Abstract
Cerebral arteriovenous malformations (AVMs) have abnormally enlarged arteries and veins prone to spontaneous hemorrhage. Immediately following surgical excision of a cerebral AVM, even normal brain tissue surrounding the lesion is subject to hemorrhage, a phenomenon termed normal perfusion pressure breakthrough (NPPB) syndrome. According to this theory, arteries supplying cerebral AVMs become dilated and lose their capacity to dilate or constrict to autoregulate pressure. Acutely after removal of a cerebral AVM, excessive blood pressure in these arterial feeders can cause normal brain tissue to bleed. However, this theory remains controversial. We present a patient with a cerebral AVM that demonstrated cerebrovascular reactivity and argues against an assumption underlying the theory of NPPB syndrome.
Keywords: Arteriovenous malformation, Autoregulation, Normal perfusion pressure breakthrough, Subarachnoid hemorrhage, Vasospasm
INTRODUCTION
Cerebral arteriovenous malformations (AVMs) have abnormally enlarged arteries and veins prone to spontaneous hemorrhage. Immediately following surgical excision of a cerebral AVM, the remaining normal brain tissue surrounding the lesion is subject to hemorrhage, a phenomenon termed normal perfusion pressure breakthrough (NPPB) syndrome[1]. The theory proposed to explain this phenomenon remains controversial. We present a patient with cerebral AVM that demonstrated cerebrovascular reactivity and argues against an assumption underlying the theory of NPPB syndrome.
CASE REPORT
A 47 year-old woman with chronic headaches and a previously diagnosed right occipital cerebral AVM developed a severe headache followed 5 d later by left-sided weakness and neglect. Computed tomography and magnetic resonance imaging brain scans showed subacute subarachnoid hemorrhage and ischemic changes in the right middle cerebral artery distribution (Figure 1A and B).
Figure 1.
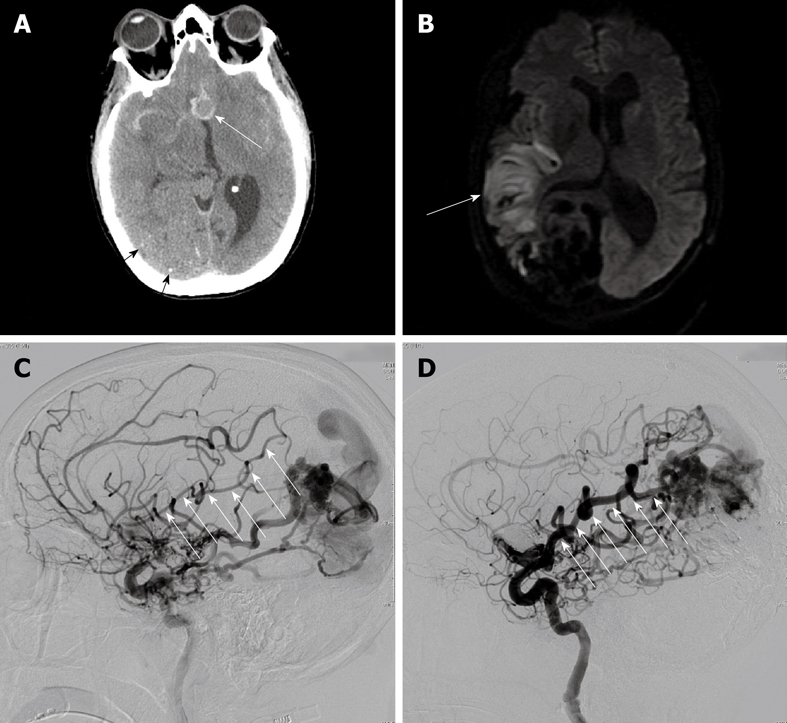
A 47-year-old woman with ruptured anterior communicating artery aneurysm associated with a large arteriovenous malformation. A: Computed tomography brain scan shows the 12 mm anterior communicating artery aneurysm in relief surrounded by high-density subarachnoid hemorrhage (white arrow). Also note small stippled calcifications in the large parietal-occipital arteriovenous malformation (black arrows); B: Diffusion-weighted magnetic resonance imaging shows area of restricted diffusion in the posterior right frontal lobe (white arrow) corresponding with the patient’s left-sided weakness and hemi-neglect; C: Catheter arteriography of the right internal carotid artery in the lateral projection at the time of initial presentation approximately 1 wk following subarachnoid hemorrhage shows significant narrowing in the angular and Rolandic branches of the right middle cerebral artery, both of which supply the arteriovenous malformation (white arrows); D: Catheter arteriography of the right internal carotid artery in the lateral projection 4 mo after initial presentation and following complete neurological recovery shows resolution of vasospasm in the angular and parietal branches of the right middle cerebral artery supplying the arteriovenous malformation (white arrows).
Catheter arteriography demonstrated a 12 mm anterior communicating artery aneurysm, a 5 cm right occipital cerebral AVM, and relatively slow flow through small caliber arteries to the cerebral AVM representing vasospasm (Figure 1C). Endovascular coil occlusion of the aneurysm was immediately performed, and the vasospasm was treated with verapamil. Subsequent arteriography for treatment of the cerebral AVM showed interval resolution of the narrowed arteries in the distribution of the stroke (Figure 1D). Despite her deficits at the time of original presentation with stroke, the patient made a full neurological recovery, even after embolization and resection of the cerebral AVM.
DISCUSSION
Cerebral AVMs can cause pathological states including hemorrhage, vascular steal, chronic hypoperfusion, and low-grade ischemia, all of which were demonstrated by the patient reported here. In 1978, Spetzler et al[1] published the theory of NPPB, positing that chronic hypoperfusion causes branches of the AVM feeding arteries, that supply normal tissue, to become markedly dilated, remaining paralyzed due to lost autoregulatory capacity. After AVM extirpation, these branches in the remaining normal tissue experience increased perfusion but lack ability to constrict and autoregulate cerebral blood flow. They argued that, in some cases, this vascular dysfunction leads to hyperemia and compromise of capillary beds, resulting in massive edema and hemorrhage.
Several studies using various methodologies have supported the claims of NPPB[2-9]. However, more recent investigations contradict many aspects of the theory, casting doubt on the link between impaired vasoreactivity and postoperative complications. NPPB theory rests on the assumption that lost autoregulatory capacity persists following resection of a cerebral AVM. Nevertheless, several studies demonstrate a postoperative return to normal CO2 reactivity in vessels with previously diminished reactivity[3,10-16]. Young et al[12,13] showed intact CO2 reactivity both before and after resection. Similarly, Ogasawara demonstrated postoperative hyperperfusion in areas with normal response to acetazolamide in the preoperative period[17].
Young et al[18] reported that cerebral AVM removal improved perfusion in the hemisphere ipsilateral to the lesion, but increasing mean arterial pressure pharmacologically did not increase CBF, suggesting intact cerebral autoregulation. This was true for uncomplicated cases, as well as patients with NPPB-like complications. Demonstrating intact vasoreactivity both before and after resection, Young et al[13,18,19] suggested the true problem is a leftward shift of the autoregulatory curve such that normal pressure exceeds the upper limit of autoregulation.
Batjer demonstrated the ability of arteries to vasodilate but an inability to vasoconstrict in response to increased pressure, a finding confirmed in a rat model[11,20,21]. We suggest that the vasoconstriction in response to SAH in our patient demonstrated that the impairment of vasoconstriction in response to elevated pressure, which has been argued to underlie NPPB syndrome, is not due to some global inability of the vasculature to constrict. This agrees with both Batjer’s findings and Young’s proposal of a leftward shift of the autoregulation curve. However, we must maintain the possibility that vasospasm in response to SAH and the vessel properties underlying autoregulation, which have been argued are impaired in NPPB-like states, might result from two distinct mechanisms.
Another condition of Spetzler’s theory that has come under scrutiny is that NPPB-like complications occur in areas adjacent to the malformation that formerly shared the arterial blood supply with it, and that such former feeders are prone to complications because of the chronic stress placed upon them by the cerebral AVM. Barnett et al[3] showed the worst steal effect exists in tissue 2-4 cm from the malformation, and higher flow occurred in tissue distal to the location of the cerebral AVM. To the contrary, Young et al[22] showed that increased cerebral blood flow following cerebral AVM resection occurs throughout the entire brain, not just in regions that shared vascular supply with the cerebral AVM. This suggests that mechanisms implicating preoperative focal hypoperfusion due to vascular steal are not the sole cause of postoperative complications. Indeed, our patient demonstrated vasospasm, infarction, and edema both ipsilateral and contralateral to her AVM, consistent with such findings.
The findings in this case illustrate that arteries surrounding cerebral AVMs can maintain vasoconstrictive capacity, although the contractile activity in response to subarachnoid hemorrhage and autoregulation might be different processes. Future studies should compare microvascular changes between procedures with uneventful cerebral AVM resection and those with these complications. However, comparison will prove difficult given the relative rarity of these complications and the resultant difficulty designing adequately powered investigations. Furthermore, better diagnostic definitions for these complications must be standardized. Differing interpretations of what constitutes an NPPB-like state have undoubtedly made studies difficult to compare. Better characterization of blood flow parameters surrounding cerebral AVMs will lead to improved prevention, prompt identification, and treatment.
Footnotes
Peer reviewer: Panagiotis Antoniou, PhD, MSc, Medical Physics, Certified Medical Physicist, School of Medicine Democritus University of Thrace, 31 Irinis Str. Alexandroupolis 68100, Greece
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 2.Nornes H, Grip A. Hemodynamic aspects of cerebral arteriovenous malformations. J Neurosurg. 1980;53:456–464. doi: 10.3171/jns.1980.53.4.0456. [DOI] [PubMed] [Google Scholar]
- 3.Barnett GH, Little JR, Ebrahim ZY, Jones SC, Friel HT. Cerebral circulation during arteriovenous malformation operation. Neurosurgery. 1987;20:836–842. doi: 10.1227/00006123-198706000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Muraszko K, Wang HH, Pelton G, Stein BM. A study of the reactivity of feeding vessels to arteriovenous malformations: correlation with clinical outcome. Neurosurgery. 1990;26:190–199; discussion 199-200. doi: 10.1097/00006123-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Pennings FA, Ince C, Bouma GJ. Continuous real-time visualization of the human cerebral microcirculation during arteriovenous malformation surgery using orthogonal polarization spectral imaging. Neurosurgery. 2006;59:167–171; discussion 167-171. doi: 10.1227/01.NEU.0000219242.92669.3B. [DOI] [PubMed] [Google Scholar]
- 6.Chyatte D. Normal pressure perfusion breakthrough after resection of arteriovenous malformation. J Stroke Cerebrovasc Dis. 1997;6:130–136. doi: 10.1016/s1052-3057(97)80229-7. [DOI] [PubMed] [Google Scholar]
- 7.Luessenhop AJ, Ferraz FM, Rosa L. Estimate of the incidence and importance of circulatory breakthrough in the surgery of cerebral arteriovenous malformations. Neurol Res. 1982;4:177–190. doi: 10.1080/01616412.1982.11739622. [DOI] [PubMed] [Google Scholar]
- 8.Asgari S, Röhrborn HJ, Engelhorn T, Fauser B, Stolke D. Intraoperative measurement of cortical oxygen saturation and blood volume adjacent to cerebral arteriovenous malformations using near-infrared spectroscopy. Neurosurgery. 2003;52:1298–1304; discussion 1304-1306. doi: 10.1227/01.neu.0000064801.78895.86. [DOI] [PubMed] [Google Scholar]
- 9.Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg. 1987;67:17–28. doi: 10.3171/jns.1987.67.1.0017. [DOI] [PubMed] [Google Scholar]
- 10.Hassler W, Steinmetz H. Cerebral hemodynamics in angioma patients: an intraoperative study. J Neurosurg. 1987;67:822–831. doi: 10.3171/jns.1987.67.6.0822. [DOI] [PubMed] [Google Scholar]
- 11.Batjer HH, Devous MD Sr, Meyer YJ, Purdy PD, Samson DS. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery. 1988;22:503–509. doi: 10.1227/00006123-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Young WL, Prohovnik I, Ornstein E, Ostapkovich N, Sisti MB, Solomon RA, Stein BM. The effect of arteriovenous malformation resection on cerebrovascular reactivity to carbon dioxide. Neurosurgery. 1990;27:257–266; discussion 266-267. doi: 10.1097/00006123-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Young WL, Solomon RA, Prohovnik I, Ornstein E, Weinstein J, Stein BM. 133Xe blood flow monitoring during arteriovenous malformation resection: a case of intraoperative hyperperfusion with subsequent brain swelling. Neurosurgery. 1988;22:765–769. doi: 10.1227/00006123-198804000-00028. [DOI] [PubMed] [Google Scholar]
- 14.De Salles AA, Manchola I. CO2 reactivity in arteriovenous malformations of the brain: a transcranial Doppler ultrasound study. J Neurosurg. 1994;80:624–630. doi: 10.3171/jns.1994.80.4.0624. [DOI] [PubMed] [Google Scholar]
- 15.Diehl RR, Henkes H, Nahser HC, Kühne D, Berlit P. Blood flow velocity and vasomotor reactivity in patients with arteriovenous malformations. A transcranial Doppler study. Stroke. 1994;25:1574–1580. doi: 10.1161/01.str.25.8.1574. [DOI] [PubMed] [Google Scholar]
- 16.Massaro AR, Young WL, Kader A, Ostapkovich N, Tatemichi TK, Stein BM, Mohr JP. Characterization of arteriovenous malformation feeding vessels by carbon dioxide reactivity. AJNR Am J Neuroradiol. 1994;15:55–61. [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasawara K, Yoshida K, Otawara Y, Kobayashi M, Yasuda S, Doi M, Ogawa A. Cerebral blood flow imaging in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Surg Neurol. 2001;56:380–384. doi: 10.1016/s0090-3019(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 18.Young WL, Kader A, Prohovnik I, Ornstein E, Fleischer LH, Ostapkovich N, Jackson LD, Stein BM. Pressure autoregulation is intact after arteriovenous malformation resection. Neurosurgery. 1993;32:491–496; discussion 496-497. doi: 10.1227/00006123-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Young WL, Pile-Spellman J, Prohovnik I, Kader A, Stein BM. Evidence for adaptive autoregulatory displacement in hypotensive cortical territories adjacent to arteriovenous malformations. Columbia University AVM Study Project. Neurosurgery. 1994;34:601–610; discussion 610-611. doi: 10.1227/00006123-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Batjer HH, Devous MD Sr. The use of acetazolamide-enhanced regional cerebral blood flow measurement to predict risk to arteriovenous malformation patients. Neurosurgery. 1992;31:213–217; discussion 217-218. doi: 10.1227/00006123-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Irikura K, Morii S, Miyasaka Y, Yamada M, Tokiwa K, Yada K. Impaired autoregulation in an experimental model of chronic cerebral hypoperfusion in rats. Stroke. 1996;27:1399–1404. doi: 10.1161/01.str.27.8.1399. [DOI] [PubMed] [Google Scholar]
- 22.Young WL, Kader A, Ornstein E, Baker KZ, Ostapkovich N, Pile-Spellman J, Fogarty-Mack P, Stein BM. Cerebral hyperemia after arteriovenous malformation resection is related to "breakthrough" complications but not to feeding artery pressure. The Columbia University Arteriovenous Malformation Study Project. Neurosurgery. 1996;38:1085–093; discussion 1093-1095. doi: 10.1097/00006123-199606000-00005. [DOI] [PubMed] [Google Scholar]


