Abstract
Advances in magnetic resonance (MR) and computed tomography (CT) imaging have improved visualization of acute and scar infarct. Over the past decade, there have been and continues to be many significant technical advancements in cardiac MR and multi-detector computed tomography (MDCT) technologies. The strength of MR imaging relies on a variety of pulse sequences and the ability to noninvasively provide information on myocardial structure, function and perfusion in a single imaging session. The recent technical developments may also allow CT technologies to rise to the forefront for evaluating clinical ischemic heart disease. Components of reperfusion injury including myocardial edema, hemorrhage, calcium deposition and microvascular obstruction (MO) have been demonstrated using MR and CT technologies. MR imaging can be used serially and noninvasively in assessing acute and chronic consequences of reperfusion injury because there is no radiation exposure or administration of radioactive materials. MDCT is better suited for assessing coronary artery stenosis and as an alternative technique for assessing viability in patients where MR imaging is contraindicated. Changes in left ventricular (LV) volumes and function measured on cine MR are directly related to infarct size measured on delayed contrast enhanced images. Recent MR studies found that transmural infarct, MO and peri-infarct zone are excellent predictors of poor post-infarct recovery and mortality. Recent MR studies provided ample evidence that growth factor genes and stem cells delivered locally have beneficial effects on myocardial viability, perfusion and function. The significance of deposited calcium in acute infarct detected on MDCT requires further studies. Cardiac MR and MDCT imaging have the potential for assessing reperfusion injury components and manifestations.
Keywords: Calcium deposits in myocardium, Magnetic resonance imaging, Multi-detector computed tomography, Myocardial micro and macro-infarct, Reperfusion injury, Vascular injury
INTRODUCTION
Ischemic heart disease remains the leading cause of death worldwide and accounts for the majority (almost 70%) of congestive heart failure cases. Reperfusion therapy, which includes thrombolytic therapy, angioplasty and stent placement, is the greatest advancement in the treatment of acute myocardial infarction. On the other hand, reperfusion therapy induces myocardial injury. Recent studies have shown that interstitial edema, infarct dimensions (size, circumferential extent and transmurality), peri-infarct zone, microvascular obstruction (MO), interstitial hemorrhage and calcium deposition are major components of reperfusion injury and these components predict the short and long term survival.
The common denominator magnetic resonance (MR) sequences used in clinical viability protocols involves evaluating left ventricular (LV) function on cine techniques and myocardial infarct size on delayed contrast enhancement (DE) imaging. Other pulse sequences, such as first pass perfusion, tagged, velocity encoded cine, T2-weighted turbo spin echo and T2*-susceptibility MR imaging have also been used for assessment of the consequences of post-infarct reperfusion. MR imaging has also been useful in defining the etiology of non-ischemic diseases, such as amyloidosis[1], viral myocarditis[2] and hypertrophic cardiomyopathy[3]. DE-MR imaging also eliminates the exposure of patients to ionizing radiation used in computed tomography (CT) imaging[4].
On the other hand, the clinical indications for implantable cardiac defibrillators and biventricular pacing therapy continue to expand, and the development and validation of alternative imaging modalities with similar abilities for assessing LV function, perfusion and viability, are needed to accommodate such a growing population of patients who are unfavorable candidates for MR imaging. The application of multi-detector computed tomography (MDCT) does not suffer from relative contraindications (such as implanted active permanent pacemakers or defibrillators (or retained components of either, due to their potential to become dysfunctional) and/or unwanted conductors (e.g. induction of ectopy or heating capable of burning) within the rapidly changing magnetic and radiofrequency environments during imaging commonly confronting MR imaging in routine clinical cardiac imaging. Furthermore, there is no limitation of basic life-support and physiologic-monitoring equipment in the vicinity of the CT scanner. MR and MDCT imaging have been used to assess cardiac anatomy[5], measure left and right ventricular volumes and function[6], regional perfusion[7,8] and myocardial viability[9,10]. The focus of this review is on the manifestations of reperfusion injury using MR and MDCT imaging.
COMPONENTS OF REPERFUSION INJURY
Myocardial edema
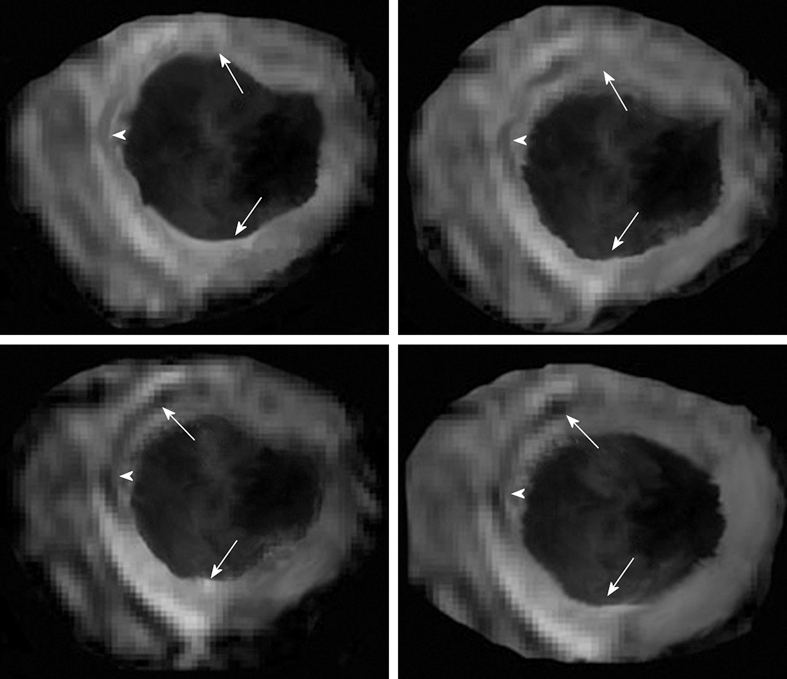
Reperfusion of previously ischemic myocardium causes edema related to the leakage of blood macromolecules into interstitium. Edema is one of the features of the salvageable area at risk[11,12]. The increase in mobile water content (edema) within the ischemic region causes a prolongation of T2 relaxation time[13]. The salvaged area at risk in reperfused infarct has been visualized on T2-weighted turbo spin echo MR imaging as a hyperintense area (Figure 1)[14-18].
Figure 1.
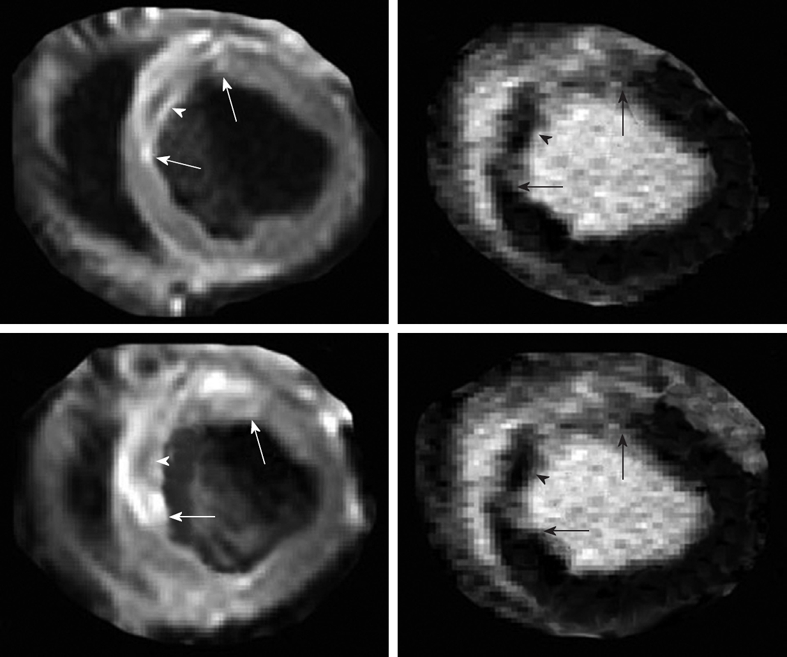
Multi-slice T2-weighted turbo spin echo imaging demonstrates the hyperintense edematous area at risk (white arrows in the left images) with hypoenhanced zone, which may represent microvascular and/or interstitial hemorrhage zone (arrowhead in the left images). Delayed contrast enhancement magnetic resonance (DE-MR) imaging confirmed the presence of microvascular and/or interstitial hemorrhage zone in the core (black arrowhead in the right images) of hyperenhanced infarcted myocardium (black arrows in the right images).
Accurate assessment of the area at risk is required to compare different revascularization techniques or for studies aimed at improving myocardial salvage[19-22] as an end point. MR imaging has documented regional myocardial edema in patients with normal coronary angiograms[23] and stunning[24]. Kwong et al[25] combined the assessment of LV function, myocardial perfusion and infarct in patients who presented to the emergency room with chest pain. The investigators found that MR imaging is the strongest predictor for the diagnosis of acute coronary syndrome compared with a standard workup. The sensitivity and specificity of MR imaging for detecting acute coronary syndrome was 84% and 85%, respectively. Moreover, multiple logistic regression analysis revealed that MR imaging had independent diagnostic value over clinical parameters, including ECG and initial troponin I levels. In another adenosine perfusion MR study, Ingkanisorn et al[26] evaluated the diagnostic value of adenosine in 135 patients who presented to the emergency room with chest pain with no elevation in troponin levels. MR imaging data indicated that there was no evidence of significant ischemic heart disease in these patients. Patients were contacted 1 year later to determine the incidence of coronary artery stenosis (> 50%) on invasive coronary angiography, abnormal correlative stress test, new infarct, or death. Based on this survey, MR perfusion imaging showed 100% sensitivity and 93% specificity for the detection of myocardial ischemia. It was concluded that MR imaging had significant prognostic value in predicting a future diagnosis of ischemia, infarct, or death. First pass perfusion imaging can be used to discriminate ischemic myocardium. A recent MR-impact study in 234 patients reported improved detection of ischemic myocardium distal to coronary stenosis compared to single photon emission computed tomography in a multicenter and multivendor randomized trial[27].
MDCT imaging has also been used in the evaluation of cardiac function, myocardial viability and plaque morphology[28-30]. A preclinical study demonstrated that this modality has the potential to detect infarct heterogeneity in the peri-infarct zone[31]. Recent experimental studies using modern MDCT technology confirmed the potential of the technique in depicting ischemic myocardium during the first pass perfusion of iodinated contrast media[10,32].
HOMOGENEOUS MYOCARDIAL INFARCT
Several studies indicated that there is a close correlation between homogeneous myocardial infarct size, dimensions (size, circumferential extent and transmurality) and LV remodeling. Inversion-recovery low-angle-shot MR imaging and helical MDCT imaging have been recently introduced and performed following the intravenous administration of contrast media with a delay of 5-10 min to define myocardial infarct dimensions[7,9,10,33-39] (Figure 2). Investigators found that differentially contrast enhanced regions on MR and MDCT imaging correlate well with areas of decreased flow[32,40] and dobutamine stress on echocardiography[41]. Furthermore, the combined use of cine and DE-MR imaging are able to differentiate regional transitional dysfunction in stunned and hibernating myocardium from permanent dysfunction on contrast enhanced infarct[42,43]. A recent study showed multi-contrast MR imaging enables simultaneous assessment of wall motion, MO and viability[44].
Figure 2.
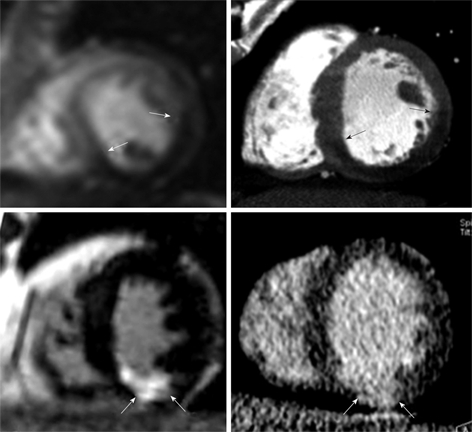
Head-to-head comparison between first pass perfusion MR (top left) and first pass multi-detector computed tomography (MDCT) imaging (top right) in a 42-year-old man with acute reperfused infarct. Ischemic myocardium (arrows) appears as a hypoenhanced region with comparable extent on both imaging modalities. Bottom: Head-to-head comparison of DE-MR (bottom left) and DE-MDCT imaging (bottom right) shows a bright region comparable in size to enhanced inferior infarct (arrows). Note that the enhanced infarct on DE-imaging is substantially smaller than ischemic myocardium[38].
Cine and DE-MR imaging have been used to determine contractile reserve in transmural and non-transmural infarct[45,46]. These studies have also indicated the substantial improvement in regional function in segments with 50% transmural enhancement and global LV improvement in transmural enhancement of less than 25% of LV wall thickness[45-47]. Others found that 75% of the patients with transmural enhancement died within 26-36 mo of diagnosis[48]. Tarantini et al[49] demonstrated in 76 patients with reperfused infarct that transmural enhancement using DE-MR imaging is associated with LV remodeling. These findings were confirmed by Roes et al[50] who showed that the size of the infarct scar in 231 patients is a stronger predictor of all-cause mortality than LV ejection fraction and LV volumes. Thus, extensive transmural enhancement is an excellent predictor of poor recovery.
Contrast enhanced T1-weighted and non-contrast T2-weighted MR imaging is useful in discriminating acute from chronic myocardial infarct[51]. In a study of 73 patients with acute and chronic infarct by Abdel-Aty et al[51] MR imaging was effective (96% sensitive) in discriminating acute from chronic infarct. In a preclinical study, Saeed et al[52] observed lack of deferential enhancement of chronic infarct after administration of blood pool MR contrast media, but not after clinically approved extracellular MR contrast media. Unlike acute reperfused infarct, chronic infarct lacks edema, MO or hemorrhage because they are resorbed.
Expanding the use of coronary MDCT into clinical practice has sparked interest in using the modality for assessing myocardial viability[53]. Gerber et al[9] showed the similarity between infarct size measured on DE-MDCT and DE-MR imaging in a series of patients. The investigators demonstrated good agreement (82%, k = 0.61, P < 0.001) between the two measurements (Figure 2). Nikolaou et al[54] demonstrated the diagnostic power of MDCT in assessing the presence, age, and size of myocardial infarct in 106 patients. Myocardial infarct was found in 27 of 106 patients. MDCT detected 23 of 27 patients with infarct with a sensitivity of 85%, specificity of 91% and accuracy of 90%.
Transfer of angiogenic genes to ischemic myocardium is a promising approach under development for the treatment of myocardial infarct. MR and CT imaging may be a useful tool for defining myocardial infarct and for use in targeting the infarct for gene and stem cell therapies[55-60]. Catheter-based fluoroscopic MR and MDCT imaging has been recently used for delivering these therapies transendocardially[37,58]. Sequential cine and DE-MR imaging showed great sensitivity in detecting improvement in ejection fraction, reduction of LV volumes and infarct size (Figures 3 and 4) after intramyocardial delivery of different angiogenic genes[58-60]. Figure 5 demonstrates the increase in vascular density of infarcted myocardium 8 wk after intramyocardial delivery of vascular growth factor gene. Thus, MR imaging provides great promise in evaluating gene and cell therapies[58,61-63].
Figure 3.
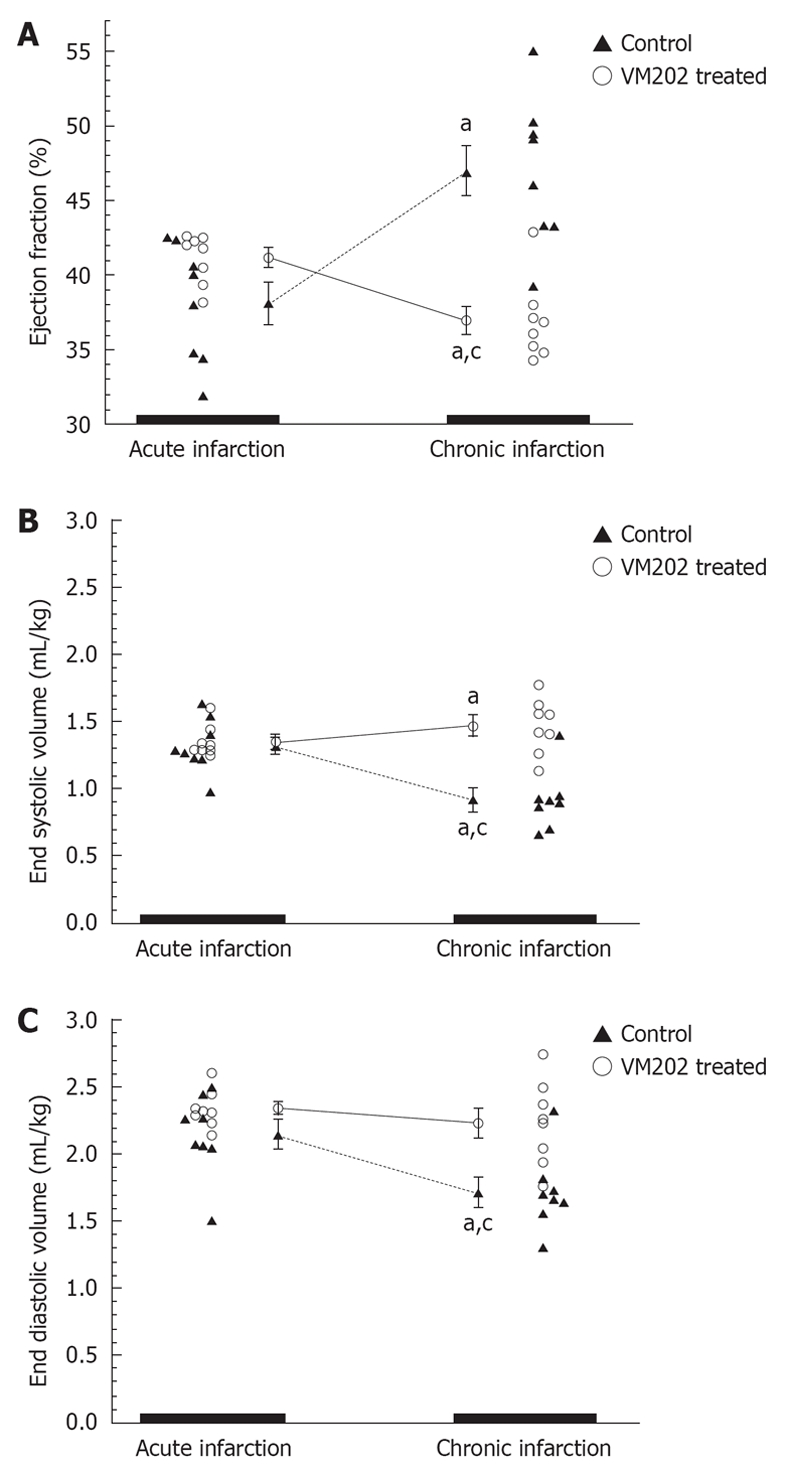
The ejection fraction (A), end systolic volumes (B) and end diastolic volumes (C) are shown for control and hepatocyte growth factor gene (VM202) treated animals. The hepatocyte growth factor gene administered at 3 d after reperfusion significantly decreased end diastolic (mL/kg) and end systolic volumes at 8 wk compared to 3 d infarct (aP < 0.05) and control group (cP < 0.05). Control animals at 8 wk showed a significant decrease in ejection fraction and significant increase in end systolic and end diastolic volumes compared with 3 d infarct[59].
Figure 4.
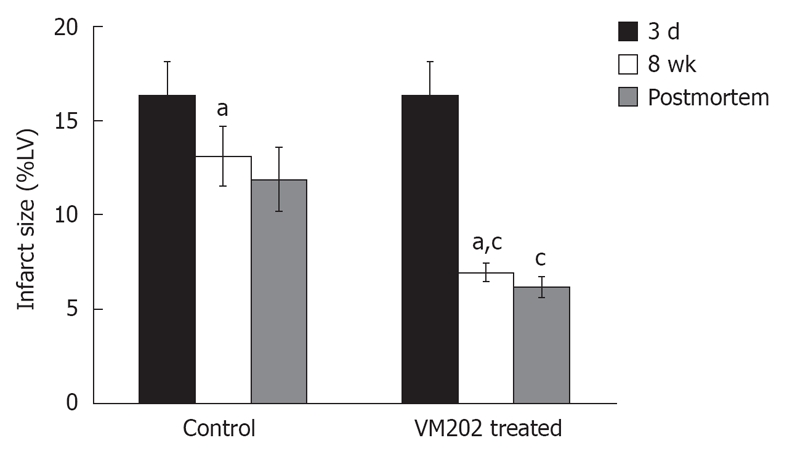
Histogram shows the difference in infarct size prior to intramyocardial gene delivery at 3 d and 8 wk after infarction in control animals (left block) and animals treated with hepatocyte growth factor gene (VM202) (right block) measured on DE-MR imaging (black and white bars) and postmortem (gray bars). Note the decline in infarct size was greater in gene treated animals compared with control animals. aP < 0.05 compared with 3 d acute infarction. cP < 0.05 compared with 8 wk chronic infarction in control animals. %LV: Percentage of LV mass[59].
Figure 5.
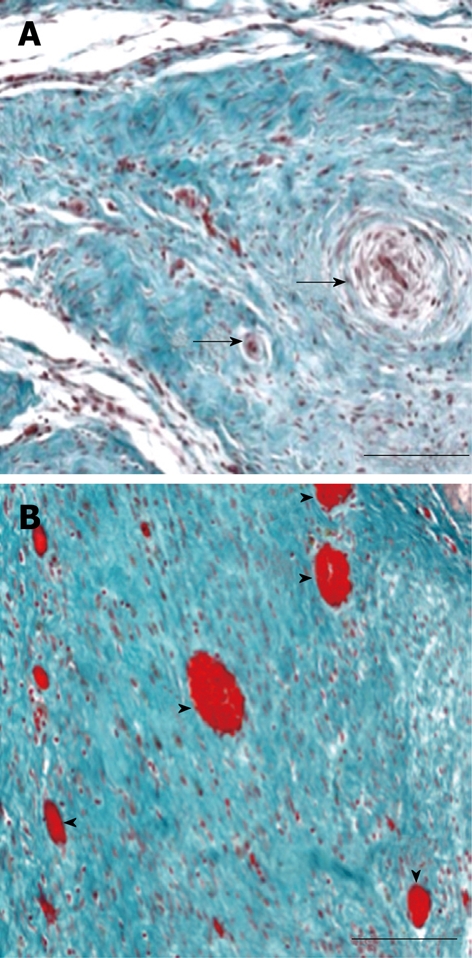
Micrographs of representative infarcts in control and VEGF-gene treated swine. A: The infarct in the control animal shows no appreciable angiogenesis and residual blood vessels have been remodeled, as shown by the thick vascular wall and small lumen (black arrows). B: VEGF-gene treated animal contains numerous blood vessels (arrowheads) in linear array representing injection track (calibration bar = 200 μm)[60].
A preliminary experimental study investigated MDCT for the assessment of the efficacy of stem cells in infarcted myocardium and showed that this technique has the capability to elucidate new therapies[37]. The radiation doses in MDCT may limit such application in patients because therapeutic studies need a minimum of two imaging sessions. The potential advantages of using MDCT in assessing myocardial viability may be related to faster acquisition time compared with cardiac MR imaging and the ability to scan claustrophobic or uncooperative patients. Additionally, MDCT angiography is the method of choice for direct visualization of the coronary arteries, coronary calcium and atherosclerosis in its earliest stages; when treatment can be most effective in preventing subsequent heart attacks or sudden death. On the other hand, MR imaging has other advantages over MDCT including: (1) the absence of radiation exposure; (2) the lack of nephrotoxic iodinated contrast media; and (3) it allows for repeated scans, particularly in pediatric patients. It should be noted that MR contrast media cause nephrogenic systemic fibrosis in patients with compromised renal function[64].
MANIFESTATIONS OF REPERFUSION INJURY
MO zone
In the setting of an acute myocardial infarction, treatment strategies have primarily focused on the management of culprit occlusions in the epicardial coronary arteries[65]. Interventional cardiologists, however, found that the benefits of revascularization of the epicardial coronary artery is limited and later discovered that MO is a major component of infarction, which is frequently seen after revascularization of the epicardial coronary artery. Investigators found that the formation of MO is related to plaque emboli, endothelial swelling, inflammation, extravascular edema and microvascular spasm[66].
How best to measure MO in terms of predictive values is an important question. A variety of techniques, flow or frame count[67,68], myocardial blush grade[69] coronary Doppler imaging[70], contrast echocardiography[68], contrast-enhanced MR imaging[71,72] and contrast enhanced MDCT[32,38], have been used to detect MO zone in patients with TIMI (thrombosis in myocardial infarction). The quality of some of these techniques, however, is suboptimal due to poor spatial resolution.
MR and MDCT imaging delineated MO as a hypoenhanced zone in the core of acutely reperfused infarct (Figure 6). The delineation is attributed to inadequate contrast media delivery during first pass perfusion (17 s blood recirculation time)[46], early (1-2 min; equilibrium phase of contrast medium in the blood and tissue interstitium)[72] and delayed (10 min; peak enhancement of myocardial infarct) MR imaging[73]. The extent of MO after bolus administration of contrast media is time dependent and varies between first pass, early contrast enhancement and DE-imaging because it is governed by 2 processes namely: perfusion and passive diffusion. Figure 6 illustrates the comparable MO extent measured on DE-MR and DE-MDCT imaging in a patient subjected to reperfusion.
Figure 6.

Head-to-head comparison of DE-MR (A) and DE-MDCT images (B) showing the dark MO zone (arrowheads) surrounded by a bright enhanced infarct in a reperfused patient (arrows)[38].
Both early and delayed persistent MO has been shown to predict post-infarct LV remodeling and outcome in patients with ST-elevation myocardial infarction (STEMI)[72-76]. A recent study showed that MO detected on DE-MR imaging is more frequently observed in patients with the most severe LV dyfunction[77]. A clinical study in 25 patients demonstrated that delayed persistent MO is also high (32%) in the No-STEMI population after successful percutaneous coronary intervention[78], but less than that observed in STEMI patients[73,79]. Recent studies indicated that MO is predictive of increasing recurrent myocardial infarct, congestive heart failure, stroke and death up to 16 mo after the event[72,73,75].
Preclinical studies showed that the extent of MO in reperfused infarct is less variable in the first 10 min after administration of blood pool MR contrast media, which may be attributed to slow convection of the contrast medium in the interstitium and its retention in the blood pool[80].
INTERSTITIAL HEMORRHAGE
Interstitial hemorrhage is another component of reperfusion injury in patients with ST-segment elevation due to acute infarct. Its presence is an important marker for myocardial and microvascular damage. Interstitial hemorrhage causes signal loss on T2*-weighted images, which depends on the status of hemoglobin (oxyhemoglobin, deoxyhemoglobin, or methemoglobin) and the presence of blood products such as ferritin and hemosiderin[15,81]. Figure 7 demonstrates intensive hypointense interstitial hemorrhage 3 d after reperfusion in a swine model on T2*-weighted (susceptibility) turbo spin echo MR imaging. O’Regan et al[82] quantified the extent of interstitial hemorrhage on T2*-weighted mapping and compared it with other indices of ischemic injury, such as area at risk and infarct size.
Figure 7.
Multislice T2*-weighted (susceptibility) turbo spin echo images show severe interstitial hemorrhage in the core (arrowheads) of the hyperintense edematous area at risk (arrows) 3 d after reperfusion.
Although non-enhancing myocardium within the infarct is thought to represent MO[46], it is possible that the presence of blood products may also contribute to its low signal seen on MR images[15]. Ganame et al[83] also used T2-weighted MR imaging to measure the extent of hemorrhage and area at risk in 98 patients with a large reperfused infarct. Based on this technique, the investigators demonstrated a high prevalence of myocardial hemorrhage of 25% in this patient cohort, more common amongst patients with large transmural infarct and severe LV global and regional dysfunction.
PERI-INFARCT ZONE
A mixed population of viable and non-viable myocytes has been found around acutely infarcted myocardium, a territory previously described as the peri-infarct zone[84,85]. Microscopic studies indicated that the peri-infarct zone has leaky microvessels[84-88]. The peri-infarct zone has consistently been substantiated by a variety of modalities, including echocardiography[89], radiopaque bead arrays[90] and MR imaging[82]. The physiological correlates of the peri-infarct zone using MR imaging have been described[91].
MR or CT contrast media have been used to define the peri-infarct zone[31,88], identify patients who are susceptible to ventricular arrhythmias[92] and predict post-infarct mortality[93]. Using preclinical necrosis-specific (mesoporphyrin) and extracellular MR contrast medium in a seminal animal study, Saeed et al[94] demonstrated MR characterization of the peri-infarct zone. They found that the enhanced region on DE-MR imaging is larger than the true infarct delineated on TTC staining, which was identical to regions enhanced by the necrosis-specific contrast medium. The difference in enhancement regions demarcated by the 2 contrast media was considered the peri-infarct zone. At that time our findings were in contrast to other groups who demonstrated that differentially enhanced myocardium represents necrotic tissue. Recent clinical MR studies confirmed our findings[31,92,95] and went further to report the associations between infarct size, the peri-infarct zone and inducible ventricular arrhythmias[31,92,93,95]. Yan et al[93] found that the extent of the peri-infarct provides prognostic value for mortality incremental to that offered by ejection fraction and LV end-diastolic volume. On the other hand, the existence of viable myocytes in a large peri-infarct zone may raise an interesting hypothesis that reperfusion could be beneficial by reducing arrhythmogenic triggers, despite the apparent lack of measurable improvement in contractile function. Furthermore, implantable cardioverter-defibrillator therapy may be warranted in such high-risk patients identified by MR due to the creation of multiple action potential circuits derived from the peri-infarct zone.
CALCIUM DEPOSIT
Considering the deleterious effects of calcium overload in reperfused myocardium[96,97], the development of a noninvasive technique to visualize calcium deposits in infarcted myocardium may have clinical value. Noninvasive imaging techniques that directly incorporate the spatial distribution of calcium in infarcted myocardium may help our understanding of the relationship between calcium deposits in the myocardium, the rate of infarct resorption, and LV function[98].
Calcium deposited in infarcted myocardium has previously been used as a target for 99m-Tc pyrophosphate scintigraphy to delineate reperfused myocardial infarcts in patients[99,100]. For over a decade, electron-beam CT has been clinically used for calcium scoring in the coronary arteries of patients. In addition to calcium scoring and detection of coronary stenosis, modern MDCT scanners have been used to assess the extent of acute and chronic infarct[10,101].
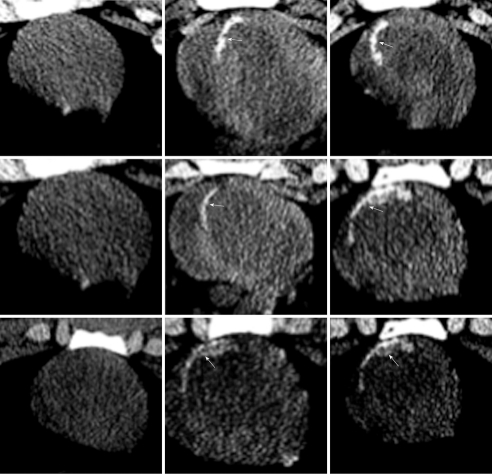
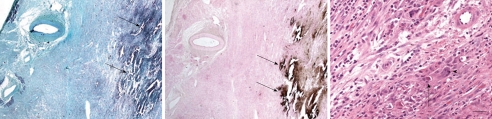
In a recent animal study, non-contrast MDCT images depicted calcium deposits as “hot-spots” 1 wk after reperfusion (Figure 8). The presence of calcium deposits on non-contrast MDCT images, however, was transient and specific to acute infarct because the calcium was resorbed from the infarct scar at 8 wk as shown on CT imaging and histopathology (Figure 9). Histopathology confirmed the engulfment of the deposited calcium by macrophages[102]. Noninvasive evaluation of the beneficial administration of calcium channel blockers on calcium overload during reperfusion may be possible using MDCT.
Figure 8.
Non-contrast enhanced MDCT imaging demonstrates deposited calcium 7 d after reperfusion (right block arrows). In all 3 animals (the 3 rows) the deposited calcium was not evident at 2-3 h after reperfusion on non-contrast enhanced MDCT (left block)[102].
Figure 9.
Histopathology from at 1-wk-old reperfused infarct shows calcium deposits as a black-brown precipitation product (arrows) on Masson trichrome (A) and the special calcium stain (von Kossa stain) (B). At 8 wk the stain shows traces of calcium deposits (C, arrow) surrounded by giant cell (arrowhead). Apparently the giant macrophages digest deposited calcium (calibration bars = 20 mm)[102].
HETEROGENOUS MICROINFARCT
Heterogeneous microinfarct results from showers of microemboli shed following coronary intervention. Clinical studies showed that 42% of patients experience major cardiac problems, such as heart failure and sudden death, after percutaneous coronary angioplasty[103,104]. High incidences (30%-50%) of defects on myocardial perfusion scintigraphy have also been detected soon after coronary balloon angioplasty and with optimally implanted stents[105,106] and these events continued during follow-up[107-109]. The emboli sizes, collected by distal protection devices during percutaneous coronary intervention, differ widely (47-2503 μm)[110] and the size and number of ruptured atherosclerotic plaques is a key event in the pathogenesis of heterogeneous microinfarct[111]. A recent clinical study demonstrated that the volume of embolized material relates directly to the volume of new necrosis detected by delayed-enhancement MR imaging[112]. The American College of Cardiology and the European Society of Cardiology recently recognized the detrimental consequences of coronary microembolization in patients in their 2007 guidelines[113].
In a preclinical study, a microinfarcted region was detected on first pass perfusion imaging 2-3 h as well as 7 d after embolization[114]. DE-MR imaging failed to define microinfarct early but at 7 d it was clearly visible. Microinfarct was visualized as bright heterogeneous sub-regions on DE-MR imaging (Figure 8). Furthermore, DE-MR and DE-MDCT imaging is sensitive in detecting the direction of embolized vessels in experimental animals (Figure 9).
Several studies demonstrated the potential of DE-MR imaging in visualizing heterogeneous microinfarct in patients[102,112,114-118]. Ricciardi et al[118] and Choi et al[119] have demonstrated heterogeneous microinfarct in patients on DE-MR imaging. The investigators found a link between MR visualization of microinfarct and impaired myocardial perfusion (Figure 10)[119]. Selvanayagam et al[120] demonstrated that the extent of elevated troponin I levels 24 h after coronary intervention is directly related to the extent of microinfarct on DE-MR imaging. More recently, they examined myocardial perfusion and microinfarct serially after percutaneous coronary intervention using MR technique[117]. They found that myocardial perfusion is reduced in myocardial segments with new microinfarct 24 h after percutaneous coronary intervention. It has been shown that microinfarct causes severe and persistent LV dysfunction and in some cases sudden death[40]. Investigators concluded that even small amounts of infarct (microinfarct) detected on DE-images provides prognostic value beyond the routine clinical, angiographic and functional predictors[25].
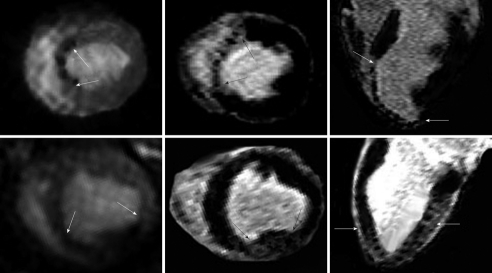
Figure 10.
Preclinical study shows the patchy enhanced embolized region in two animals after delivery of embolic materials (7200 microsphere count, 100-300 μm diameters). Animal one received the embolic materials in the left anterior descending coronary artery causing perfusion deficit in the antero-septal wall 2-3 h after delivery of the embolic materials (top left, arrows), while animal two received the embolic materials in the left circumflex coronary artery causing perfusion deficit in the inferior wall (bottom left, arrows). Short (center images) and long (right images) axis views of DE-MR imaging illustrates the microinfarct of the same ischemic territory 7 d after embolization[114].
Heterogeneous microinfarct is not limited to percutaneous coronary intervention for atherosclerosis but include a wide range of diseases, such as valvular disease, prosthetic valve, endocarditis, cardiomyopathy with mural thrombus, arrhythmias and during heart-lung-bypass[121-125]. This pathology has also been reported in patients with hypertension, diabetes[126], systemic lupus erythematosus[127] and sickle cell disease, where abnormally shaped erythrocytes obstructing the capillaries and small arterioles may cause myocardial fibrosis[128]. Therefore, early detection and subsequent effects of microinfarct need highly sensitive imaging modalities.
CONCLUSION
The clinical role of MR and MDCT imaging continues to expand supported by the advances in software and hardware. The strength of MR imaging relies on the variety of pulse sequences and the ability to noninvasively provide information on myocardial structure, function and perfusion in a single imaging session. The complementary use of both MR and MDCT imaging allows the components and manifestations of reperfusion injury including myocardial edema, interstitial hemorrhage, calcium deposition and MO to be visualized. MR imaging can be used serially and noninvasively in assessing the consequences of reperfusion injury because there is no radiation exposure or administration of radioactive materials. MDCT is better suited for assessing coronary artery stenosis and as an alternative technique for assessing viability in patients where MR imaging is contraindicated. Clinical MR studies found that the presence of transmural infarct, MO and peri-infarct are excellent predictor of poor post-infarct recovery and mortality[31,92,93,95,129]. Heterogeneous cardiac microinfarct detected on contrast enhanced MR and MDCT imaging has a prolonged effect on LV function and perfusion (Figure 11)[130]. The clinical significance of deposited calcium, detected on MDCT, in acute homogeneous infarct requires further studies. Recent preclinical MR studies provided ample evidence that angiogenic genes and stem cells, delivered transendocardially under MR-guidance, have beneficial effects on myocardial function, perfusion and viability. Imaging protocols are in progress to monitor the long-term efficacy of such therapeutic agents. Cardiac MR and MDCT imaging can characterize reperfusion injury components and manifestations.
Figure 11.
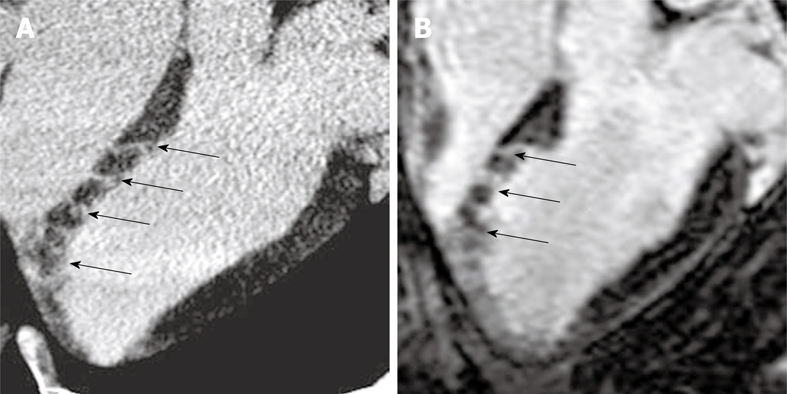
DE-multi-slice MDCT (A) and DE-MR (B) images from experimentally embolized LAD coronary artery show good correspondence between the modalities in defining heterogenous microinfarct. Both modalities show enhanced stripes (arrows) of microinfarct extending from the epicardium to the endocardium mapping occluded microvessels.
Footnotes
Peer reviewers: Yahya Paksoy, MD, Professor, Department of Radiology, Selcuk University Meram Chool of Medicine, 42085 Konya, Turkey; Patrick M Colletti, MD, Professor of Radiology and Medicine, Director Nuclear Medicine Fellowship, USC Keck School of Medicine, Professor of Biokinesiology, Professor of Pharmacology and Pharmaceutical Sciences, Chief of MRI, LAC+USC Imaging Science Center, University of Southern California, 1200 N State Street Room 3566, Los Angeles, CA 90033, United States
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
References
- 1.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 3.Amano Y, Takayama M, Kumita S. Contrast-enhanced myocardial T1-weighted scout (Look-Locker) imaging for the detection of myocardial damages in hypertrophic cardiomyopathy. J Magn Reson Imaging. 2009;30:778–784. doi: 10.1002/jmri.21921. [DOI] [PubMed] [Google Scholar]
- 4.Sakuma H, Suzawa N, Ichikawa Y, Makino K, Hirano T, Kitagawa K, Takeda K. Diagnostic accuracy of stress first-pass contrast-enhanced myocardial perfusion MRI compared with stress myocardial perfusion scintigraphy. AJR Am J Roentgenol. 2005;185:95–102. doi: 10.2214/ajr.185.1.01850095. [DOI] [PubMed] [Google Scholar]
- 5.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.van der Vleuten PA, Willems TP, Götte MJ, Tio RA, Greuter MJ, Zijlstra F, Oudkerk M. Quantification of global left ventricular function: comparison of multidetector computed tomography and magnetic resonance imaging. a meta-analysis and review of the current literature. Acta Radiol. 2006;47:1049–1057. doi: 10.1080/02841850600977760. [DOI] [PubMed] [Google Scholar]
- 7.George RT, Silva C, Cordeiro MA, DiPaula A, Thompson DR, McCarthy WF, Ichihara T, Lima JA, Lardo AC. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153–160. doi: 10.1016/j.jacc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Mahnken AH, Bruners P, Katoh M, Wildberger JE, Günther RW, Buecker A. Dynamic multi-section CT imaging in acute myocardial infarction: preliminary animal experience. Eur Radiol. 2006;16:746–752. doi: 10.1007/s00330-005-0057-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 10.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404. doi: 10.1161/CIRCULATIONAHA.105.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Dorado D, Oliveras J. Myocardial oedema: a preventable cause of reperfusion injury? Cardiovasc Res. 1993;27:1555–1563. doi: 10.1093/cvr/27.9.1555. [DOI] [PubMed] [Google Scholar]
- 12.García-Dorado D, Oliveras J, Gili J, Sanz E, Pérez-Villa F, Barrabés J, Carreras MJ, Solares J, Soler-Soler J. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 13.Boxt LM, Hsu D, Katz J, Detweiler P, Mclaughlin S, Kolb TJ, Spotnitz HM. Estimation of myocardial water content using transverse relaxation time from dual spin-echo magnetic resonance imaging. Magn Reson Imaging. 1993;11:375–383. doi: 10.1016/0730-725x(93)90070-t. [DOI] [PubMed] [Google Scholar]
- 14.Arai AE. Using magnetic resonance imaging to characterize recent myocardial injury: utility in acute coronary syndrome and other clinical scenarios. Circulation. 2008;118:795–796. doi: 10.1161/CIRCULATIONAHA.108.797373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basso C, Corbetti F, Silva C, Abudureheman A, Lacognata C, Cacciavillani L, Tarantini G, Marra MP, Ramondo A, Thiene G, et al. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol. 2007;100:1322–1327. doi: 10.1016/j.amjcard.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Stork A, Lund GK, Muellerleile K, Bansmann PM, Nolte-Ernsting C, Kemper J, Begemann PG, Adam G. Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur Radiol. 2006;16:2350–2357. doi: 10.1007/s00330-006-0232-3. [DOI] [PubMed] [Google Scholar]
- 18.Tilak GS, Hsu LY, Hoyt RF Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 19.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 20.Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334–341. doi: 10.1161/01.cir.92.3.334. [DOI] [PubMed] [Google Scholar]
- 21.Santoro GM, Bisi G, Sciagrà R, Leoncini M, Fazzini PF, Meldolesi U. Single photon emission computed tomography with technetium-99m hexakis 2-methoxyisobutyl isonitrile in acute myocardial infarction before and after thrombolytic treatment: assessment of salvaged myocardium and prediction of late functional recovery. J Am Coll Cardiol. 1990;15:301–314. doi: 10.1016/s0735-1097(10)80053-1. [DOI] [PubMed] [Google Scholar]
- 22.Wackers FJ, Gibbons RJ, Verani MS, Kayden DS, Pellikka PA, Behrenbeck T, Mahmarian JJ, Zaret BL. Serial quantitative planar technetium-99m isonitrile imaging in acute myocardial infarction: efficacy for noninvasive assessment of thrombolytic therapy. J Am Coll Cardiol. 1989;14:861–873. doi: 10.1016/0735-1097(89)90456-7. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro MD, Guarraia DL, Moloo J, Cury RC. Evaluation of acute coronary syndromes by cardiac magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:25–32. doi: 10.1097/RMR.0b013e31816fd81d. [DOI] [PubMed] [Google Scholar]
- 24.Bragadeesh T, Jayaweera AR, Pascotto M, Micari A, Le DE, Kramer CM, Epstein FH, Kaul S. Post-ischaemic myocardial dysfunction (stunning) results from myofibrillar oedema. Heart. 2008;94:166–171. doi: 10.1136/hrt.2006.102434. [DOI] [PubMed] [Google Scholar]
- 25.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 26.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 28.Achenbach S, Daniel WG. Current role of cardiac computed tomography. Herz. 2007;32:97–107. doi: 10.1007/s00059-007-2961-8. [DOI] [PubMed] [Google Scholar]
- 29.Kopp AF, Heuschmid M, Reimann A, Kuettner A, Beck T, Ohmer M, Burgstahler C, Brodoefel H, Claussen CD, Schroeder S. Evaluation of cardiac function and myocardial viability with 16- and 64-slice multidetector computed tomography. Eur Radiol. 2005;15 Suppl 4:D15–D20. doi: 10.1007/s10406-005-0133-6. [DOI] [PubMed] [Google Scholar]
- 30.Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, Serruys PW, Krestin GP, de Feyter PJ. Noninvasive assessment of coronary plaque burden using multislice computed tomography. Am J Cardiol. 2005;95:1165–1169. doi: 10.1016/j.amjcard.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Schuleri KH, Centola M, George RT, Amado LC, Evers KS, Kitagawa K, Vavere AL, Evers R, Hare JM, Cox C, et al. Characterization of peri-infarct zone heterogeneity by contrast-enhanced multidetector computed tomography: a comparison with magnetic resonance imaging. J Am Coll Cardiol. 2009;53:1699–1707. doi: 10.1016/j.jacc.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furtado AD, Carlsson M, Wintermark M, Ordovas K, Saeed M. Identification of residual ischemia, infarction, and microvascular impairment in revascularized myocardial infarction using 64-slice MDCT. Contrast Media Mol Imaging. 2008;3:198–206. doi: 10.1002/cmmi.253. [DOI] [PubMed] [Google Scholar]
- 33.Baks T, Cademartiri F, Moelker AD, van der Giessen WJ, Krestin GP, Duncker DJ, de Feyter PJ. Assessment of acute reperfused myocardial infarction with delayed enhancement 64-MDCT. AJR Am J Roentgenol. 2007;188:W135–W137. doi: 10.2214/AJR.05.1176. [DOI] [PubMed] [Google Scholar]
- 34.Mahnken AH, Bruners P, Mühlenbruch G, Emmerich M, Hohl C, Günther RW, Wildberger JE. Low tube voltage improves computed tomography imaging of delayed myocardial contrast enhancement in an experimental acute myocardial infarction model. Invest Radiol. 2007;42:123–129. doi: 10.1097/01.rli.0000251577.68223.84. [DOI] [PubMed] [Google Scholar]
- 35.Kondo C, Mori S, Endo M, Kusakabe K, Suzuki N, Hattori A, Kusakabe M. Real-time volumetric imaging of human heart without electrocardiographic gating by 256-detector row computed tomography: initial experience. J Comput Assist Tomogr. 2005;29:694–698. doi: 10.1097/01.rct.0000173844.89988.37. [DOI] [PubMed] [Google Scholar]
- 36.Sanz J, Weeks D, Nikolaou K, Sirol M, Rius T, Rajagopalan S, Dellegrottaglie S, Strobeck J, Fuster V, Poon M. Detection of healed myocardial infarction with multidetector-row computed tomography and comparison with cardiac magnetic resonance delayed hyperenhancement. Am J Cardiol. 2006;98:149–155. doi: 10.1016/j.amjcard.2006.01.093. [DOI] [PubMed] [Google Scholar]
- 37.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 38.Jacquier A, Boussel L, Amabile N, Bartoli JM, Douek P, Moulin G, Paganelli F, Saeed M, Revel D, Croisille P. Multidetector computed tomography in reperfused acute myocardial infarction. Assessment of infarct size and no-reflow in comparison with cardiac magnetic resonance imaging. Invest Radiol. 2008;43:773–781. doi: 10.1097/RLI.0b013e318181c8dd. [DOI] [PubMed] [Google Scholar]
- 39.Jacquier A, Revel D, Saeed M. MDCT of the myocardium: a new contribution to ischemic heart disease. Acad Radiol. 2008;15:477–487. doi: 10.1016/j.acra.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, Schnackenburg B, Delius W, Mudra H, Wolfram D, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 41.Zamorano J, Delgado J, Almería C, Moreno R, Gómez Sánchez M, Rodrigo J, Fernández C, Ferreiros J, Rufilanchas J, Sánchez-Harguindey L. Reason for discrepancies in identifying myocardial viability by thallium-201 redistribution, magnetic resonance imaging, and dobutamine echocardiography. Am J Cardiol. 2002;90:455–459. doi: 10.1016/s0002-9149(02)02513-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim RJ, Hillenbrand HB, Judd RM. Evaluation of myocardial viability by MRI. Herz. 2000;25:417–430. doi: 10.1007/s000590050034. [DOI] [PubMed] [Google Scholar]
- 43.Weiss CR, Aletras AH, London JF, Taylor JL, Epstein FH, Wassmuth R, Balaban RS, Arai AE. Stunned, infarcted, and normal myocardium in dogs: simultaneous differentiation by using gadolinium-enhanced cine MR imaging with magnetization transfer contrast. Radiology. 2003;226:723–730. doi: 10.1148/radiol.2263012196. [DOI] [PubMed] [Google Scholar]
- 44.Connelly KA, Detsky JS, Graham JJ, Paul G, Vijayaragavan R, Dick AJ, Wright GA. Multicontrast late gadolinium enhancement imaging enables viability and wall motion assessment in a single acquisition with reduced scan times. J Magn Reson Imaging. 2009;30:771–777. doi: 10.1002/jmri.21907. [DOI] [PubMed] [Google Scholar]
- 45.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 46.Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Müller V, Mester J, Higgins CB, Adam G, Meinertz T. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology. 2004;232:49–57. doi: 10.1148/radiol.2321031127. [DOI] [PubMed] [Google Scholar]
- 47.Sandstede JJ, Lipke C, Beer M, Harre K, Pabst T, Kenn W, Neubauer S, Hahn D. Analysis of first-pass and delayed contrast-enhancement patterns of dysfunctional myocardium on MR imaging: use in the prediction of myocardial viability. AJR Am J Roentgenol. 2000;174:1737–1740. doi: 10.2214/ajr.174.6.1741737. [DOI] [PubMed] [Google Scholar]
- 48.Kwong RY, Yucel EK. Cardiology patient pages. Computed tomography scan and magnetic resonance imaging. Circulation. 2003;108:e104–e106. doi: 10.1161/01.CIR.0000086899.32832.EC. [DOI] [PubMed] [Google Scholar]
- 49.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, Marra MP, Napodano M, Ramondo A, Iliceto S. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, et al. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 52.Saeed M, Weber O, Lee R, Do L, Martin A, Saloner D, Ursell P, Robert P, Corot C, Higgins CB. Discrimination of myocardial acute and chronic (scar) infarctions on delayed contrast enhanced magnetic resonance imaging with intravascular magnetic resonance contrast media. J Am Coll Cardiol. 2006;48:1961–1968. doi: 10.1016/j.jacc.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 53.Nieman K, Shapiro MD, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Gold HK, Jang IK, Brady TJ, Cury RC. Reperfused myocardial infarction: contrast-enhanced 64-Section CT in comparison to MR imaging. Radiology. 2008;247:49–56. doi: 10.1148/radiol.2471070332. [DOI] [PubMed] [Google Scholar]
- 54.Nikolaou K, Flohr T, Knez A, Rist C, Wintersperger B, Johnson T, Reiser MF, Becker CR. Advances in cardiac CT imaging: 64-slice scanner. Int J Cardiovasc Imaging. 2004;20:535–540. doi: 10.1007/s10554-004-7015-1. [DOI] [PubMed] [Google Scholar]
- 55.Dick AJ, Lederman RJ. MRI-guided myocardial cell therapy. Int J Cardiovasc Intervent. 2005;7:165–170. doi: 10.1080/14628840500342946. [DOI] [PubMed] [Google Scholar]
- 56.Dicks D, Saloner D, Martin A, Carlsson M, Saeed M. Percutaneous transendocardial VEGF gene therapy: MRI guided delivery and characterization of 3D myocardial strain. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 57.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saeed M, Martin A, Jacquier A, Bucknor M, Saloner D, Do L, Ursell P, Su H, Kan YW, Higgins CB. Permanent coronary artery occlusion: cardiovascular MR imaging is platform for percutaneous transendocardial delivery and assessment of gene therapy in canine model. Radiology. 2008;249:560–571. doi: 10.1148/radiol.2491072068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeed M, Martin A, Ursell P, Do L, Bucknor M, Higgins CB, Saloner D. MR assessment of myocardial perfusion, viability, and function after intramyocardial transfer of VM202, a new plasmid human hepatocyte growth factor in ischemic swine myocardium. Radiology. 2008;249:107–118. doi: 10.1148/radiol.2483071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saeed M, Saloner D, Martin A, Do L, Weber O, Ursell PC, Jacquier A, Lee R, Higgins CB. Adeno-associated viral vector-encoding vascular endothelial growth factor gene: effect on cardiovascular MR perfusion and infarct resorption measurements in swine. Radiology. 2007;243:451–460. doi: 10.1148/radiol.2432060928. [DOI] [PubMed] [Google Scholar]
- 61.Carlsson M, Wilson M, Martin AJ, Saeed M. Myocardial microinfarction after coronary microembolization in swine: MR imaging characterization. Radiology. 2009;250:703–713. doi: 10.1148/radiol.2503081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacquier A, Higgins CB, Martin AJ, Do L, Saloner D, Saeed M. Injection of adeno-associated viral vector encoding vascular endothelial growth factor gene in infarcted swine myocardium: MR measurements of left ventricular function and strain. Radiology. 2007;245:196–205. doi: 10.1148/radiol.2451061077. [DOI] [PubMed] [Google Scholar]
- 63.Saeed M, Martin AJ, Lee RJ, Weber O, Revel D, Saloner D, Higgins CB. MR guidance of targeted injections into border and core of scarred myocardium in pigs. Radiology. 2006;240:419–426. doi: 10.1148/radiol.2402051086. [DOI] [PubMed] [Google Scholar]
- 64.Weinreb JC, Abu-Alfa AK. Gadolinium-based contrast agents and nephrogenic systemic fibrosis: why did it happen and what have we learned? J Magn Reson Imaging. 2009;30:1236–1239. doi: 10.1002/jmri.21979. [DOI] [PubMed] [Google Scholar]
- 65.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 66.Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LW, Braunwald E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation. 1980;62:945–952. doi: 10.1161/01.cir.62.5.945. [DOI] [PubMed] [Google Scholar]
- 67.Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 68.Ito H, Taniyama Y, Iwakura K, Nishikawa N, Masuyama T, Kuzuya T, Hori M, Higashino Y, Fujii K, Minamino T. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33:654–660. doi: 10.1016/s0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 69.Gibson CM, Murphy SA, Rizzo MJ, Ryan KA, Marble SJ, McCabe CH, Cannon CP, Van de Werf F, Braunwald E. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Circulation. 1999;99:1945–1950. doi: 10.1161/01.cir.99.15.1945. [DOI] [PubMed] [Google Scholar]
- 70.Iwakura K, Ito H, Takiuchi S, Taniyama Y, Nakatsuchi Y, Negoro S, Higashino Y, Okamura A, Masuyama T, Hori M, et al. Alternation in the coronary blood flow velocity pattern in patients with no reflow and reperfused acute myocardial infarction. Circulation. 1996;94:1269–1275. doi: 10.1161/01.cir.94.6.1269. [DOI] [PubMed] [Google Scholar]
- 71.Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–1014. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 72.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 73.Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 74.Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734–2741. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 75.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 76.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 77.Ørn S, Manhenke C, Greve OJ, Larsen AI, Bonarjee VV, Edvardsen T, Dickstein K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J. 2009;30:1978–1985. doi: 10.1093/eurheartj/ehp219. [DOI] [PubMed] [Google Scholar]
- 78.Mewton N, Bonnefoy E, Revel D, Ovize M, Kirkorian G, Croisille P. Presence and extent of cardiac magnetic resonance microvascular obstruction in reperfused non-ST-elevated myocardial infarction and correlation with infarct size and myocardial enzyme release. Cardiology. 2009;113:50–58. doi: 10.1159/000167042. [DOI] [PubMed] [Google Scholar]
- 79.Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ, van Rossum AC. Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology. 2009;250:363–370. doi: 10.1148/radiol.2502080739. [DOI] [PubMed] [Google Scholar]
- 80.Saeed M, Higgins CB, Geschwind JF, Wendland MF. T1-relaxation kinetics of extracellular, intracellular and intravascular MR contrast agents in normal and acutely reperfused infarcted myocardium using echo-planar MR imaging. Eur Radiol. 2000;10:310–318. doi: 10.1007/s003300050050. [DOI] [PubMed] [Google Scholar]
- 81.van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, Duncker DJ, Wielopolski PA. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J. 2006;27:1620–1626. doi: 10.1093/eurheartj/ehl059. [DOI] [PubMed] [Google Scholar]
- 82.O'Regan DP, Ahmed R, Karunanithy N, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA. Reperfusion hemorrhage following acute myocardial infarction: assessment with T2* mapping and effect on measuring the area at risk. Radiology. 2009;250:916–922. doi: 10.1148/radiol.2503081154. [DOI] [PubMed] [Google Scholar]
- 83.Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–1449. doi: 10.1093/eurheartj/ehp093. [DOI] [PubMed] [Google Scholar]
- 84.Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698–708. doi: 10.1148/radiology.211.3.r99jn41698. [DOI] [PubMed] [Google Scholar]
- 85.Saeed M, Wendland MF, Masui T, Connolly AJ, Derugin N, Brasch RC, Higgins CB. Myocardial infarction: assessment with an intravascular MR contrast medium. Work in progress. Radiology. 1991;180:153–160. doi: 10.1148/radiology.180.1.2052685. [DOI] [PubMed] [Google Scholar]
- 86.Dymarkowski S, Ni Y, Miao Y, Bogaert J, Rademakers F, Bosmans H, Marchal G. Value of t2-weighted magnetic resonance imaging early after myocardial infarction in dogs: comparison with bis-gadolinium-mesoporphyrin enhanced T1-weighted magnetic resonance imaging and functional data from cine magnetic resonance imaging. Invest Radiol. 2002;37:77–85. doi: 10.1097/00004424-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Krombach GA, Higgins CB, Chujo M, Saeed M. Blood pool contrast-enhanced MRI detects suppression of microvascular permeability in early postinfarction reperfusion after nicorandil therapy. Magn Reson Med. 2002;47:896–902. doi: 10.1002/mrm.10149. [DOI] [PubMed] [Google Scholar]
- 88.Saeed M, Lund G, Wendland MF, Bremerich J, Weinmann H, Higgins CB. Magnetic resonance characterization of the peri-infarction zone of reperfused myocardial infarction with necrosis-specific and extracellular nonspecific contrast media. Circulation. 2001;103:871–876. doi: 10.1161/01.cir.103.6.871. [DOI] [PubMed] [Google Scholar]
- 89.Jackson BM, Gorman JH 3rd, Salgo IS, Moainie SL, Plappert T, St John-Sutton M, Edmunds LH Jr, Gorman RC. Border zone geometry increases wall stress after myocardial infarction: contrast echocardiographic assessment. Am J Physiol Heart Circ Physiol. 2003;284:H475–H479. doi: 10.1152/ajpheart.00360.2002. [DOI] [PubMed] [Google Scholar]
- 90.Van Leuven SL, Waldman LK, McCulloch AD, Covell JW. Gradients of epicardial strain across the perfusion boundary during acute myocardial ischemia. Am J Physiol. 1994;267:H2348–H2362. doi: 10.1152/ajpheart.1994.267.6.H2348. [DOI] [PubMed] [Google Scholar]
- 91.Pennell D. Myocardial salvage: retrospection, resolution, and radio waves. Circulation. 2006;113:1821–1823. doi: 10.1161/CIRCULATIONAHA.105.618942. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 94.Saeed M, Bremerich J, Wendland MF, Wyttenbach R, Weinmann HJ, Higgins CB. Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular MR contrast media in rats. Radiology. 1999;213:247–257. doi: 10.1148/radiology.213.1.r99se30247. [DOI] [PubMed] [Google Scholar]
- 95.Rubenstein JC, Ortiz JT, Wu E, Kadish A, Passman R, Bonow RO, Goldberger JJ. The use of periinfarct contrast-enhanced cardiac magnetic resonance imaging for the prediction of late postmyocardial infarction ventricular dysfunction. Am Heart J. 2008;156:498–505. doi: 10.1016/j.ahj.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Rodríguez-Sinovas A, Abdallah Y, Piper HM, Garcia-Dorado D. Reperfusion injury as a therapeutic challenge in patients with acute myocardial infarction. Heart Fail Rev. 2007;12:207–216. doi: 10.1007/s10741-007-9039-9. [DOI] [PubMed] [Google Scholar]
- 97.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 98.Saeed M, Lee RJ, Weber O, Do L, Martin A, Ursell P, Saloner D, Higgins CB. Scarred myocardium imposes additional burden on remote viable myocardium despite a reduction in the extent of area with late contrast MR enhancement. Eur Radiol. 2006;16:827–836. doi: 10.1007/s00330-005-0052-x. [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto T, Kambara H, Fudo T, Tamaki S, Nohara R, Takatsu Y, Hattori R, Tokunaga S, Kawai C. Early estimation of acute myocardial infarct size soon after coronary reperfusion using emission computed tomography with technetium-99m pyrophosphate. Am J Cardiol. 1987;60:952–957. doi: 10.1016/0002-9149(87)90331-6. [DOI] [PubMed] [Google Scholar]
- 100.Wheelan K, Wolfe C, Corbett J, Rude RE, Winniford M, Parkey RW, Buja LM, Willerson JT. Early positive technetium-99m stannous pyrophosphate images as a marker of reperfusion after thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 1985;56:252–256. doi: 10.1016/0002-9149(85)90844-6. [DOI] [PubMed] [Google Scholar]
- 101.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Günther RW, Kühl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–2047. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 102.Carlsson M, Ursell PC, Saloner D, Saeed M. Multidetector computed tomography for characterization of calcium deposits in reperfused myocardial infarction. Acta Radiol. 2009;50:396–405. doi: 10.1080/02841850902756540. [DOI] [PubMed] [Google Scholar]
- 103.Antoniucci D, Valenti R, Migliorini A, Moschi G, Parodi G, Dovellini EV, Bolognese L, Santoro GM. Comparison of impact of emergency percutaneous revascularization on outcome of patients > or =75 to those < 75 years of age with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2003;91:1458–1461, A6. doi: 10.1016/s0002-9149(03)00397-7. [DOI] [PubMed] [Google Scholar]
- 104.Bolognese L, Carrabba N, Santoro GM, Valenti R, Buonamici P, Antoniucci D. Angiographic findings, time course of regional and global left ventricular function, and clinical outcome in diabetic patients with acute myocardial infarction treated with primary percutaneous transluminal coronary angioplasty. Am J Cardiol. 2003;91:544–549. doi: 10.1016/s0002-9149(02)03302-7. [DOI] [PubMed] [Google Scholar]
- 105.Hardoff R, Shefer A, Gips S, Merdler A, Flugelman MY, Halon DA, Lewis BS. Predicting late restenosis after coronary angioplasty by very early (12 to 24 h) thallium-201 scintigraphy: implications with regard to mechanisms of late coronary restenosis. J Am Coll Cardiol. 1990;15:1486–1492. doi: 10.1016/0735-1097(90)92815-j. [DOI] [PubMed] [Google Scholar]
- 106.Wijns W, Serruys PW, Reiber JH, de Feyter PJ, van den Brand M, Simoons ML, Hugenholtz PG. Early detection of restenosis after successful percutaneous transluminal coronary angioplasty by exercise-redistribution thallium scintigraphy. Am J Cardiol. 1985;55:357–361. doi: 10.1016/0002-9149(85)90375-3. [DOI] [PubMed] [Google Scholar]
- 107.Holmes DR Jr. Very early prediction of restenosis after successful coronary angioplasty: how early is early and can we identify it? J Am Coll Cardiol. 1990;15:265–266. doi: 10.1016/s0735-1097(10)80045-2. [DOI] [PubMed] [Google Scholar]
- 108.Rodés-Cabau J, Candell-Riera J, Domingo E, Castell-Conesa J, Anívarro I, Angel J, Aguadé-Bruix S, Padilla F, Soto A, Soler-Soler J. Frequency and clinical significance of myocardial ischemia detected early after coronary stent implantation. J Nucl Med. 2001;42:1768–1772. [PubMed] [Google Scholar]
- 109.Jaffe R, Haim SB, Karkabi B, Front A, Gips S, Weisz G, Khader N, Merdler A, Flugelman MY, Halon DA, et al. Myocardial perfusion abnormalities early (12-24 h) after coronary stenting or balloon angioplasty: implications regarding pathophysiology and late clinical outcome. Cardiology. 2002;98:60–66. doi: 10.1159/000064680. [DOI] [PubMed] [Google Scholar]
- 110.Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, Figulla HR. Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 2007;115:600–608. doi: 10.1161/CIRCULATIONAHA.106.660779. [DOI] [PubMed] [Google Scholar]
- 111.Skyschally A, Schulz R, Erbel R, Heusch G. Reduced coronary and inotropic reserves with coronary microembolization. Am J Physiol Heart Circ Physiol. 2002;282:H611–H614. doi: 10.1152/ajpheart.00797.2001. [DOI] [PubMed] [Google Scholar]
- 112.Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, Neubauer S, Banning AP. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006;114:662–669. doi: 10.1161/CIRCULATIONAHA.105.593210. [DOI] [PubMed] [Google Scholar]
- 113.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 114.Carlsson M, Martin AJ, Ursell PC, Saloner D, Saeed M. Magnetic resonance imaging quantification of left ventricular dysfunction following coronary microembolization. Magn Reson Med. 2009;61:595–602. doi: 10.1002/mrm.21869. [DOI] [PubMed] [Google Scholar]
- 115.Nassenstein K, Breuckmann F, Bucher C, Kaiser G, Konorza T, Schäfer L, Konietzka I, de Greiff A, Heusch G, Erbel R, et al. How much myocardial damage is necessary to enable detection of focal late gadolinium enhancement at cardiac MR imaging? Radiology. 2008;249:829–835. doi: 10.1148/radiol.2493080457. [DOI] [PubMed] [Google Scholar]
- 116.Breuckmann F, Nassenstein K, Bucher C, Konietzka I, Kaiser G, Konorza T, Naber C, Skyschally A, Gres P, Heusch G, et al. Systematic analysis of functional and structural changes after coronary microembolization: a cardiac magnetic resonance imaging study. JACC Cardiovasc Imaging. 2009;2:121–130. doi: 10.1016/j.jcmg.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Selvanayagam JB, Cheng AS, Jerosch-Herold M, Rahimi K, Porto I, van Gaal W, Channon KM, Neubauer S, Banning AP. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007;116:1458–1464. doi: 10.1161/CIRCULATIONAHA.106.671909. [DOI] [PubMed] [Google Scholar]
- 118.Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 119.Choi JW, Gibson CM, Murphy SA, Davidson CJ, Kim RJ, Ricciardi MJ. Myonecrosis following stent placement: association between impaired TIMI myocardial perfusion grade and MRI visualization of microinfarction. Catheter Cardiovasc Interv. 2004;61:472–476. doi: 10.1002/ccd.20024. [DOI] [PubMed] [Google Scholar]
- 120.Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, Banning AP. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027–1032. doi: 10.1161/01.CIR.0000156328.28485.AD. [DOI] [PubMed] [Google Scholar]
- 121.Caraballo V. Fatal myocardial infarction resulting from coronary artery septic embolism after abortion: unusual cause and complication of endocarditis. Ann Emerg Med. 1997;29:175–177. doi: 10.1016/s0196-0644(97)70325-1. [DOI] [PubMed] [Google Scholar]
- 122.Garg RK, Jolly N. Acute myocardial infarction secondary to thromboembolism in a patient with atrial fibrillation. Int J Cardiol. 2007;123:e18–e20. doi: 10.1016/j.ijcard.2006.11.095. [DOI] [PubMed] [Google Scholar]
- 123.Quinn EG, Fergusson DJ. Coronary embolism following aortic and mitral valve replacement: successful management with abciximab and urokinase. Cathet Cardiovasc Diagn. 1998;43:457–459. doi: 10.1002/(sici)1097-0304(199804)43:4<457::aid-ccd24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 124.Takenaka T, Horimoto M, Igarashi K, Yoshie H, Tsujino I, Morihira M. Multiple coronary thromboemboli complicating valvular heart disease and atrial fibrillation. Am Heart J. 1996;131:194–196. doi: 10.1016/s0002-8703(96)90070-8. [DOI] [PubMed] [Google Scholar]
- 125.Yutani C, Imakita M, Ueda-Ishibashi H, Katsuragi M, Fujita H. Coronary artery embolism with special reference to invasive procedures as the source. Mod Pathol. 1992;5:244–249. [PubMed] [Google Scholar]
- 126.Erbel R. Spontaneous and interventional coronary microembolisation. Heart. 2003;89:986–989. doi: 10.1136/heart.89.9.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mandell BF. Cardiovascular involvement in systemic lupus erythematosus. Semin Arthritis Rheum. 1987;17:126–141. doi: 10.1016/0049-0172(87)90035-7. [DOI] [PubMed] [Google Scholar]
- 128.Westwood MA, Shah F, Anderson LJ, Strange JW, Tanner MA, Maceira AM, Howard J, Porter JB, Walker JM, Wonke B, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26:564–568. doi: 10.1002/jmri.21018. [DOI] [PubMed] [Google Scholar]
- 129.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 130.Carlsson M, Saloner D, Martin A, Ursell P, Saeed M. Heterogeneous microinfarcts caused by coronary microemboli: Evaluation with multidetector CT and MR imaging in swine model. Radiology. 2010:In press. doi: 10.1148/radiol.09090527. [DOI] [PMC free article] [PubMed] [Google Scholar]