Abstract
As a result of many advantages such as the absence of radiation exposure, non-invasiveness, low cost, safety, and ready availability, transthoracic ultrasonography (TUS) represents an emerging and useful technique in the management of pleural and pulmonary diseases. In this second part of a comprehensive review that deals with the role of TUS in pleuropulmonary pathology, the normal findings, sonographic artifacts and morphology of the most important and frequent pulmonary diseases are described. In particular, the usefulness of TUS in diagnosing or raising suspicion of pneumonia, pulmonary embolism, atelectasis, diffuse parenchymal diseases, adult and newborn respiratory distress syndrome, lung cancer and lung metastases are discussed, as well as its role in guidance for diagnostic and therapeutic interventional procedures. Moreover, the preliminary data about the role of contrast enhanced ultrasonography in the study of pulmonary pleural-based lesions are also reported. Finally, the limits of TUS when compared with chest computed tomography are described, highlighting the inability of TUS to depict lesions that are not in contact with the pleura or are located under bony structures, poor visualization of the mediastinum, and the need for very experienced examiners to obtain reliable results.
Keywords: Ultrasonography, Pleural diseases, Lung diseases
INTRODUCTION
In the first part of this review on the role of transthoracic ultrasonography (TUS) in the diagnostic workup of pleuropulmonary pathology, we described the ultrasound equipment, examination technique and limits of TUS, as well as the normal findings and artifacts that originate at the boundary between the pleura and the normally aerated lung pleura (i.e. the pleural line, gliding sign, horizontal reverberation artifacts, and comet-tail artifacts)[1].
In this second part of the review, we deal with the pathological lung. Indeed, as a consequence of its non-invasiveness, TUS can be very useful in lung pathology and can be used for the diagnosis of lung diseases and monitoring progress of sonographically visible processes, thus avoiding radiation exposure. Moreover, the relatively low cost and wide availability of standard conventional ultrasound machines, as well as the increasing use of modern portable devices, allow examination at almost any location and at any time. This makes TUS a valuable tool in many difficult situations, such as in an ambulance, emergency room, or directly at the bedside of critically ill patients.
Pulmonary pathology can be divided into diffuse interstitial (or parenchymal) lung diseases and peripheral or subpleural lung diseases. Peripheral or subpleural diseases commonly appear as pleural-based consolidations, whereas the presence of diffuse interstitial syndromes can be inferred by the detection of different artifacts with respect to normal lung. In particular, multiple comet-tail artifacts departing from the pleural line (much more numerous and diffuse than in the normally aerated lung) and ring-down vertical artifacts (characterized by a series of hyperechoic narrow-based bands spreading from the pleural line into the lung) can be visualized in these cases[2-8]. Interstitial lung diseases include pulmonary edema (due to heart failure, trauma, or inflammation) and interstitial fibrosis. Peripheral diseases with peculiar consolidation aspects are common in pneumonia, atelectasis, and pulmonary infarction. Adult respiratory distress syndrome (ARDS) generally shows mixed TUS features. Finally, neoplastic lesions that abut the pleura (both primary neoplasms and metastases) usually appear as hypoechoic, sometimes inhomogeneous, non-aerated masses.
PULMONARY DISEASES
For the purposes of this article, the main pulmonary diseases are divided into diffuse parenchymal pulmonary diseases, infection diseases, peripheral pulmonary artery embolisms and pulmonary infarction, atelectasis, adult and newborn distress respiratory syndrome, bronchopulmonary tumors and pulmonary metastases.
Diffuse parenchymal lung diseases
Diffuse parenchymal lung diseases (DPLDs) can be divided into those of known cause (e.g. collagen vascular disease), idiopathic interstitial pneumopathy (idiopathic and non-idiopathic pulmonary fibrosis), granulomatous (e.g. sarcoidosis), and other forms[9,10]. High-resolution computed tomography (HRCT) should be considered the gold standard technique to diagnose DPLD, and many other noninvasive and invasive procedures concur in clinical practice to define and characterize DPLD, such as chest radiography, laboratory and serological tests (e.g. angiotensin-converting enzyme and antinuclear antibodies), pulmonary function testing, bronchoscopy with bronchoalveolar lavage, and transbronchial lung biopsy. However, some studies have demonstrated that TUS, as a consequence of its well-known advantages (absence of radiation exposure, ready availability, and cost-effectiveness), can play a complementary role in the diagnosis of DPLD, especially when chest radiography or HRCT are not readily available or undesirable, for instance during pregnancy[9,10]. Moreover, TUS can be useful in monitoring the course of the disease in patients with confirmed DPLD (thus avoiding unnecessary overload of radiation exposure), and confirming the clinical-radiological suspicion of acute pulmonary edema due to heart failure, trauma, or inflammation. Indeed, radiological evidence of ground glass areas that are suggestive of pulmonary edema has often been associated with the presence of sonographic artifacts, such as multiple comet-tail artifacts and vertical ring-down artifacts. Likewise, typical radiological findings and corresponding sonographic findings can be observed in fibrosing pulmonary disease. Fibrosis usually involves the pleural surface as well as the intralobular and interlobular septa, therefore, thickened subpleural intralobular and interlobular septa, and ground glass areas, represent common findings at radiological examination[11]. In the same setting, TUS enables one to document multiple ring-down artifacts (Figure 1) and comet-tail artifacts that depart from a thickened and irregular pleural line (Figure 2). Indeed, in patients with diffuse alveolar-interstitial syndrome, multiple comet-tail artifacts can be observed all over the lung surface, and this finding has been reported to have high sensitivity. In a study of Reissig et al[9], almost all the patients with DPLD showed more than six comet-tail artifacts per scan associated with an irregular and fragmented pleural line. Comet-tail artifacts become visible when there is a marked difference in acoustic impedance between an object and its surroundings (as happens at the boundary between the pleural surface and the lung), which demarcates the edge of the normally aerated lung. These artifacts are best visible under real-time conditions, as they might appear less pronounced on frozen sonograms, which makes quantification of the alterations difficult. Lichtenstein et al[3] have attributed the origin of the comet-tail artifacts to the thickening of the subpleural interlobular septa, which would cause fragmentation of the pleural reflector at the points of greatest impedance. However, it must be highlighted that pleural alterations and multiple comet-tail artifacts are nonspecific TUS features. They might also occur in patients with chronic obstructive lung disease, bronchiolitis obliterans-organizing pneumonia, and pulmonary alveolar proteinosis, as well as after pneumonia and pulmonary embolism. Some authors have suggested that the number of comet-tail artifacts is smaller and the affected area is more localized in these conditions[8,9,12]. In DPLD, these abnormalities are commonly detected on both sides of the lung, which reflects the diffuse fibrosing process, and corresponds to the findings on HRCT. However, the degree of the pleural involvement can vary within the same patient, and usually a thickened and fragmented pleural line with multiple comet-tail artifacts is better detectable in the lower part of the lung, which reflects the extent of the fibrosis. In conclusion, although diagnosis of DPLD requires chest radiography, HRCT, and often bronchoalveolar lavage and transbronchial biopsy, TUS can play some complementary role in the diagnostic workup. Multiple comet-tail artifacts distributed over the entire lung surface in combination with a thickened, irregular and fragmented pleural line are strongly suggestive of the presence of DPLD.
Figure 1.
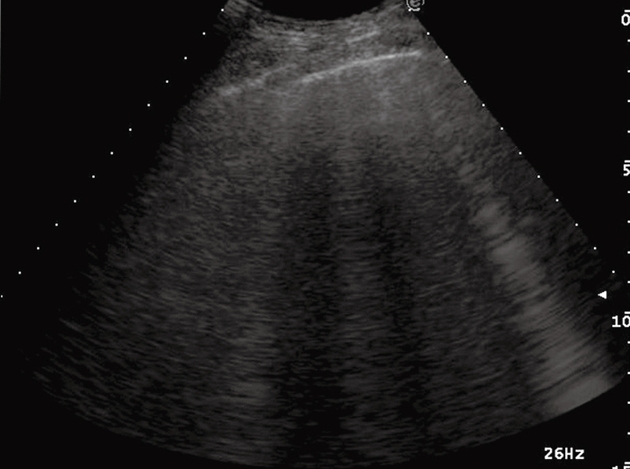
Ring-down artifacts, characterized by a series of hyperechoic, narrow-based bands spreading from the pleural line into the lung.
Figure 2.
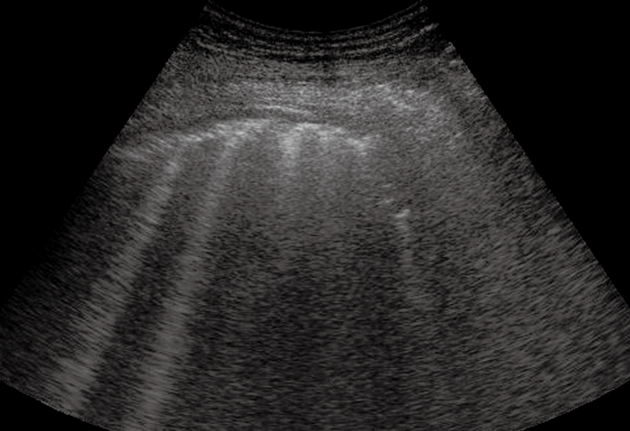
Pulmonary fibrosis. Multiple comet-tail artifacts departing from a thickened and irregular pleural line.
Infectious diseases
As previously described, peripheral pulmonary lesions in contact with the visceral pleura can easily be visualized by TUS[1,2,13-15]. However, the part of consolidation that can be visualized with TUS is generally smaller than that documented by chest radiography or CT, because just the areas on the level of the pleura can be detected. Indeed, at present pneumonia is mostly diagnosed by X-ray, and CT is considered the gold standard for the diagnosis of infectious lung diseases. However, in the case of a chest X-ray on only one plane, or in the case of a patient in the lying position, the summation image often cannot provide exact information. As concerns CT, its use is limited by the high radiation exposure and cost. TUS shows several advantages, such as its feasibility, low cost, and the possibility of monitoring disease progress, because it can either document resolution or detect complications such as lung abscesses, parapneumonic effusion, empyema, and pleural fibrosis. Moreover, TUS is the method of choice to guide transthoracic aspiration or drainage of pleural effusion, empyema, and pulmonary abscesses in contact with the pleura, playing a very useful role in both diagnosis and treatment of infectious diseases and their complications. In this regard, TUS has recently been reported to be as effective as chest CT in detecting loculated effusion and lung necrosis or abscess that results from complicated pneumonia in children[16]. As a consequence of its efficacy, reliability, and minimal radiation exposure, TUS has been recommended by the British Thoracic Society guidelines for the management of pediatric empyema as the first-line approach for detecting pleural effusion and guiding drain placement in children[17].
In this section, TUS findings in pneumonia, lung abscesses, and tuberculosis are described. Pneumonia is commonly visualized by TUS as a hypoechoic consolidated area of varying size and shape, with irregular borders. The echotexture can appear homogeneous or inhomogeneous[14-18]. The most common sonographic feature of pneumonia is the air bronchogram, which is characterized by lens-shape internal echoes within the hypodense area or echogenic lines (Figure 3), and corresponds to air inclusions or air-filled bronchioles and bronchi. Conversely, the fluid bronchogram is characterized by anechoic or hypoechoic tubular structures in the bronchial tree (Figure 4), without perfusion signs inside at color Doppler examination[1,2,13,19-21]. This feature suggests post-stenotic pneumonia that requires further investigation. Besides these common findings, some pleural signs are often present in infectious disease, as well as some vascular features[18]. The pleural line can appear interrupted, fragmented, and hypoechoic where the pneumonic infiltrate is present. A local pleural effusion occurs in about 9% of patients with pneumonia, and a basal pleural effusion appears in about 60% of patients[22]. As concerns vascular criteria, color Doppler sonography can show increased branch-like vessel visualization that corresponds to the segment branches of the pulmonary artery. Contrast-enhanced ultrasonography (CEUS) of pneumonia usually shows a short wash-in period during the arterial phase and a prolonged and marked degree of contrast agent accumulation during the parenchymal phase in the case of classic pneumonia (Figure 5)[18]. These findings suggest preferential pulmonary arterial vascularization, as reported by some authors[23-26]. CEUS can also be helpful to detect complications like sequestration, to demarcate surrounding fluid (Figure 6), and to achieve a possible differential diagnosis with respect to pulmonary infarction and malignant lesions, which show different CEUS patterns, as described in the respective sections of this review.
Figure 3.
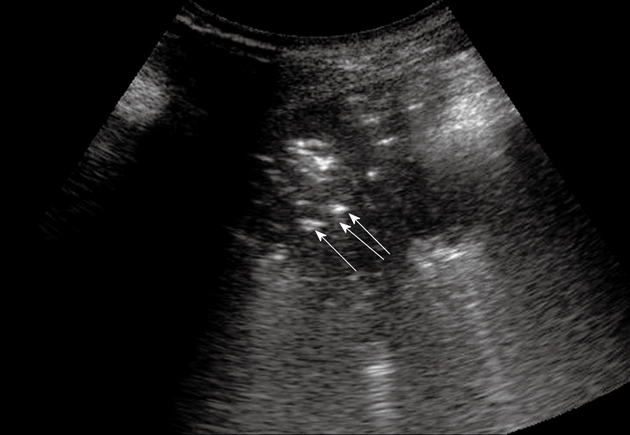
Pneumonia. Posterior intercostal scan shows a hypoechoic consolidated area that contains multiple echogenic lines that represent an air bronchogram (arrows).
Figure 4.
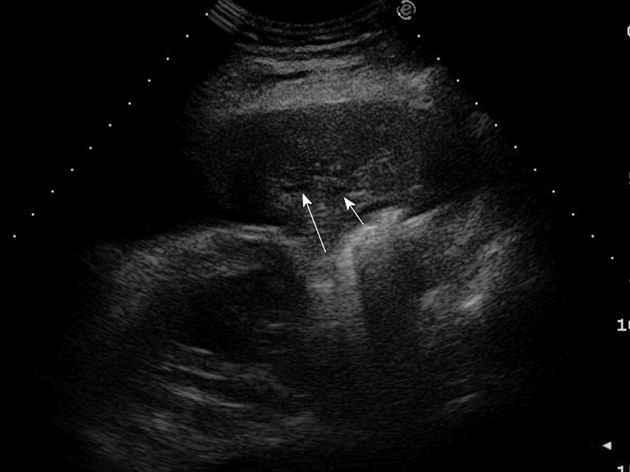
Post-stenotic pneumonia. Posterior intercostal scan shows a hypoechoic consolidated area that contains anechoic, branched tubular structures in the bronchial tree (fluid bronchogram) (arrows).
Figure 5.
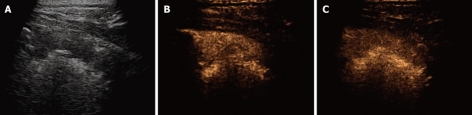
Contrast-enhanced ultrasonography of pneumonia. A: Baseline scan shows a hypoechoic consolidated area; B: Seven seconds after iv bolus of contrast agent, the lesion shows marked and homogeneous enhancement; C: The lesion remains substantially unmodified after 90 s.
Figure 6.
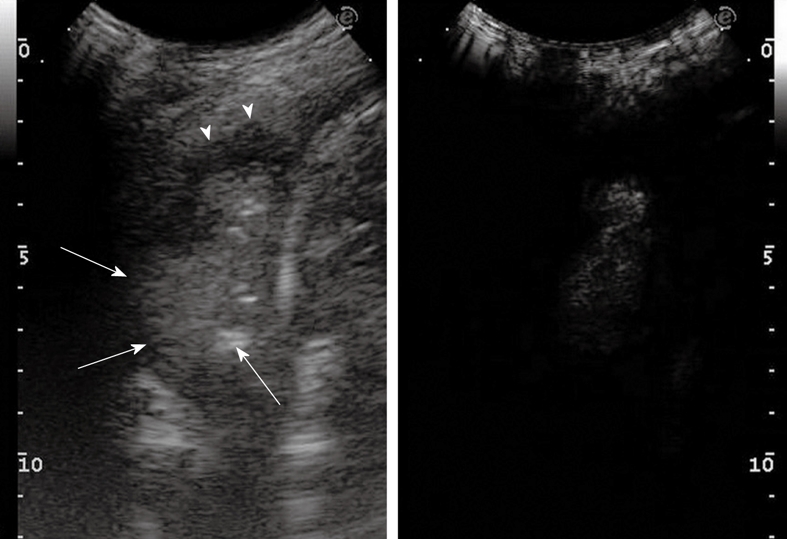
Contrast-enhanced ultrasonography evaluation of pneumonia with pleural effusion. Baseline scan shows parenchymal consolidation with air bronchogram (arrows) and subtle surrounding effusion (arrowheads) (left side of the split-screen). After iv bolus of contrast agent, the consolidation is enhanced and better demarcated from the effusion (right side of the split-screen).
Lung abscesses typically appear as round or oval, largely anechoic lesions[14,18]. In the early stage, small abscesses are visible as a pathological collection of fluid irregularly settled in a consolidated, liver-like infiltrate (Figure 7). Microabscesses are often visible as anechoic areas within the pneumonic consolidation. Commonly, a small pleural effusion is associated with lung abscesses. Depending on the capsule formation, the edge of the abscess can be smooth and echodense (Figure 8A). At CEUS evaluation, lung abscesses show lack of contrast agent uptake (Figure 8B) and this finding can be particularly useful in the detection of microabscesses, and in the differential diagnosis between lung abscesses and malignant lesions[18,23]. The presence of air inside the lesion produces high amplitude echoes, and highly echogenic small air inclusions moving in the fluid with breathing can be observed especially in the case of abscesses due to gas-producing microorganisms, or in the case of connection to the bronchial system (Figure 9). If septa develop inside the lesion, they are visualized as echodense, fluttering threads.
Figure 7.
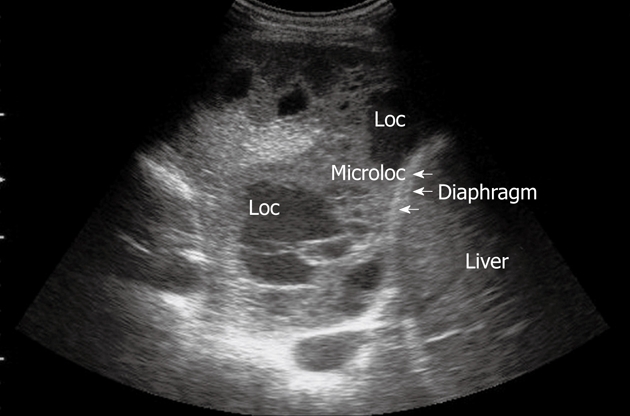
Pneumonia complicated by abscesses. Multiple small collections of fluid are irregularly settled in a consolidated liver-like infiltrate. Loc: Loculation; Microloc: Microloculation.
Figure 8.
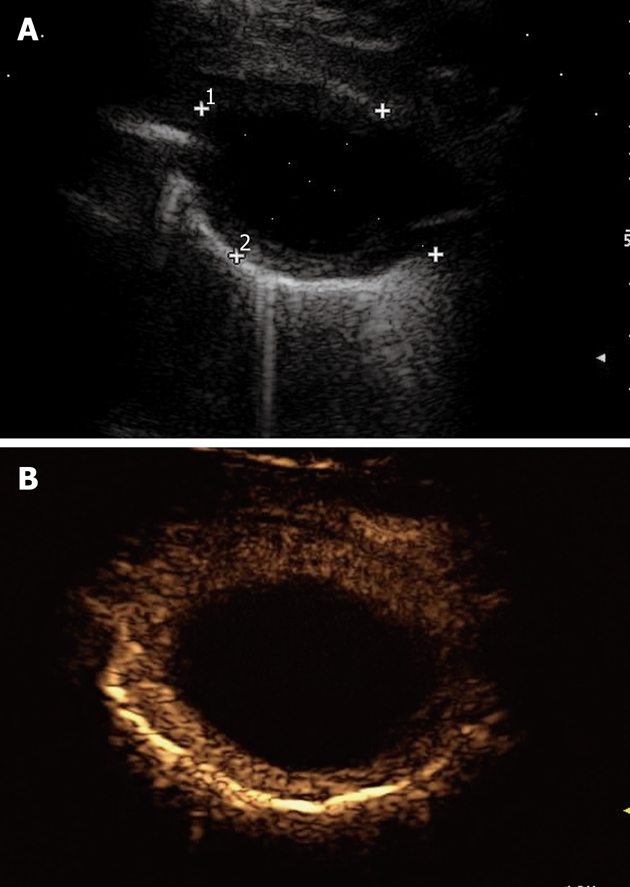
Lung abscess. A: An anechoic oval lesion is surrounded by an echodense capsule; B: After iv bolus of contrast agent, the lesion shows no contrast agent uptake, whereas the capsule is strongly enhanced.
Figure 9.
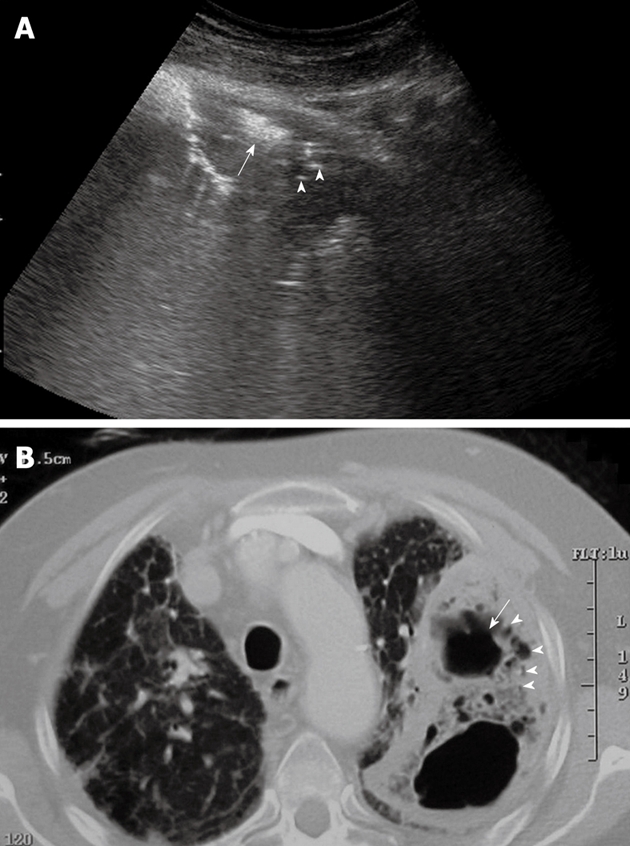
Lung abscess with air inside the lesion. A: High amplitude echoes are clearly visible (arrow), as well as multiple echogenic small air inclusions (arrowheads); B: Corresponding computed tomography scan shows the same findings.
Tubercular lung lesions appear as round or irregularly shaped lesions and show a relatively homogeneous texture. Depending on the size, air inclusions can be visible inside the lesions, but a pronounced air bronchogram, as in the case of pneumonia, is only rarely present. Miliary tuberculosis is characterized by multiple subpleural nodules several millimeters in size. Pleural effusions often occur at the beginning of the disease. Frequently, an initially anechoic pleural effusion shows internal echoes over the course of the disease, and pleural thickening is often associated. However, no specific TUS pattern of pulmonary tuberculosis can be identified, and no definitive CEUS data have been reported in the literature.
Peripheral pulmonary artery embolism and pulmonary infarction
Chest radiography is still considered the first diagnostic step in patients with suspected pulmonary embolism (PE). It can show infiltrates, atelectasis, and effusion, but such features represent just the consequence of PE on the periphery and are nonspecific, because the central embolism cannot be visualized. Consequently, ventilation-perfusion scintigraphy and CT angiography are needed to confirm the diagnosis[27]. However, the Prospective Investigation of Pulmonary Embolism Diagnosis Study has shown that ventilation-perfusion scintigraphy is not sufficiently conclusive. In that study, high-probability scans resulted in detection in only a minority of patients with PE, whereas PE was present in 12% of patients with low-probability scans[28]. In recent years, CT pulmonary angiography (CTPA) has been established as the method of choice for the diagnosis of central PE up to the level of the segmental arteries, because it enables one to show the thromboembolic obstruction directly. Spiral CT scanning shows a sensitivity and specificity of 90% in depicting thrombi within the central pulmonary vessels, and can reveal characteristic consolidations in patients with acute PE[29-31]. Pulmonary angiography has long been considered the gold standard for diagnosing PE, but nowadays, it is rarely used because of its invasive nature and the risk of complications[32], and it has been almost completely replaced by CTPA. However, despite their usefulness, in some circumstances critically ill patients may not tolerate any of these procedures, and occasionally, adequate equipment might not be available. In these cases, TUS can play some useful roles, because it shows the major advantage of bedside availability in the emergency room and in the intensive care unit, and it has been reported to have good accuracy in the diagnosis of PE[33-35]. The sensitivity of TUS for PE has been estimated to range from 80% to 94%, the specificity from 84% and 92%, and the overall accuracy from 82% to 91%[33,36,37]. A recent meta-analysis has reported sensitivity and specificity of 80% and 93%, respectively[38].
Typical sonographic findings in peripheral PE are multiple, hypoechoic lesions that are visible at the level of the pleura, which can usually be well demarcated from the surrounding parenchyma. About two-thirds of PE-based consolidations can be visualized in the dorsal basal position for anatomical and hemodynamic reasons, with a preference for the right lung. Frequently, the lesions show a triangular or wedge shape. In a recent prospective multicenter study of 352 patients, the sonographic morphology was mainly triangular, with the vertex towards the hilum of the lung in 58% of the cases, and rounded or mixed in 42%. In that study, a small pleural effusion was seen in 49%, a basal effusion in 33%, and a focal effusion in 16% of the cases[34]. The lesions are almost always pleural-based, are freely subject to respiratory excursions, and their size usually exceeds 2 cm in diameter. When the lesions are > 3 cm in size, central bronchial echoes can be seen as central hyperechoic structures that indicate the presence of air in the affected bronchioles, which is considered a sign of segmental involvement (Figure 10). Lesions visualized on TUS are smaller than on CT angiography because of the presence of air artifacts at the deeper margins and at the top of the infarct cone, but TUS can depict either a larger number of lesions or smaller lesions than CT scan[34]. Although the specificity of sonographic morphology of the lesions is limited, the detection of two or more triangular or rounded lesions with a pleural base, 0.5-3 cm in size, can often represent a confirmation of clinically suspected PE, and the contemporary presence of a small pleural effusion makes the diagnosis of PE very likely.
Figure 10.
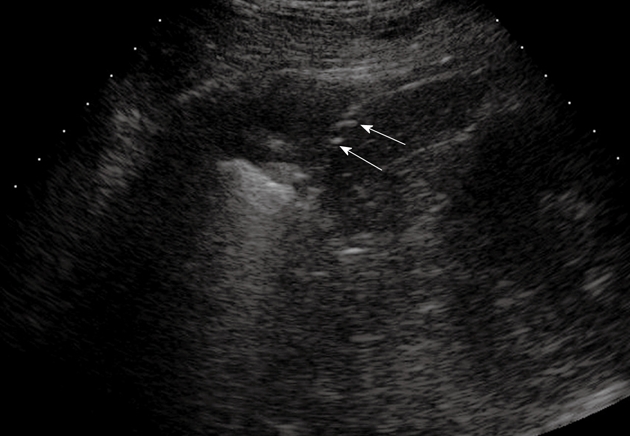
Pulmonary infarction. Posterior intercostal scan shows a triangular-shaped hypoechoic lesion with central hyperechoic structures that indicate the presence of air occupying the affected bronchiole (arrows).
Thromboembolism is often a dynamic event that is associated with recurrent embolization and spontaneous lysis (besides the pharmacologically induced lysis). Moreover, 70%-90% of emboli are associated with peripheral pulmonary hemorrhage, the so-called incomplete or partial infarction, which can be reabsorbed within a few hours or days[34]. Consequently, follow-up imaging techniques can document the disappearance of previously depicted pleural-based pulmonary lesions (Figure 11). On color Doppler sonography, PE-based peripheral lesions do not show flow signals inside, a phenomenon defined as “consolidation with little perfusion”[35]. On the other hand, recanalization of incomplete infarction that results from anticoagulation treatment or intrinsic lysis can be demonstrated by the reappearance of a blood flow signal on follow-up. In addition, a congested thromboembolic vessel (vascular sign) might occasionally be visible, and corresponds to a pulmonary artery with thickened vessel walls around an intraluminal organizing embolus[35]. Corresponding to these findings, initial studies in patients with confirmed PE have reported that the lesions show a lack of contrast agent uptake in the arterial phase at CEUS examination, which suggests the absence of pulmonary arterial blood supply (Figure 12). However, in patients with PE and pleural effusion or in those with chronic PE, mixed enhancement might be observed[18,23,25].
Figure 11.
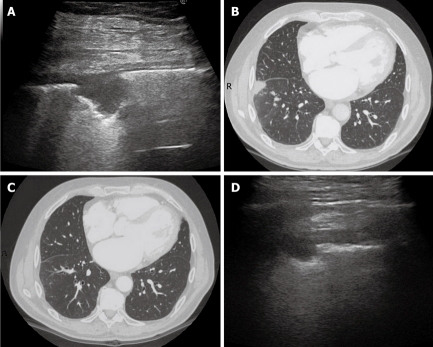
Dynamic course of pulmonary infarction. A: Lateral intercostal scan of the right lung shows a typical triangular-shaped peripheral lesion; B: Likewise, computed tomography scan of the lateral segment of the lower right lobe shows a triangular pleural-based lesion with the vertex towards the hilum; C: After 40 d, the lesion is no longer visible by computed tomography scan; D: The lesion appears reduced in size at transthoracic ultrasonography examination.
Figure 12.

Contrast-enhanced ultrasonography of pulmonary infarction. After iv bolus of contrast agent, the lesion (the same one as in Figure 11A) shows no contrast agent uptake in the arterial phase, which suggests the absence of blood supply.
In conclusion, although CTPA is undoubtedly the method of choice to obtain a definitive diagnosis of PE, TUS should be taken into consideration in some circumstances, particularly in critically ill patients who might not tolerate transport for other imaging modalities. Moreover, even though a negative result does not rule out PE, TUS can play a central role in patients with presumed PE in cases of pregnancy, contrast agent allergy, or renal failure.
Atelectasis
Atelectasis is defined as the absence of ventilation in part of the lung or the entire lung, and therefore it can be visualized by sonography. It can be divided into compression atelectasis due to voluminous pleural effusion, and obstructive or resorptive atelectasis caused by bronchial block of air entry[39]. At TUS examination, compression atelectasis appears as a largely apneumatic consolidation with liver-like echotexture. It shows the shape of a jelly bag cap, and can be monoconcave or biconcave (Figure 13). Typically, compression atelectasis can be seen floating in the effusion like a waving hand, and is partially ventilated during breathing. Obstructive atelectasis shows a liver-like and inhomogeneous echotexture with secretion-filled bronchi (fluid bronchogram) and variable shape (Figure 4).
Figure 13.
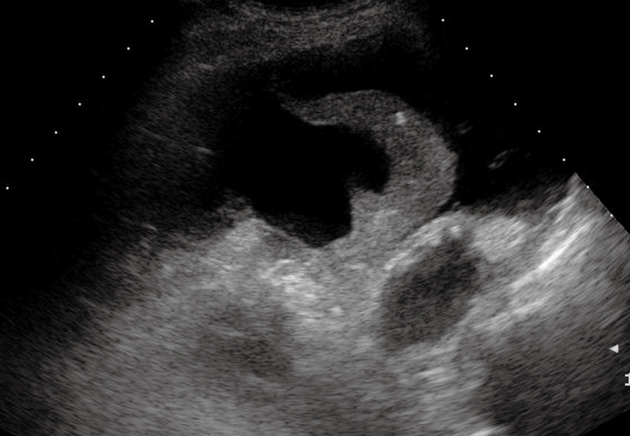
Compression atelectasis. Posterior intercostal scan shows a liver-like consolidation with the typical shape of a jelly bag cap surrounded by pleural effusion.
The air content inside the consolidation typically does not change during inspiration. In patients with alveolar consolidations that show air bronchograms, the real-time TUS visualization of bronchograms during breathing movements can often enable one to distinguish between obstructive atelectasis and pneumonia[39]. The presence of the dynamic air bronchogram indicates pneumonia, while a static air bronchogram suggests obstructive atelectasis. In a recent study by Lichtenstein et al, the dynamic air bronchogram showed a specificity of 94% and a sensitivity of 61%. In the same study, the authors described early and late signs suggestive for obstructive atelectasis. Early signs included the disappearance of lung sliding associated with the presence of the lung pulse, a cardiac activity visible throughout abolished lung sliding, and the presence of a standstill cupola that demonstrated the absence of lung expansion. The late sign appeared when the air inside the consolidation was progressively absorbed, which yielded a loss of volume of the lesion with the typical static air bronchogram inside[39]. The capability of TUS to detect the air bronchogram represents a major advantage, particularly in critically ill patients, because TUS enables one to visualize this sign also in ventilated newborns, thus reducing the necessity for ionizing radiation exposure. Moreover, TUS is probably more accurate than chest radiography in the detection of air bronchograms[39,40].
Pleural effusion is almost always associated with compression atelectasis and frequently with obstructive atelectasis. In the case of compression atelectasis, the effusion is typically larger compared to that associated with obstructive atelectasis.
At color Doppler evaluation, compression atelectasis shows increased branch-like vessel visualization when compared to the liver. The corresponding findings at CEUS examination include short time to enhancement, which indicates predominant pulmonary arterial vascularization, and marked enhancement during the arterial and parenchymal phase (Figure 14). In patients with compression atelectasis, the contrast agent apparently remains trapped in lung tissue after wash-out of the blood pool, in comparison with splenic enhancement. In the case of obstructive atelectasis, color Doppler sonography almost always shows increased vessel visualization inside the consolidation, and this finding can be useful for distinguishing central space-occupying neoplastic lesions from atelectatic lung parenchyma, because tumor tissue is characterized by minimal representation of flow signals. At CEUS examination, new obstructive atelectasis has been reported to show a short time to enhancement, with accumulation of the contrast agent similar to that in the spleen, which suggests predominant pulmonary arterial vascularization[18,23,25]. However, in a recent study, patients with tumor-associated obstructive atelectasis showed different CEUS patterns, including either a marked extent of enhancement and short time to enhancement, or a delayed time to enhancement and nearly complete absence of extent of enhancement[23]. In the patients with central lung cancer, the delayed time to enhancement could be secondary to transmural tumor growth, with intraluminal cell formation in the pulmonary artery branches, with consequent obliteration and occlusion of pulmonary arteries. The presence of a central tumor can be identified and more clearly distinguished from the atelectatic parenchyma, because it shows a delayed time to enhancement and a reduced extent of enhancement in comparison with the surrounding atelectatic parenchyma.
Figure 14.
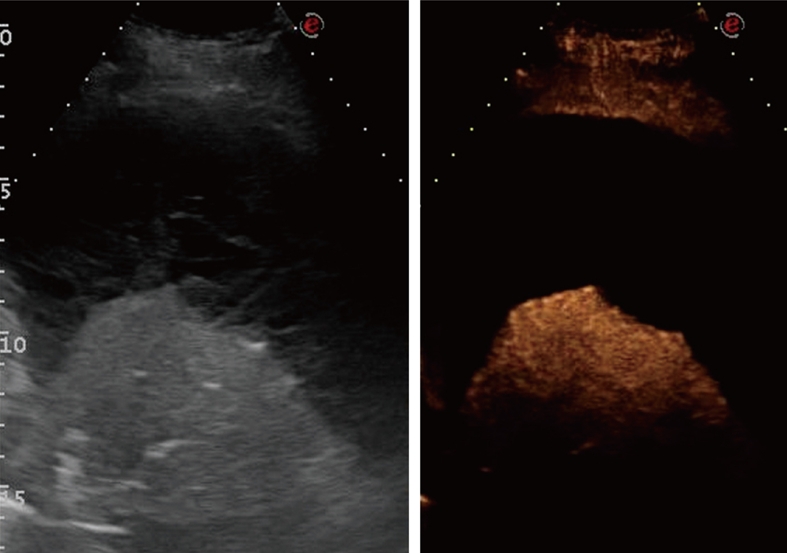
Contrast-enhanced ultrasonography evaluation of compression atelectasis. Baseline scan shows a liver-like consolidation surrounded by multiloculated pleural effusion (left side of the split-screen). Twelve seconds after iv bolus of contrast agent, the consolidation shows marked and homogeneous enhancement, whereas pleural effusion shows no enhancement.
In both compression and obstructive atelectasis, TUS is able to monitor the course of the disease. In particular, the pleural effusion that causes compression atelectasis can be monitored during diuretic treatment, as well as after TUS-guided aspiration. After effusion puncture, compression atelectasis can become smaller or no longer visible, depending on the capability of the lung to re-expand. In the case of obstructive atelectasis, aspiration of pleural effusion can result in formation of a pneumothorax ex vacuo, which can be also detected by TUS.
ARDS and newborn respiratory distress syndrome
CT scanning is traditionally considered the gold standard in the evaluation of both ARDS and newborn respiratory distress syndrome (NRDS), and recruitment of atelectatic lung regions after therapy, because it is the only available technique that can directly measure the extension of the affected and healthy lung tissue[41-43]. However, TUS enables a dynamic evaluation of lung recruitment and can be easily performed at the bedside. Such ability feature is particularly useful in patients affected by ARDS/NRDS because they are frequently too instable to be moved to the CT room. Moreover, TUS can be repeated as much as is needed to monitor the course of the disease. Although at present only few preliminary data have been reported, some authors have recently described the important role of TUS in critically ill patients affected by ARDS, as well as in NRDS patients[41-44].
The main features of ARDS/NRDS are diffuse alveolar infiltration and lung consolidation. TUS findings depend on the decreased aeration of the alveoli because of atelectasis, so that the ultrasound beam can be transmitted further beyond the phreno-pulmonary border into the lung parenchyma. Conversely, in normally aerated lungs, the ultrasound beam is completely reflected by the phreno-pulmonary border. The ultrasound beam is then completely reverberated by the dilated and aerated bronchioles and alveolar ducts, which gives rise to the typical TUS pattern of intense retro-phrenic hyperechogenicity that can be observed in patients with ARDS[43,45,46] (Figure 15). In a study of Bober et al[43], 131 newborns with clinical signs of respiratory failure underwent ultrasound examination, by application of the transducer in the left and right epigastric regions, and scans were obtained through the right lobe of the liver and the spleen. The pattern of echogenicity above the diaphragm was observed sequentially during numerous respiratory cycles. Alterations in lung aeration suggestive of NRDS were found in 109/131 subjects, and the diagnosis was confirmed in 101 cases by chest X-ray. TUS showed a sensitivity of 100%, and a specificity of 92% in diagnosing NRDS.
Figure 15.
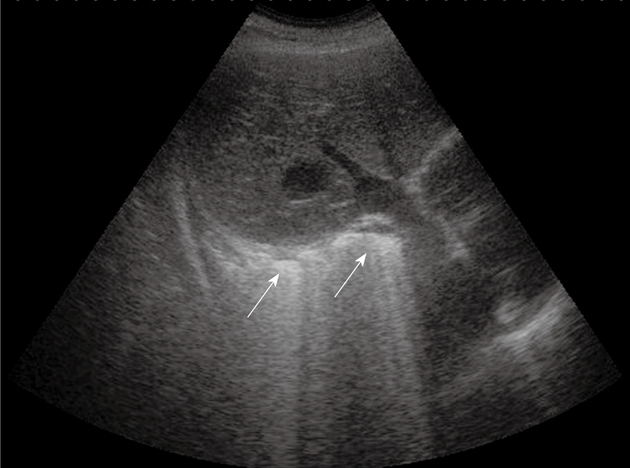
Adult respiratory distress syndrome. Oblique subcostal scan through the right lobe of the liver shows the typical transthoracic ultrasonography pattern of intense retro-phrenic hyperechogenicity due to complete reverberation of the US beam (arrows).
Numerous B-lines are typically present in the areas of ventilated parenchyma near the consolidation[41,42]. TUS allows the assessment of lung consolidation adjacent to the visceral pleura, but only parenchymal abnormalities adjacent to the pleura can be evaluated. However, notwithstanding this major limitation, patients with ARDS due to extrapulmonary causes generally show symmetrical consolidations adjacent to the visceral pleura and set behind at the base of the lung[41,44]. Dynamic air bronchogram is often present within the consolidation, which can help in excluding the obstructive origin of the consolidation and in supporting the diagnosis of ARDS.
Primary lung carcinoma
Although differentiating chronic benign lung consolidations from malignant tumors is difficult at TUS examination, and further investigations are always mandatory, TUS enables one to gather a lot of information about malignant peripheral lung lesions. Moreover, the capability of TUS to guide percutaneous biopsy of peripheral lesions, and its feasibility, as well as the interesting preliminary data about CEUS evaluation of lung lesions[18,23-26], make TUS a useful and innovative technique in the study of pulmonary malignancies.
Peripheral bronchial carcinomas usually appear at TUS as round or oval, sometimes polycyclic, hypoechoic consolidated lesions, with relatively well delineated borders[14] (Figure 16). The lesions can appear both homogeneous and inhomogeneous, while air bronchograms typically cannot be detected, because solid carcinomas do not contain aerated lung parenchyma[14,18]. If tumor necrosis occurs, it can be visualized as a particularly hypoechoic to anechoic region within the tumor. CEUS can help to define better necrotic areas that are depicted as anechoic regions inside the enhanced viable tumor[18,23-26] (Figure 17). In some cases, a diffuse or locally infiltrating growth can be observed. The infiltrative growth of solid tissue without regard to anatomical structures is characteristic of malignancy. Indeed, only rare inflammatory diseases, such as actinomycosis or nocardiosis, can also spread in this manner. Typically, malignant consolidations move rigidly and do not change their shape during respiration. Nevertheless, the gliding sign can still be observed if the tumor has not crossed the visceral pleura. Conversely, respiratory excursion can no longer be detected if the tumor infiltrates the thoracic wall. Frequently, the pleural line near the lesion appears fragmented or interrupted. Malignant lesions that abut the pleura commonly form an acute angle with the pleural line, and this finding might be helpful in differentiating malignant from benign lesions, as described in the first part of this review[1]. Bronchial carcinomas are often accompanied by pleural effusion, frequently hemorrhagic, as an expression of pleural carcinomatosis[18]. Pleural metastases from bronchogenic carcinoma are usually too small to be detected by imaging techniques[1]. However, when their size is large enough to allow their visualization by TUS, they appear as hypoechoic nodules attached to the pleura, and are well delimited from the pleural effusion[18]. In the case of central bronchial carcinomas, only the atelectasis due to obstruction can be identified by TUS[14,39]. For long-lasting obstruction, a fluid bronchogram can be observed within the consolidated lung parenchyma. Some preliminary reports have suggested that CEUS can often enable one to demarcate central tumor lesions from the atelectatic parenchyma, as previously described[18,23-26].
Figure 16.
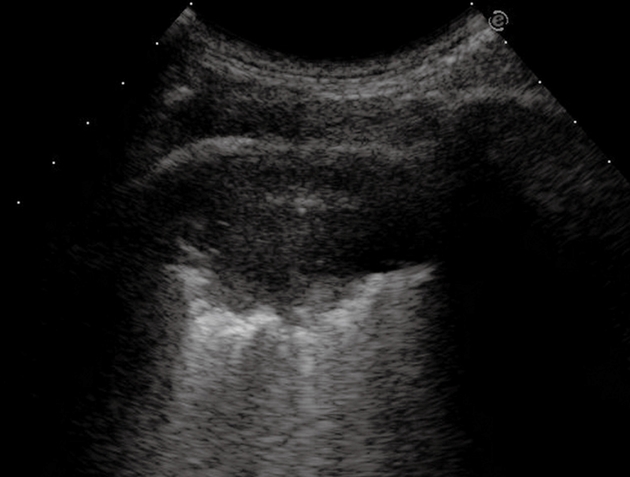
Peripheral bronchial carcinoma. Posterior intercostal scan shows a hypoechoic consolidation with relatively well-delineated borders. The air bronchogram is absent.
Figure 17.
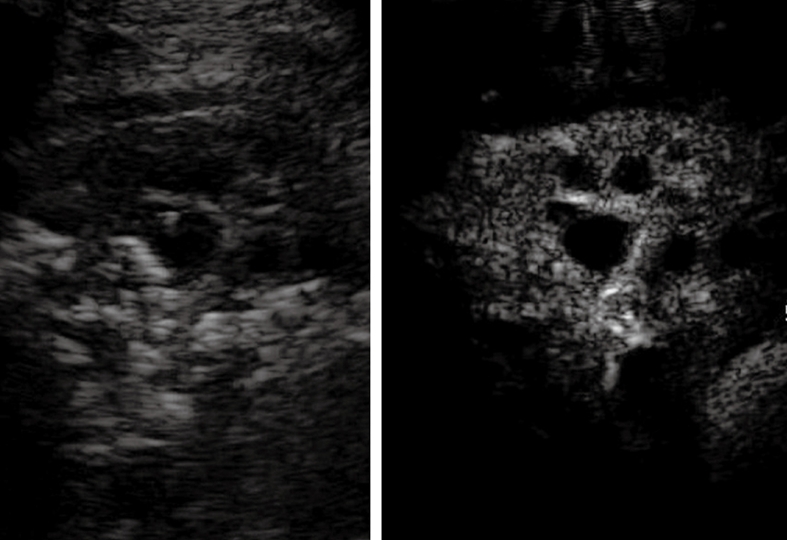
Contrast-enhanced ultrasonography evaluation of bronchial carcinoma. Baseline scan shows consolidation with inhomogeneous echotexture (left side of the split-screen). Twenty seconds after iv bolus of contrast agent, necrotic areas can be depicted as anechoic regions inside the enhanced viable tumor (right side of the split-screen).
A recent review has discussed the role of TUS in diagnosing and staging lung cancer[47]. In particular, the authors have stressed the usefulness of TUS in staging both local tumor spread (T) and distant lymph node invasion (N), as well as in detecting distant metastases (M). Identification of infiltration of the thoracic wall results in a change of the surgical approach because, in such cases, the affected sections of the thoracic wall must be resected together with the primary tumor. In this regard, TUS has been reported to have a significantly higher sensitivity than chest CT in diagnosing thoracic wall infiltration (89%-100% vs 42%-68%)[48,49]. Both direct evidence of infiltration of the wall structures and rib destruction can be considered TUS criteria for infiltration of the thoracic wall (Figure 18), whereas an interruption of the pleural line and/or limited respiratory movement of the consolidated lesion provide a suspicion but not a proof of infiltration of the thoracic wall[47,48]. The presence of pleural effusion in a patient with bronchial carcinoma usually indicates pleural involvement, and corresponds to a T4 stage tumor[47]. Less frequently, pleural effusion is a para-malignancy that accompanies effusion, due to lymphatic drainage dysfunction, atelectasis, or hypoproteinemia[50]. It follows that correct staging requires differentiation between malignant pleural effusion and reactive accompanying effusion, and TUS is considered the method of choice to identify pleural effusion, as well as to guide aspiration for cytological examination. With respect to N stage, TUS enables one to identify supraclavicular or cervical lymph node involvement, and this finding has a central role in the staging of bronchial carcinoma because lymph node metastases can be identified in 16%-26% of patients[51-53]. Finally, US examination of the abdomen can identify the presence of distant metastases in the liver or adrenal glands, thus contributing to M staging of bronchial carcinoma[47]. However, notwithstanding the usefulness of TUS and the central role played in detecting infiltration of the thoracic wall, CT examination is mandatory for correct and complete staging of lung cancer.
Figure 18.
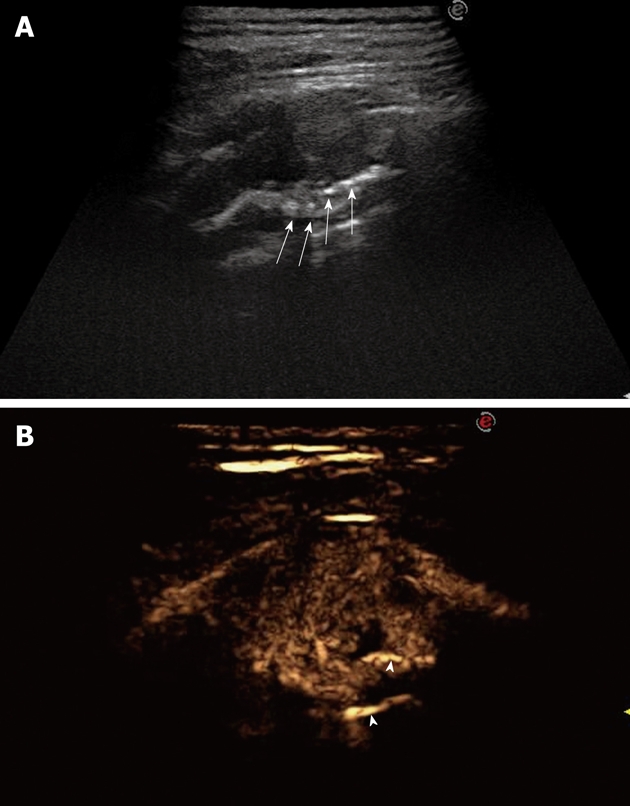
Bronchial carcinoma infiltrating the pleural wall. A: Posterior intercostal scan shows a hypoechoic lesion accompanied by rib destruction (arrows); B: Twenty-four seconds after iv bolus of contrast agent, the lesion appears inhomogeneously enhanced; the disrupted rib appears more echogenic than the tumor (arrowheads), as a consequence of the incomplete tissue suppression due to the strong echogenicity of bone tissue.
At color Doppler examination, malignant tumors can present with reduced vessel visualization[18]. However, as a rule, color Doppler sonography is not suitable for differentiating benign from malignant peripheral lesions. Based on the dual arterial supply of the lung, CEUS is able to characterize pulmonary arterial and bronchial arterial lung vascularity. Although a definitive differentiation between benign and malignant lesions is not yet possible, preliminary data in the literature support our personal experience in suggesting a possible role of CEUS in the diagnostic workup of patients with pleural-based pulmonary nodules of unknown origin[18,23-26]. Typical CEUS findings in pleural-based lung cancer are a delayed time to enhancement and variable extent of enhancement (Figure 19), which indicates a preferential bronchial arterial supply, which plays a major role in the tumor neoangiogenesis of growing cancer[18,23-26].
Figure 19.
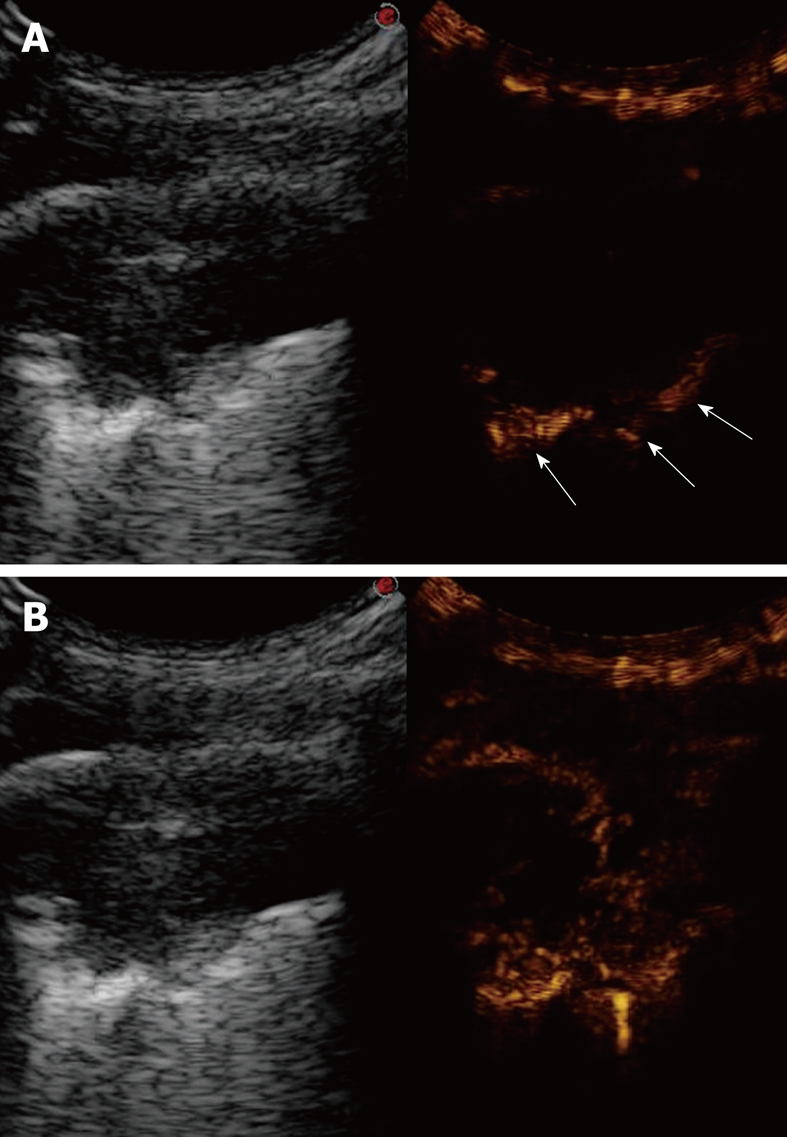
Contrast-enhanced ultrasonography of bronchial carcinoma. A: Baseline scan shows a hypoechoic lesion with irregular borders (left side of the split-screen). Ten seconds after iv bolus of contrast agent, the pulmonary parenchyma near the lesion is already enhanced (arrows), whereas the lesions is still unenhanced (right side of the split-screen); B: Twenty seconds later, the lesion shows delayed inhomogeneous enhancement, which indicates a preferential bronchial arterial supply (right side of the split-screen).
Pulmonary metastases
The presence of one or more pulmonary nodules in a patient with a history of underlying malignancy is almost always suggestive of metastatic disease. Spiral CT scanning is the most accurate imaging modality for detecting nodules over the entire lung[14,54-56]. TUS presents the limit of an incomplete view of the lung parenchyma. However, TUS enables one to visualize even small peripheral metastatic lesions, and represents the method of choice to guide the biopsy of peripheral lung lesions that abut the pleura[1].
Pulmonary metastases can appear at TUS with varying echotexture, round shape, and typically clear borders[18] (Figure 20). A reliable differentiation between pulmonary metastases and metastases of the parietal pleura is possible on the basis of the lack of respiratory excursion. However, metastases of the visceral pleura that do not involve the parietal pleura can present respiration-dependent movement, like pulmonary metastases. Peripheral pulmonary metastases are typically small, therefore, it is usually not possible to derive flow signals at color Doppler sonography[18]. Furthermore, respiration-dependent or pulsatile motion artifacts are often present, which makes the value of color Doppler examination hardly useful for the evaluation of peripheral pulmonary metastases. Compared to color Doppler sonography, CEUS can also be performed for small lesions. The arterial supply of lung metastases derives from systemic arterial circulation, therefore, at CEUS examination, they are usually characterized by delayed contrast enhancement and reduced contrast agent extent with respect to the pulmonary parenchyma (Figure 21)[18,23-26].
Figure 20.
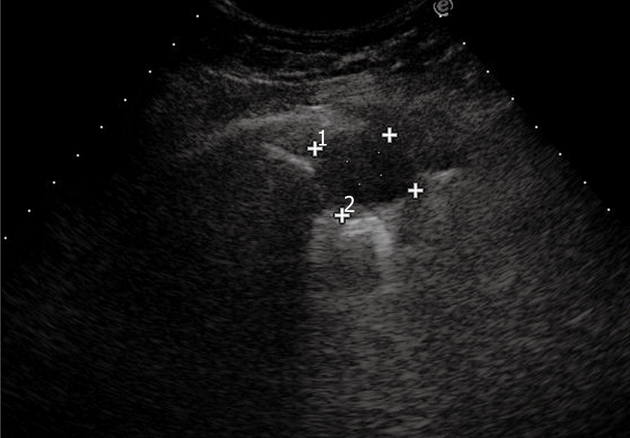
Pulmonary metastasis. Posterior intercostal scan shows a round-shaped, clear-bordered lesion.
Figure 21.
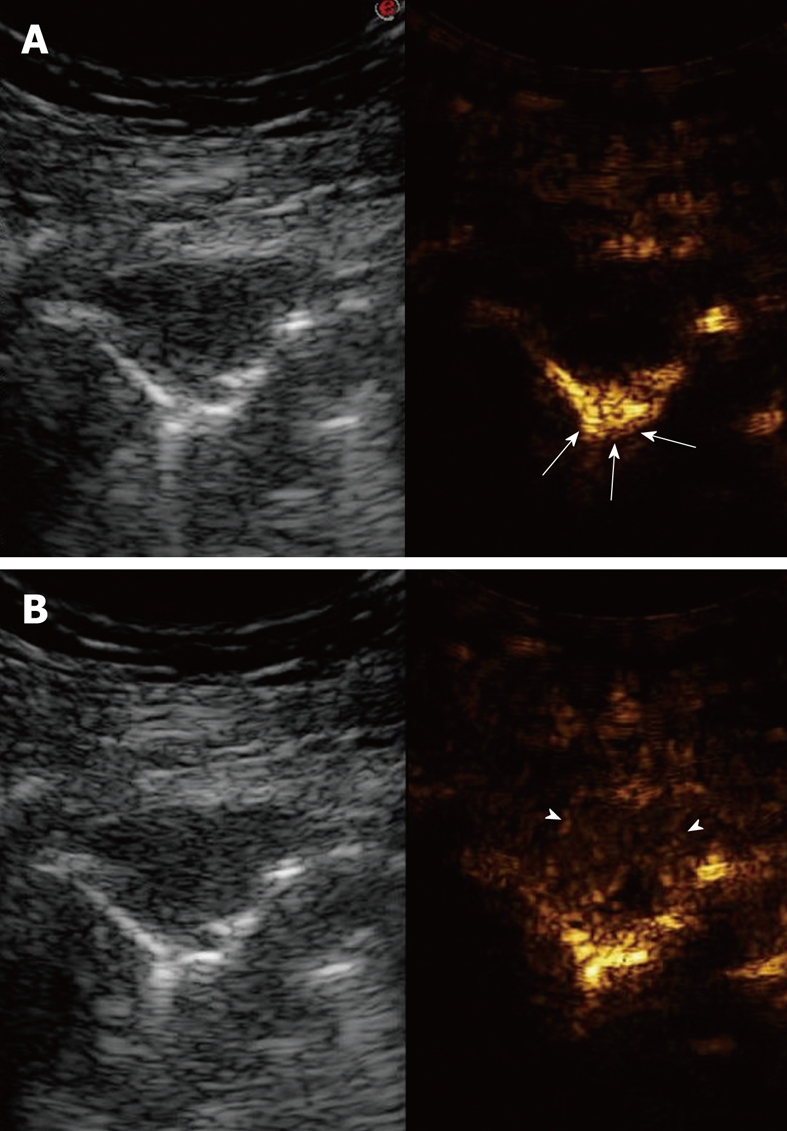
Contrast-enhanced ultrasonography of pulmonary metastasis. A: Baseline scan shows a small hypoechoic lesion (left side of the split-screen). Ten seconds after iv bolus of contrast agent, the lesion appears unenhanced with respect to the early enhancement of the pulmonary parenchyma (arrows) (right side of the split-screen); B: Fifty seconds later, the lesion shows inhomogeneous enhancement with reduced contrast agent extent (arrowhead) with respect to the pulmonary parenchyma (right side of the split-screen).
CONCLUSION
The limits of ultrasonography in the study of the lung are well established. First, the sonographic waves are hindered by air and bony structures, therefore, TUS cannot detect subscapular, paravertebral and retrosternal lesions, nor provide any diagnostic information in the presence of subcutaneous emphysema, and achieves poor visualization of the mediastinum. Second, it can only depict processes at the level of the pleura, thus, centrally located lesions cannot be detected by TUS. Finally, TUS is strictly operator dependent, and a lot of experience is needed to perform a reliable evaluation of pulmonary diseases. It follows that TUS cannot replace chest CT in the study of the lung.
However, certain diseases can be diagnosed with TUS (pneumonia with or without accompanying pleural effusion, PE, and atelectasis), other diseases can be suspected (diffuse parenchymal diseases, ARDS/NRDS, lung cancer and lung metastases that abut the pleura), and some preliminary experiences suggest that the use of second-generation ultrasound contrast agents provides useful information in the differential diagnosis between neoplastic and inflammatory diseases. Moreover, diagnostic and therapeutic interventional procedures can be performed under sonographic guidance. In this regard, TUS is considered the method of choice to guide aspiration and drainage of pleural effusion and empyema, and TUS-guided biopsy has been proved to be as effective as CT-guided biopsy of peripheral pulmonary lesions when they are in contact with the pleura and provide an adequate acoustic window. Finally, the well-known advantages of ultrasonography, such as the lack of radiation exposure, easy availability, and possibility of performing examination at almost any location, make TUS a valuable tool to be used without restriction in pregnant women, newborns, and at the bedside in critically ill patients, as well as to monitor the course of the disease.
In conclusion, TUS of the lung and pleura should be considered a very useful method that is complementary to chest CT in the diagnostic workup of pleuropulmonary pathology, and some effort should be encouraged in clinical and radiological departments to train skilled examiners with wide experience in TUS.
Footnotes
Peer reviewers: Jai Soung Park, MD, PhD, Professor, Department of Radiology, Soonchunhyang University Buchen Hospital, 1174 jung-dong, Wonmi-gu, Bucheon, Gyeonggi-do 420-767, South Korea; Ritesh Agarwal, MD, DM, MAMS, FCCP, Assistant Professor, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Sector-12, Chandigarh 160012, India
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pleuropulmonary pathology. World J Radiol. 2010;2:83–90. doi: 10.4329/wjr.v2.i2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soldati G, Copetti R. Ecografia toracica. Torino: CG Edizioni Medico-Scientifiche; 2006. pp. 12–21. [Google Scholar]
- 3.Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 4.Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–1270. doi: 10.1016/j.amjcard.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 6.Avruch L, Cooperberg PL. The ring-down artifact [Abstract] J Ultrasound Med. 1985;4:21–28. doi: 10.7863/jum.1985.4.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Soldati G, Rossi M. Wet and dry lungs: a useful sonographic distinction [abstract] Crit Care. 1999;3 Suppl 1:61. [Google Scholar]
- 8.Kroegel C, Reissig A, Hengst U. [Diagnosis of parenchymal lung diseases. Possibilities and limits of transthoracic sonography] Dtsch Med Wochenschr. 1999;124:765–772. doi: 10.1055/s-2007-1024410. [DOI] [PubMed] [Google Scholar]
- 9.Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003;22:173–180. doi: 10.7863/jum.2003.22.2.173. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 11.Miller WT Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. AJR Am J Roentgenol. 2005;184:613–622. doi: 10.2214/ajr.184.2.01840613. [DOI] [PubMed] [Google Scholar]
- 12.Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med. 2009;28:163–174. doi: 10.7863/jum.2009.28.2.163. [DOI] [PubMed] [Google Scholar]
- 13.Soldati G. Lung sonography: artifact, movement or echotexture. J Ultrasound. 2001;4:329–338. [Google Scholar]
- 14.Beckh S, Bölcskei PL, Lessnau KD. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759–1773. doi: 10.1378/chest.122.5.1759. [DOI] [PubMed] [Google Scholar]
- 15.Acunas B, Celik L, Acunas A. Chest sonography. Differentiation of pulmonary consolidation from pleural disease. Acta Radiol. 1989;30:273–275. [PubMed] [Google Scholar]
- 16.Kurian J, Levin TL, Han BK, Taragin BH, Weinstein S. Comparison of ultrasound and CT in the evaluation of pneumonia complicated by parapneumonic effusion in children. AJR Am J Roentgenol. 2009;193:1648–1654. doi: 10.2214/AJR.09.2791. [DOI] [PubMed] [Google Scholar]
- 17.Balfour-Lynn IM, Abrahamson E, Cohen G, Hartley J, King S, Parikh D, Spencer D, Thomson AH, Urquhart D. BTS guidelines for the management of pleural infection in children. Thorax. 2005;60 Suppl 1:i1–i21. doi: 10.1136/thx.2004.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reissig A, Görg C, Mathis G. Transthoracic sonography in the diagnosis of pulmonary diseases: a systematic approach. Ultraschall Med. 2009;30:438–454; quiz 455-456. doi: 10.1055/s-0028-1109703. [DOI] [PubMed] [Google Scholar]
- 19.Yang PC, Luh KT, Chang DB, Yu CJ, Kuo SH, Wu HD. Ultrasonographic evaluation of pulmonary consolidation. Am Rev Respir Dis. 1992;146:757–762. doi: 10.1164/ajrccm/146.3.757. [DOI] [PubMed] [Google Scholar]
- 20.Targhetta R, Chavagneux R, Bourgeois JM, Dauzat M, Balmes P, Pourcelot L. Sonographic approach to diagnosing pulmonary consolidation. J Ultrasound Med. 1992;11:667–672. doi: 10.7863/jum.1992.11.12.667. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg B, Diakoumakis EE, Kass EG, Seife B, Zvi ZB. The air bronchogram: sonographic demonstration. AJR Am J Roentgenol. 1986;147:593–595. doi: 10.2214/ajr.147.3.593. [DOI] [PubMed] [Google Scholar]
- 22.Reissig A, Kroegel C. Sonographic diagnosis and follow-up of pneumonia: a prospective study. Respiration. 2007;74:537–547. doi: 10.1159/000100427. [DOI] [PubMed] [Google Scholar]
- 23.Görg C. Transcutaneous contrast-enhanced sonography of pleural-based pulmonary lesions. Eur J Radiol. 2007;64:213–221. doi: 10.1016/j.ejrad.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Sperandeo M, Sperandeo G, Varriale A, Filabozzi P, Decuzzi M, Dimitri L, Vendemiale G. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: a preliminary study. Ultrasound Med Biol. 2006;32:1467–1472. doi: 10.1016/j.ultrasmedbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Görg C, Kring R, Bert T. Transcutaneous contrast-enhanced sonography of peripheral lung lesions. AJR Am J Roentgenol. 2006;187:W420–W429. doi: 10.2214/AJR.05.0890. [DOI] [PubMed] [Google Scholar]
- 26.Görg C, Bert T, Kring R, Dempfle A. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: experience in 137 patients. Ultraschall Med. 2006;27:437–444. doi: 10.1055/s-2006-927021. [DOI] [PubMed] [Google Scholar]
- 27.Alderson PO, Martin EC. Pulmonary embolism: diagnosis with multiple imaging modalities. Radiology. 1987;164:297–312. doi: 10.1148/radiology.164.2.3299482. [DOI] [PubMed] [Google Scholar]
- 28.Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 29.Remy-Jardin M, Remy J, Artaud D, Fribourg M, Beregi JP. Spiral CT of pulmonary embolism: diagnostic approach, interpretive pitfalls and current indications. Eur Radiol. 1998;8:1376–1390. doi: 10.1007/s003300050557. [DOI] [PubMed] [Google Scholar]
- 30.Kim KI, Müller NL, Mayo JR. Clinically suspected pulmonary embolism: utility of spiral CT. Radiology. 1999;210:693–697. doi: 10.1148/radiology.210.3.r99mr01693. [DOI] [PubMed] [Google Scholar]
- 31.Garg K, Sieler H, Welsh CH, Johnston RJ, Russ PD. Clinical validity of helical CT being interpreted as negative for pulmonary embolism: implications for patient treatment. AJR Am J Roentgenol. 1999;172:1627–1631. doi: 10.2214/ajr.172.6.10350303. [DOI] [PubMed] [Google Scholar]
- 32.Goldhaber SZ. Pulmonary embolism. N Engl J Med. 1998;339:93–104. doi: 10.1056/NEJM199807093390207. [DOI] [PubMed] [Google Scholar]
- 33.Mathis G, Bitschnau R, Gehmacher O, Scheier M, Kopf A, Schwärzler B, Amann T, Doringer W, Hergan K. Chest ultrasound in diagnosis of pulmonary embolism in comparison to helical CT. Ultraschall Med. 1999;20:54–59. doi: 10.1055/s-1999-14234. [DOI] [PubMed] [Google Scholar]
- 34.Mathis G, Blank W, Reissig A, Lechleitner P, Reuss J, Schuler A, Beckh S. Thoracic ultrasound for diagnosing pulmonary embolism: a prospective multicenter study of 352 patients. Chest. 2005;128:1531–1538. doi: 10.1378/chest.128.3.1531. [DOI] [PubMed] [Google Scholar]
- 35.Reissig A, Kroegel C. Transthoracic ultrasound of lung and pleura in the diagnosis of pulmonary embolism: a novel non-invasive bedside approach. Respiration. 2003;70:441–452. doi: 10.1159/000074195. [DOI] [PubMed] [Google Scholar]
- 36.Reissig A, Heyne JP, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest. 2001;120:1977–1983. doi: 10.1378/chest.120.6.1977. [DOI] [PubMed] [Google Scholar]
- 37.Lechleitner P, Riedl B, Raneburger W, Gamper G, Theurl A, Lederer A. Chest sonography in the diagnosis of pulmonary embolism: a comparison with MRI angiography and ventilation perfusion scintigraphy. Ultraschall Med. 2002;23:373–378. doi: 10.1055/s-2002-36173. [DOI] [PubMed] [Google Scholar]
- 38.Niemann T, Egelhof T, Bongartz G. Transthoracic sonography for the detection of pulmonary embolism--a meta-analysis. Ultraschall Med. 2009;30:150–156. doi: 10.1055/s-2008-1027856. [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009;135:1421–1425. doi: 10.1378/chest.08-2281. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Gardelli G, Feletti F, Gamberini E, Bonarelli S, Nanni A, Mughetti M. Using sonography to assess lung recruitment in patients with acute respiratory distress syndrome. Emerg Radiol. 2009;16:219–221. doi: 10.1007/s10140-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 42.Caironi P, Gattinoni L. How to monitor lung recruitment in patients with acute lung injury. Curr Opin Crit Care. 2007;13:338–343. doi: 10.1097/MCC.0b013e32814db80c. [DOI] [PubMed] [Google Scholar]
- 43.Bober K, Swietliński J. Diagnostic utility of ultrasonography for respiratory distress syndrome in neonates. Med Sci Monit. 2006;12:CR440–CR446. [PubMed] [Google Scholar]
- 44.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosgrove DO, Garbutt P, Hill CR. Echoes across the diaphragm. Ultrasound Med Biol. 1978;3:385–392. doi: 10.1016/0301-5629(78)90081-9. [DOI] [PubMed] [Google Scholar]
- 46.Avni EF, Braude P, Pardou A, Matos C. Hyaline membrane disease in the newborn: diagnosis by ultrasound. Pediatr Radiol. 1990;20:143–146. doi: 10.1007/BF02012957. [DOI] [PubMed] [Google Scholar]
- 47.Prosch H, Mathis G, Mostbeck GH. Percutaneous ultrasound in diagnosis and staging of lung cancer. Ultraschall Med. 2008;29:466–478; quiz 479-484. doi: 10.1055/s-2008-1027672. [DOI] [PubMed] [Google Scholar]
- 48.Bandi V, Lunn W, Ernst A, Eberhardt R, Hoffmann H, Herth FJ. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest. 2008;133:881–886. doi: 10.1378/chest.07-1656. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki N, Saitoh T, Kitamura S. Tumor invasion of the chest wall in lung cancer: diagnosis with US. Radiology. 1993;187:39–42. doi: 10.1148/radiology.187.1.8451433. [DOI] [PubMed] [Google Scholar]
- 50.Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:131S–148S. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 51.van Overhagen H, Brakel K, Heijenbrok MW, van Kasteren JH, van de Moosdijk CN, Roldaan AC, van Gils AP, Hansen BE. Metastases in supraclavicular lymph nodes in lung cancer: assessment with palpation, US, and CT. Radiology. 2004;232:75–80. doi: 10.1148/radiol.2321030663. [DOI] [PubMed] [Google Scholar]
- 52.Fultz PJ, Feins RH, Strang JG, Wandtke JC, Johnstone DW, Watson TJ, Gottlieb RH, Voci SL, Rubens DJ. Detection and diagnosis of nonpalpable supraclavicular lymph nodes in lung cancer at CT and US. Radiology. 2002;222:245–251. doi: 10.1148/radiol.2221010431. [DOI] [PubMed] [Google Scholar]
- 53.Prosch H, Strasser G, Sonka C, Oschatz E, Mashaal S, Mohn-Staudner A, Mostbeck GH. Cervical ultrasound (US) and US-guided lymph node biopsy as a routine procedure for staging of lung cancer. Ultraschall Med. 2007;28:598–603. doi: 10.1055/s-2007-963215. [DOI] [PubMed] [Google Scholar]
- 54.Munden RF, Pugatch RD, Liptay MJ, Sugarbaker DJ, Le LU. Small pulmonary lesions detected at CT: clinical importance. Radiology. 1997;202:105–110. doi: 10.1148/radiology.202.1.8988198. [DOI] [PubMed] [Google Scholar]
- 55.Remy-Jardin M, Remy J, Giraud F, Marquette CH. Pulmonary nodules: detection with thick-section spiral CT versus conventional CT. Radiology. 1993;187:513–5200. doi: 10.1148/radiology.187.2.8475300. [DOI] [PubMed] [Google Scholar]
- 56.Webb WR. Radiologic evaluation of the solitary pulmonary nodule. A. JR Am J Roentgenol. 1990;154:701–708. doi: 10.2214/ajr.154.4.2107661. [DOI] [PubMed] [Google Scholar]