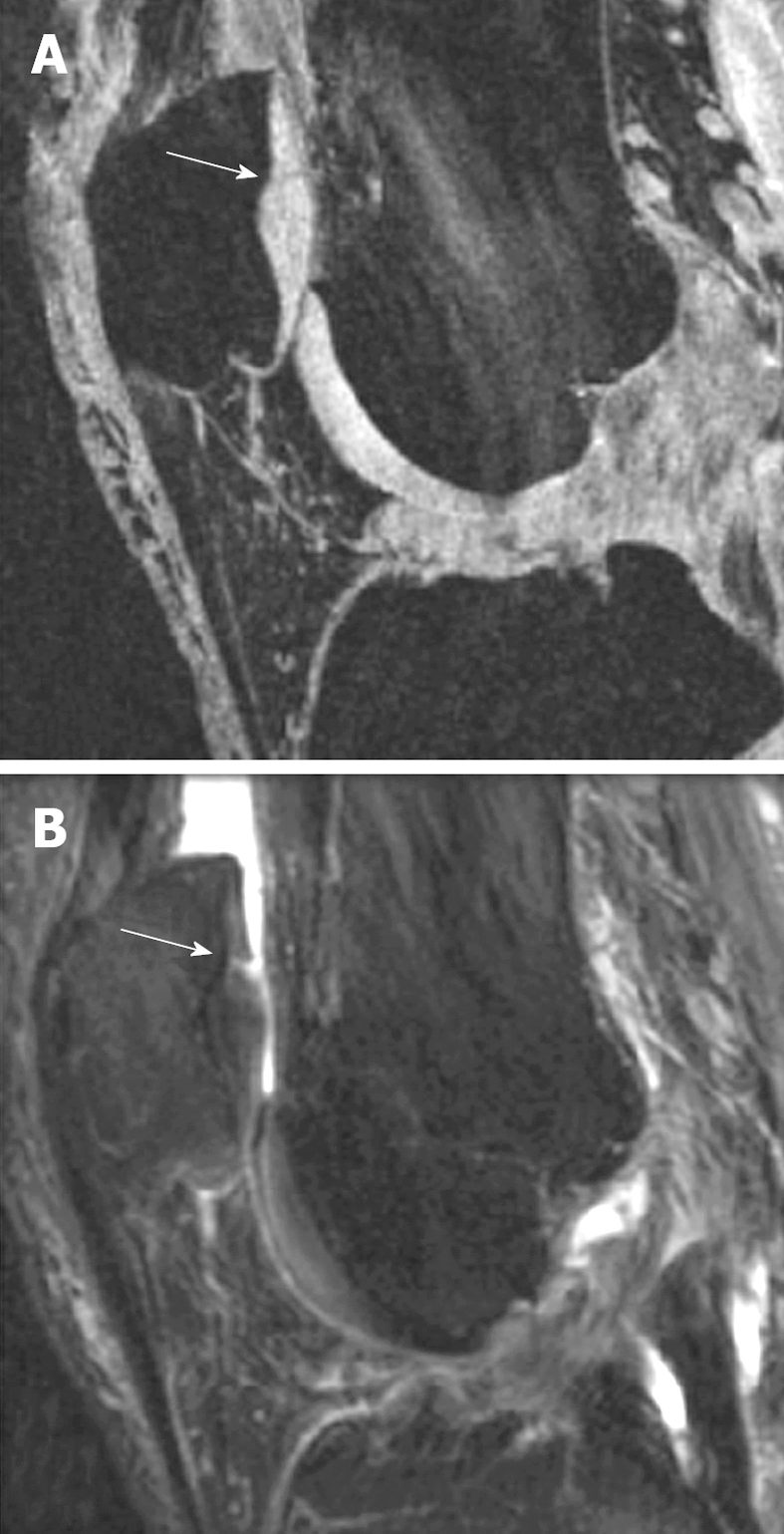
In small animals like rats, due to the small size of their knee joint, assessment of cartilage thickness is challenging but has recently proved to be feasible. With a 7T MR scanner, Faure et al[23] reported that MRI could be used to detect arthritis and joint changes at a very early stage in living rats with rheumatoid arthritis. With a rat meniscus transection OA model 44 d post surgery, MRI was able to demonstrate qualitatively the decrease in cartilage thickness and loss of cartilage in some areas, as well as focal neo-cartilage proliferation at the joint margin (Figures 4 and 5)[22].
Figure 4.
Sagittal view MRI at 4.7T of a rat knee with medial meniscal tear (A) and a rat with sham operation (B). A: Parts of the tibia cartilage become much thinner (white arrows). B: Normal tibia cartilage (white arrows). This animal model has been described in reference 22 (Wang et al[22] 2006).
Figure 5.
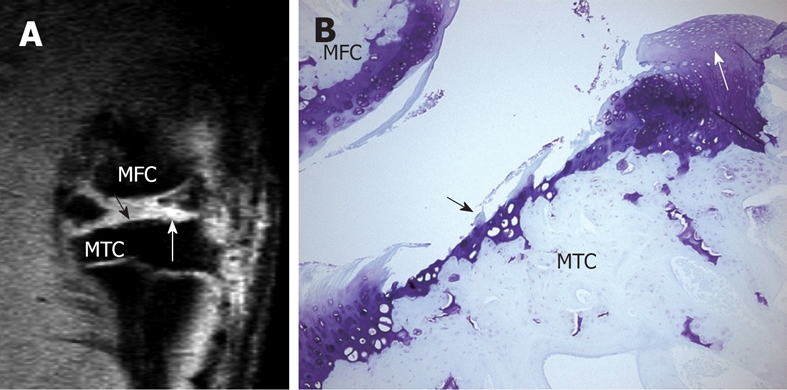
High resolution MRI demonstrates cartilage erosion and proliferation in rat knee joint. A: Sagittal view MRI of a rat knee with a medial meniscal tear. White arrow shows neo-cartilage proliferation and black arrow shows articular cartilage loss; B: Histology section of the medial tibial condyle stained with Toluidine blue (× 100), 28 d after meniscal tear. Cartilage erosion (black arrow) and neo-cartilage proliferation (white arrow) are seen in the tibia cartilage. MTC: Medial tibia condyle; MFC: Media femur condyle. This animal model has been described in reference 22 (Wang et al[22] 2006).
Recently, it was pointed out that to date MR tomographic knowledge of laboratory animal skeletal microanatomy remains limited. With MR images acquired in normal rats, many signs unfamiliar to radiologists can be noted, including notch-like bright signal areas in the epiphysis, gray signal areas in the epiphysis, and fuzzy joint surface of the epiphysis of the femur and tibia. Detailed inspection of the histology specimen showed more unfamiliar features of rat knee microanatomy, including curvy or dipped surface of the femur/tibia epiphysis, areas composed of a mixture of cartilage and bone components, normal notch structure, cyst-like structure, and a cavity between cortical lamellae under the joint cartilage (Figure 6)[57]. Further research is warranted to understand how these structures will affect articular cartilage assessment.
Figure 6.
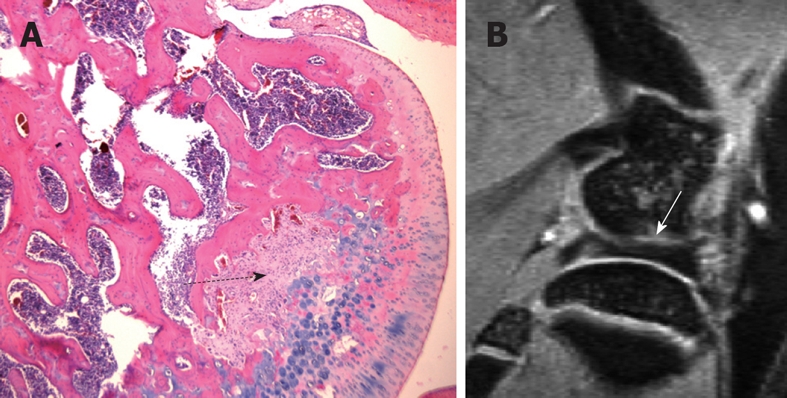
One example of the pitfalls in interpreting normal rat knee high resolution MR images. A: H&E-stained sections of a normal 3 mo rat knee, a mid-section through the femur. Dotted black arrow denotes the area of fibrous components together with their matrix, which are continuous with the cartilaginous component of the joint surface, and there is no bony component seen within this region. This region may appear as a notch or a cyst on MR images due to its higher signal; B: Sagittal MR image of a normal 3 mo old rat knee. White arrow in B denotes a gray area possibly composed of a mixture of cartilage and bone components.
CLINICAL RELEVANCE OF CARTILAGE PATHOLOGIES DEMONSTRATED BY MRI
Satisfactory specificity and sensitivity for detecting chondral lesions by MRI have been demonstrated in cadaveric knees and in vivo with arthroscopic verification[38,58,59]. Knee cartilage defect severity, as measured from sagittal T1-weighted FS SPGR sequences, has been shown to be significantly associated with urinary levels of C-terminal cross-linking telopeptide of type II collagen[60]. An association was also reported between cartilage defects and body mass index[61]. Link et al[1] showed that in patients with OA the degree of cartilage pathology seen in MR images is associated with clinical symptoms.
In a cross-sectional study, Hunter et al[62] observed a significant negative association between patellar cartilage volume and the WOMAC score in a population of 133 postmenopausal women. Wluka et al[63] reported a weak association between tibial cartilage volume and symptoms (WOMAC score) at baseline in a sample of 132 patients with symptomatic early knee OA, and worsening of symptoms over a 2 years period was associated with tibial cartilage loss. These data suggest that treatment targeted at reducing the rate of knee cartilage loss in subjects with symptomatic OA may relate to clinical outcomes and delay knee replacement.
MRI is very useful for cartilage repair surgical planning and follow-up. The combination of the clinical outcome after cartilage repair together with the morphological and composition description of the cartilage repair tissue as well as the surrounding cartilage may lead to a satisfactory follow-up evaluation. MRI can give a precise estimate of progressing degeneration and objective assessment of the long-term outcome of cartilage repair.
In animal based studies, MRI can be used to carry out in vivo longitudinal follow-up in the same animal and track the disease, monitor its progress and see how it responds to potential treatments[5]. In a recent study of a mono-iodoacetate induced arthritis model in rats[64], MRI demonstrated that intra-articular soft tissue inflammatory changes peaked at day 3 and then started to regress; bony damage appeared at day 14, peaked at day 21, with hallmarks of repair visible by day 35. A similar bi-phase pain response was observed clinically with the 1 mg mono-iodoacetate dose group, peaking at days 3 and 21. Therefore, MRI was able to characterize the pathological course of the arthritis model which enabled a link to be established between the structural changes and joint clinical discomfort. There are several “disease” or “structure” modifying OA drugs under clinical development. Although JSN on weight-bearing radiographs is still the accepted surrogate marker for demonstrating structural change by regulatory agencies, this is expected to change in the near future, given the limitations of radiography[3,4], and MRI will very likely replace radiography in this context. As the validation of MRI endpoints for OA trials in human studies is still incomplete, the design and interpretation of a clinical trial will be strengthened if the effect of the drug is shown by the same endpoint in a valid animal model. By measuring the volume of medial tibia cartilage in a spontaneous OA guinea pig model, MRI demonstrated that doxycycline treatment halved the cartilage loss as compared to the vehicle treatment[65].
CONCLUSION
MRI can semi-quantitatively assess cartilage morphology, and quantitatively evaluate regional cartilage volume and thickness. Other cartilage parameters including cartilage quality, cartilage surface smoothness, cartilage coverage, and distribution of change can also be evaluated. Progress made in MRI technology in the last few years allows longitudinal studies of human knee cartilage morphology with enough accuracy to follow the disease-caused changes and also evaluate the therapeutic effects of chondro-protective drugs[66]. For cartilage repair patients, future studies will be needed to determine whether MRI is prognostic of clinical outcome and can replace arthroscopic biopsy for monitoring repair-tissue histology. In animal experimental settings, high field MRI can non-invasively provide detailed images of joints and can be used to carry out in vivo longitudinal follow-up in the same animal and track the disease as well as see how it responds to potential treatments. There are also several MRI methods that may allow evaluation of the glycosaminoglycan matrix or collagen network of articular cartilage and may be the most sensitive method for the detection of early changes. These techniques are being further explored and validated. With the development of new therapies for OA and cartilage injury, MRI will play an important role in the diagnosis, staging, and evaluation of the effectiveness of these therapies.
References
- 1.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, Majumdar S. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 2.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, Welch G. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage. 2003;11:387–393. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 3.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, Niu J, Gale DR, Felson DT. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 4.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonté F, Beaudoin G, de Guise JA, Bloch DA, Choquette D, Haraoui B, Altman RD, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 5.Wang YX. In vivo magnetic resonance imaging of animal models of knee osteoarthritis. Lab Anim. 2008;42:246–264. doi: 10.1258/la.2007.06041e. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat Embryol (Berl) 2001;203:147–173. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- 7.Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, Eckstein F. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 9.Sittek H, Eckstein F, Gavazzeni A, Milz S, Kiefer B, Schulte E, Reiser M. Assessment of normal patellar cartilage volume and thickness using MRI: an analysis of currently available pulse sequences. Skeletal Radiol. 1996;25:55–62. doi: 10.1007/s002560050032. [DOI] [PubMed] [Google Scholar]
- 10.Eckstein F, Stammberger T, Priebsch J, Englmeier KH, Reiser M. Effect of gradient and section orientation on quantitative analysis of knee joint cartilage. J Magn Reson Imaging. 2000;11:161–167. doi: 10.1002/(sici)1522-2586(200002)11:2<161::aid-jmri13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein JD, Li JG, Majumdar S, Henkelman RM. Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. AJR Am J Roentgenol. 1997;169:1089–1096. doi: 10.2214/ajr.169.4.9308470. [DOI] [PubMed] [Google Scholar]
- 12.Hardya PA, Newmark R, Liu YM, Meier D, Norris S, Piraino DW, Shah A. The influence of the resolution and contrast on measuring the articular cartilage volume in magnetic resonance images. Magn Reson Imaging. 2000;18:965–972. doi: 10.1016/s0730-725x(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 13.Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002;46:2065–2072. doi: 10.1002/art.10460. [DOI] [PubMed] [Google Scholar]
- 14.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004;50:94–97. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 15.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, Eaton CB, Schneider E. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornaat PR, Reeder SB, Koo S, Brittain JH, Yu H, Andriacchi TP, Gold GE. MR imaging of articular cartilage at 1.5T and 3.0T: comparison of SPGR and SSFP sequences. Osteoarthritis Cartilage. 2005;13:338–344. doi: 10.1016/j.joca.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, Wirth W, Evelhoch JL. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 18.Masi JN, Sell CA, Phan C, Han E, Newitt D, Steinbach L, Majumdar S, Link TM. Cartilage MR imaging at 3.0 versus that at 1.5 T: preliminary results in a porcine model. Radiology. 2005;236:140–150. doi: 10.1148/radiol.2361040747. [DOI] [PubMed] [Google Scholar]
- 19.Link TM, Sell CA, Masi JN, Phan C, Newitt D, Lu Y, Steinbach L, Majumdar S. 3.0 vs 1.5 T MRI in the detection of focal cartilage pathology--ROC analysis in an experimental model. Osteoarthritis. Cartilage. 2006;14:63–70. doi: 10.1016/j.joca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Gold GE, McCauley TR, Gray ML, Disler DG. What's new in cartilage? Radiographics. 2003;23:1227–1242. doi: 10.1148/rg.235035113. [DOI] [PubMed] [Google Scholar]
- 21.Kneeland JB, Reddy R. Frontiers in musculoskeletal MRI: articular cartilage. J Magn Reson Imaging. 2007;25:339–344. doi: 10.1002/jmri.20811. [DOI] [PubMed] [Google Scholar]
- 22.Wang YX, Westwood FR, Moores SM, Ball A, Heapy C, Pickford R, Tessier JJ, Bowyer J. In vivo high-resolution three-dimensional magnetic resonance imaging of a rat knee osteoarthritis model induced by meniscal transection. Proceedings of the International Society for Magnetic Resonance in Medicine, Seattle. 2006. p. No. 3633. [Google Scholar]
- 23.Faure P, Doan BT, Beloeil JC. In-vivo high resolution three-dimensional MRI studies of rat joints at 7 T. NMR Biomed. 2003;16:484–493. doi: 10.1002/nbm.855. [DOI] [PubMed] [Google Scholar]
- 24.Tessier JJ, Bowyer J, Brownrigg NJ, Peers IS, Westwood FR, Waterton JC, Maciewicz RA. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11:845–853. doi: 10.1016/s1063-4584(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19:435–443. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 26.Gray ML, Burstein D. Molecular (and functional) imaging of articular cartilage. J Musculoskelet Neuronal Interact. 2004;4:365–368. [PubMed] [Google Scholar]
- 27.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 28.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17:1135–1146. doi: 10.1007/s00330-006-0453-5. [DOI] [PubMed] [Google Scholar]
- 29.Creamer P. Osteoarthritis pain and its treatment. Curr Opin Rheumatol. 2000;12:450–455. doi: 10.1097/00002281-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum. 2002;46:1223–1227. doi: 10.1002/art.10256. [DOI] [PubMed] [Google Scholar]
- 31.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, Niu J, Gale DR, Felson DT. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 32.Recht MP, Goodwin DW, Winalski CS, White LM. MRI of articular cartilage: revisiting current status and future directions. AJR Am J Roentgenol. 2005;185:899–914. doi: 10.2214/AJR.05.0099. [DOI] [PubMed] [Google Scholar]
- 33.Lee KY, Masi JN, Sell CA, Schier R, Link TM, Steinbach LS, Safran M, Ma B, Majumdar S. Computer-aided quantification of focal cartilage lesions using MRI: accuracy and initial arthroscopic comparison. Osteoarthritis Cartilage. 2005;13:728–737. doi: 10.1016/j.joca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, Belzer JP, Wischer TK, Genant HK. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172:1073–1080. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 35.McGibbon CA, Trahan CA. Measurement accuracy of focal cartilage defects from MRI and correlation of MRI graded lesions with histology: a preliminary study. Osteoarthritis Cartilage. 2003;11:483–493. doi: 10.1016/s1063-4584(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 36.Graichen H, Al-Shamari D, Hinterwimmer S, von Eisenhart-Rothe R, Vogl T, Eckstein F. Accuracy of quantitative magnetic resonance imaging in the detection of ex vivo focal cartilage defects. Ann Rheum Dis. 2005;64:1120–1125. doi: 10.1136/ard.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drapé JL, Pessis E, Auleley GR, Chevrot A, Dougados M, Ayral X. Quantitative MR imaging evaluation of chondropathy in osteoarthritic knees. Radiology. 1998;208:49–55. doi: 10.1148/radiology.208.1.9646792. [DOI] [PubMed] [Google Scholar]
- 38.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, Hospodar PP. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167:127–132. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 39.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Conaghan PG, Hunter D, Tennant A, Amin S, Clancy M, Guermazi A, Peterfy C, Genant H, Felson DT. Evaluation an MRI scoring system for osteoarthritis of the knee using modern psychometric approaches. Osteoarthritis Cartilage. 2004;12(Suppl B):S119–S120. [Google Scholar]
- 41.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, Woodworth TG, Bloem JL. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)--inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 42.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 Suppl A:A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Hunter DJ, Conaghan PG, Peterfy CG, Bloch D, Guermazi A, Woodworth T, Stevens R, Genant HK. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14 Suppl A:A112–A115. doi: 10.1016/j.joca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Hunter DJ, Niu JB, Zhang Y, LaValley M, McLennan CE, Hudelmaier M, Eckstein F, Felson DT. Premorbid knee osteoarthritis is not characterised by diffuse thinness: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2008;67:1545–1549. doi: 10.1136/ard.2007.076810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckstein F, Heudorfer L, Faber SC, Burgkart R, Englmeier KH, Reiser M. Long-term and resegmentation precision of quantitative cartilage MR imaging (qMRI). Osteoarthritis. Cartilage. 2002;10:922–928. doi: 10.1053/joca.2002.0844. [DOI] [PubMed] [Google Scholar]
- 47.Hudelmaier M, Glaser C, Englmeier KH, Reiser M, Putz R, Eckstein F. Correlation of knee-joint cartilage morphology with muscle cross-sectional areas vs. anthropometric variables. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:175–184. doi: 10.1002/ar.a.10001. [DOI] [PubMed] [Google Scholar]
- 48.Hanna F, Ebeling PR, Wang Y, O'Sullivan R, Davis S, Wluka AE, Cicuttini FM. Factors influencing longitudinal change in knee cartilage volume measured from magnetic resonance imaging in healthy men. Ann Rheum Dis. 2005;64:1038–1042. doi: 10.1136/ard.2004.029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maroudas A, Bullough P. Permeability of articular cartilage. Nature. 1968;219:1260–1261. doi: 10.1038/2191260a0. [DOI] [PubMed] [Google Scholar]
- 50.Mow V, Setton L. Mechanical properties of normal and osteoarthritis articular cartilage. In: Brandt K, Doherty M, Lohmander LS, editors. Osteoarthritis. New York: Oxford Medical Publications; 1998. pp. 108–122. [Google Scholar]
- 51.Kshirsagar AA, Watson PJ, Tyler JA, Hall LD. Measurement of localized cartilage volume and thickness of human knee joints by computer analysis of three-dimensional magnetic resonance images. Invest Radiol. 1998;33:289–299. doi: 10.1097/00004424-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005;13:782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Burgkart R, Glaser C, Hinterwimmer S, Hudelmaier M, Englmeier KH, Reiser M, Eckstein F. Feasibility of T and Z scores from magnetic resonance imaging data for quantification of cartilage loss in osteoarthritis. Arthritis Rheum. 2003;48:2829–2835. doi: 10.1002/art.11259. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Wluka AE, Cicuttini FM. The determinants of change in tibial plateau bone area in osteoarthritic knees: a cohort study. Arthritis Res Ther. 2005;7:R687–R693. doi: 10.1186/ar1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edinger DT, Hayashi K, Hongyu Y, Markel MD, Manley PA. Histomorphometric analysis of the proximal portion of the femur in dogs with osteoarthritis. Am J Vet Res. 2000;61:1267–1272. doi: 10.2460/ajvr.2000.61.1267. [DOI] [PubMed] [Google Scholar]
- 56.Calvo E, Palacios I, Delgado E, Ruiz-Cabello J, Hernández P, Sánchez-Pernaute O, Egido J, Herrero-Beaumont G. High-resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage. 2001;9:463–472. doi: 10.1053/joca.2001.0413. [DOI] [PubMed] [Google Scholar]
- 57.Wang HH, Wang YX, Griffith JF, Sun YL, Zhang G, Chan CW, Qin L, Ahuja AT, Teng LS. Pitfalls in interpreting rat knee joint magnetic resonance images and their histological correlation. Acta Radiol. 2009;50:1042–1048. doi: 10.3109/02841850903156484. [DOI] [PubMed] [Google Scholar]
- 58.Kawahara Y, Uetani M, Nakahara N, Doiguchi Y, Nishiguchi M, Futagawa S, Kinoshita Y, Hayashi K. Fast spin-echo MR of the articular cartilage in the osteoarthrotic knee. Correlation of MR and arthroscopic findings. Acta Radiol. 1998;39:120–125. doi: 10.1080/02841859809172164. [DOI] [PubMed] [Google Scholar]
- 59.Yoshioka H, Stevens K, Hargreaves BA, Steines D, Genovese M, Dillingham MF, Winalski CS, Lang P. Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat-suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging. 2004;20:857–864. doi: 10.1002/jmri.20193. [DOI] [PubMed] [Google Scholar]
- 60.Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G. Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage. 2005;13:198–205. doi: 10.1016/j.joca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Knee structural alteration and BMI: a cross-sectional study. Obes Res. 2005;13:350–361. doi: 10.1038/oby.2005.47. [DOI] [PubMed] [Google Scholar]
- 62.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 63.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63:264–268. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang YX, Heapy C, Pickford R, Tessier JJ, Ball A, Holmes A, Read SJ. MRI structural changes and joint discomfort: an investigation in the mono-iodoacetate induced arthritis model in rats. Proceedings of the European Society for Magnetic Resonance in Medicine and Biology, Basle, 2005: No. 283. [Google Scholar]
- 65.Tessier J, Bowyer J, Heapy C, Elliott J, Pickford R, Flannelly J, Maciewicz R. Doxycycline Slows MRI-Assessed Cartilage Volume Loss in the Guinea Pig Model of Osteoarthritis. Proceedings of the International Society for Magnetic Resonance in Medicine, Seattle. New York: Oxford Medical Publications; 2006. p. No. 61. [Google Scholar]
- 66.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonté F, Beaudoin G, Bloch DA, Choquette D, Haraoui B, Altman RD, Hochberg M, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]